Nut Allergy: Clinical and Allergological Features in Italian Children
Abstract
1. Background
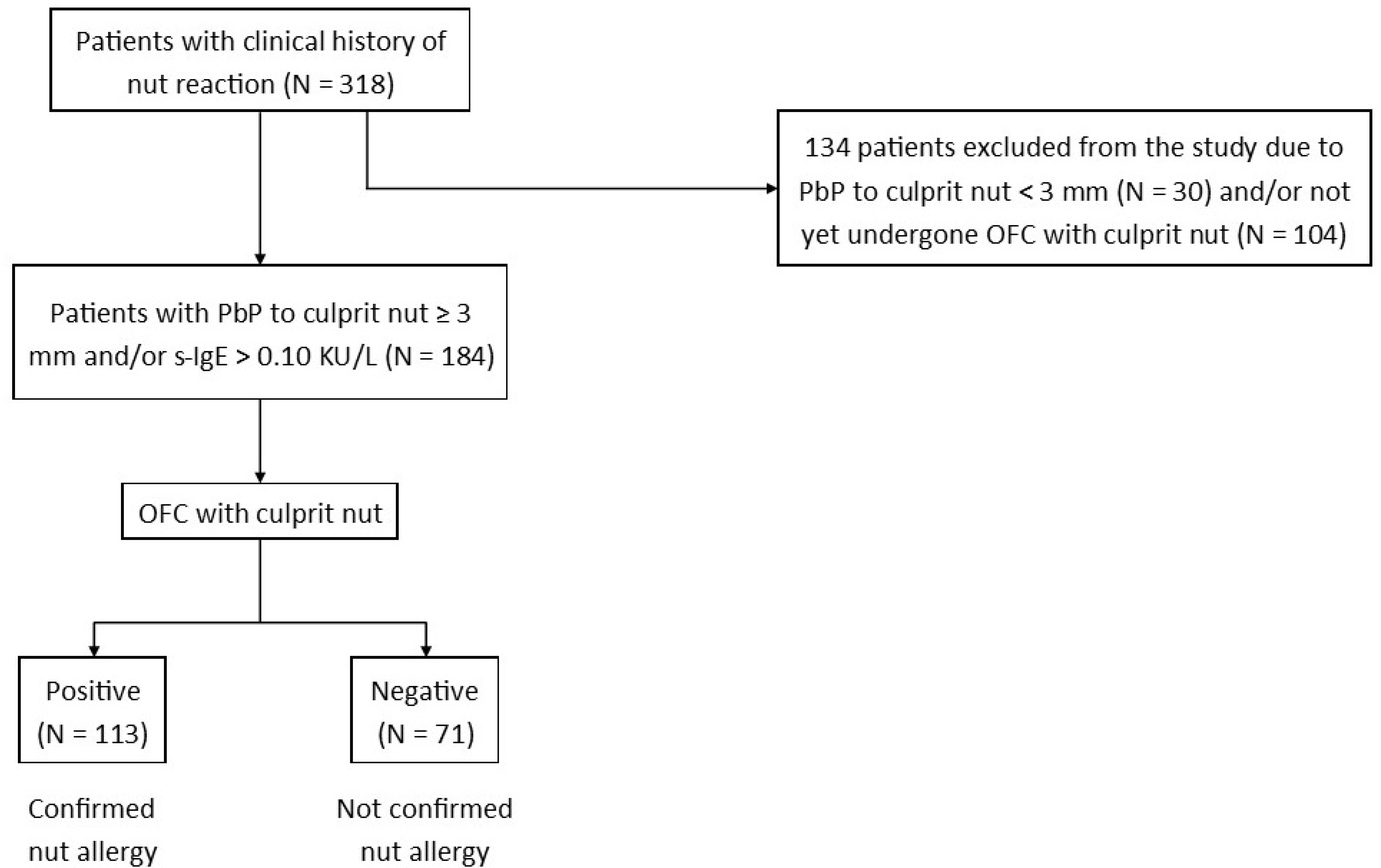
2. Materials and Methods
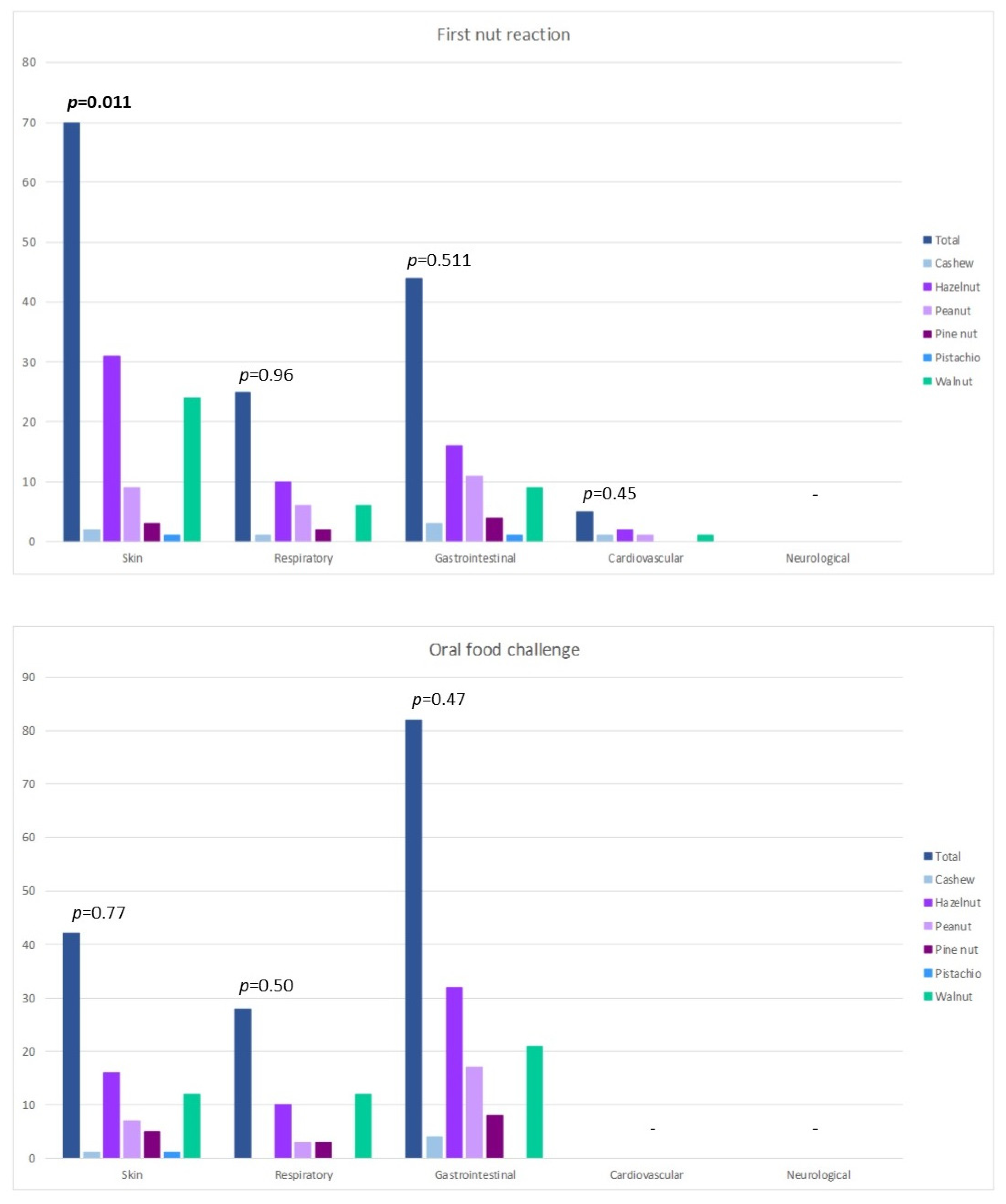
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OFC | oral food challenge |
| PbP | prick by prick |
| s-IgE | serum-specific IgE |
| SPT | skin prick test |
| TN | tree nuts |
References
- Gupta, R.; Warren, C.; Smith, B.; Blumenstock, J.; Jiang, J.; Davis, M.; Nadeau, K. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics 2018, 142, e20181235. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Munoz-Furlong, A.; Godbold, J.H.; Sampson, H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immunol. 2010, 125, 1322–1326. [Google Scholar] [CrossRef]
- Dunngalvin, A.; Dubois, A.E.J.; Blok, B.M.J.F.; Hourihane, J.O.B. The Effects of Food Allergy on Quality of Life. Chem. Immunol. Allergy 2015, 101, 235–252. [Google Scholar]
- Primeau, M.; Kagan, R.; Joseph, L.; Lim, H.; Dufresne, C.; Duffy, C.; Prhcal, D.; Clarke, A. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin. Exp. Allergy 2000, 30, 1135–1143. [Google Scholar] [CrossRef]
- Logan, K.; Du Toit, G.; Giovannini, M.; Turcanu, V.; Lack, G. Pediatric Allergic Diseases, Food Allergy, and Oral Tolerance. Annu. Rev. Cell Dev. Biol. 2020, 36, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, P.G.; Buyuktiryaki, B.; Soyer, O.; Sahiner, U.M.; Sekerel, B.E. Factors predicting anaphylaxis in children with tree nut allergies. Allergy Asthma Proc. 2019, 40, 180–186. [Google Scholar] [CrossRef]
- Eigenmann, P.A.; Lack, G.; Mazon, A.; Nieto, A.; Haddad, D.; Borugh, H.A.; Caubet, J.C. Managing Nut Allergy: A Remaining Clinical Challenge. J. Allergy Clin. Immunol. Pract. 2017, 5, 296–300. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, V.; Koplin, J.; Lodge, C.; Tang, M.; Dharmage, S.; Allen, K. The Prevalence of Tree Nut Allergy: A Systematic Review. Curr. Allergy Asthma Rep. 2015, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.A.; Muñoz-furlong, A.; Sampson, H.A. Fatalities due to anaphylactic reactions to foods. J. Allergy Clin. Immunol. 2001, 107, 191–193. [Google Scholar] [CrossRef]
- de Leon, M.P.; Rolland, J.M.; O’Hehir, R.E. The peanut allergy epidemic: Allergen molecular characterisation and prospects for specific therapy. Expert Rev. Mol. Med. 2007, 9, 1–18. [Google Scholar] [CrossRef]
- Stiefel, G.; Anagnostou, K.; Boyle, R.J.; Brathwaite, N.; Ewan, P.; Fox, A.T.; Huber, P.; Luyt, D.; Till, S.J.; Venter, C.; et al. BSACI guideline for the diagnosis and management of peanut and tree nut allergy. Clin. Exp. Allergy 2017, 47, 719–739. [Google Scholar] [CrossRef]
- Walsh, J.; O’Flynn, N. Diagnosis and assessment of food allergy in children and young people in primary care and community settings: NICE clinical guideline. Br. J. Gen. Pract. 2011, 61, 473–475. [Google Scholar] [CrossRef]
- Weinberger, T.; Sicherer, S. Current perspectives on tree nut allergy: A review. J. Asthma Allergy 2018, 11, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Elizur, A.; Appel, M.Y.; Nachshon, L.; Levy, M.B.; Epstein-Rigbi, N.; Golobov, K.; Goldberg, M. NUT Co Reactivity—ACquiring Knowledge for Elimination Recommendations (NUT CRACKER) study. Allergy 2018, 73, 593–601. [Google Scholar] [CrossRef]
- Giovannini, M.; Comberiati, P.; Piazza, M.; Chiesa, E.; Piacentini, G.L.; Boner, A.; Zanoni, G.; Peroni, D.G. Retrospective definition of reaction risk in Italian children with peanut, hazelnut and walnut allergy through component-resolved diagnosis. Allergol. Immunopathol. 2018, 47, 73–78. [Google Scholar] [CrossRef]
- Ortolani, C.; Ispano, M.; Pastorello, E.A.; Ansaloni, R.; Magri, G. Comparison of results of skin prick tests (with fresh foods and commercial food extracts) and RAST in 100 patients with oral allergic syndrome. J. Allergy Clin. Immunol. 1989, 83, 683–690. [Google Scholar] [CrossRef]
- Heinzerling, L.; Mari, A.; Bergmann, K.-C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Brockow, K.; Fernández Rivas, M.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Liccioli, G.; Sarti, L.; Giovannini, M.; Novembre, E.; Mori, F. Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina 2020, 56, 111. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, B.; Beyer, K. Time for a new grading system for allergic reactions? Allergy 2016, 71, 135–136. [Google Scholar] [CrossRef]
- Cetinkaya, P.G.; Buyuktiryaki, B.; Soyer, O.; Sahiner, U.M.; Sackesen, C.; Sekerel, B.E. Phenotypical characterization of tree nuts and peanut allergies in east Mediterranean children. Allergol. Immunopathol. 2020, 48, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Chan, E.S.; Sicherer, S. Peanut allergy: New advances and ongoing controversies. Pediatrics 2020, 145, e20192102. [Google Scholar] [CrossRef]
- Lyons, S.A.; Datema, M.R.; Le, T.M.; Asero, R.; Barreales, L.; Belohlavkova, S.; de Blay, F.; Clausen, M.; Dubakiene, R.; Fernández-Perez, C.; et al. Walnut Allergy Across Europe: Distribution of Allergen Sensitization Patterns and Prediction of Severity. J. Allergy Clin. Immunol. Pract. 2021, 9, 225–235.e10. [Google Scholar] [CrossRef]
- Calamelli, E.; Trozzo, A.; Di Blasi, E.; Serra, L.; Bottau, P. Hazelnut allergy. Medicina 2021, 57, 67. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, J.M.; Bagley, K.; Kulis, M. Tree nut allergies: Allergen homology, cross-reactivity, and implications for therapy. Clin. Exp. Allergy 2018, 48, 762–772. [Google Scholar] [CrossRef]
- Haroun-Díaz, E.; Azofra, J.; González-Mancebo, E.; de las Heras, M.; Pastor-Vargas, C.; Esteban, V.; Villalba, M.; Díaz-Perales, A.; Cuesta-Herranz, J. Nut allergy in two different areas of Spain: Differences in clinical and molecular pattern. Nutrients 2017, 9, 909. [Google Scholar] [CrossRef]
- Boyce, J.A.; Assa’ad, A.; Burks, W.A.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, H.S.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, 51–58. [Google Scholar] [CrossRef]
- Brough, H.A.; Caubet, J.-C.; Mazon, A.; Haddad, D.; Bergmann, M.M.; Wassenberg, J.; Panetta, V.; Gourgey, R.; Radulovic, S.; Nieto, M.; et al. Defining challenge-proven coexistent nut and sesame seed allergy: A prospective multicenter European study. J. Allergy Clin. Immunol. 2020, 145, 1231–1239. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Burks, A.W.; Sampson, H.A. Clinical Features of Acute Allergic Reactions to Peanut and Tree Nuts in Children. Pediatrics 1998, 102, e6. [Google Scholar] [CrossRef] [PubMed]
- Midun, E.; Radulovic, S.; Brough, H.; Caubet, J.C. Recent advances in the management of nut allergy. World Allergy Organ. J. 2021, 14, 100491. [Google Scholar] [CrossRef]
- Maloney, J.M.; Rudengren, M.; Ahlstedt, S.; Bock, S.A.; Sampson, H.A. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J. Allergy Clin. Immunol. 2008, 122, 145–151. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, V.; Peters, R.; Tang, M.L.K.; Dharmage, S.; Ponsonby, A.L.; Gurrin, L.; Perrett, K.; Koplin, J.; Allen, K.J.; Dwyer, T.; et al. Patterns of tree nut sensitization and allergy in the first 6 years of life in a population-based cohort. J. Allergy Clin. Immunol. 2019, 143, 644–650.e5. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Conover-Walker, M.K.; Matsui, E.C.; Wood, R.A. The natural history of tree nut allergy. J. Allergy Clin. Immunol. 2005, 116, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Couch, C.; Franxman, T.; Greenhawt, M. Characteristics of tree nut challenges in tree nut allergic and tree nut sensitized individuals. Ann. Allergy, Asthma Immunol. 2017, 118, 591–596. [Google Scholar] [CrossRef]
- Anagnostou, K. Safety of Oral Food Challenges in Early Life. Children 2018, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Becker, A.B. Oral food challenge outcomes in a pediatric tertiary care center. Allergy Asthma Clin. Immunol. 2017, 13, 43. [Google Scholar] [CrossRef]
- Järvinen, K.M.; Amalanayagam, S.; Shreffler, W.G.; Noone, S.; Sicherere, S.H.; Sampson, H.A.; Nowak-Wegrzyn, A. Epinephrine treatment is infrequent and biphasic reactions are rare in food-induced reactions during oral food challenges in children. J. Allergy Clin. Immunol. 2009, 124, 1267–1272. [Google Scholar] [CrossRef][Green Version]
- Lieberman, J.A.; Cox, A.L.; Vitale, M.; Sampson, H.A. Outcomes of office-based, open food challenges in the management of food allergy. J. Allergy Clin. Immunol. 2011, 128, 1120–1122. [Google Scholar] [CrossRef]
- Ballini, G.; Gavagni, C.; Guidotti, C.; Ciolini, G.; Liccioli, G.; Giovannini, M.; Sarti, L.; Ciofi, D.; Novembre, E.; Mori, F.; et al. Frequency of positive oral food challenges and their outcomes in the allergy unit of a tertiary-care pediatric hospital. Allergol. Immunopathol. 2021, 49, 120–130. [Google Scholar] [CrossRef]
| Dose (mg) | Almond (mg of Protein) | Cashew (mg of Protein) | Hazelnut (mg of Protein) | Peanut (mg of Protein) | Pine Nut (mg of Protein) | Pistachio (mg of Protein) | Walnut (mg of Protein) | |
|---|---|---|---|---|---|---|---|---|
| 5 | 1.05 | 0.9 | 0.7 | 1.3 | 0.7 | 1 | 0.75 | |
| 10 | 2.1 | 1.8 | 1.4 | 2.6 | 1.4 | 2 | 1.5 | |
| 25 | 5.25 | 4.5 | 3.5 | 6.5 | 3.5 | 5 | 3.75 | |
| 50 | 10.5 | 9 | 7 | 13 | 7 | 10 | 7.5 | |
| 100 | 21 | 18 | 14 | 26 | 14 | 20 | 15 | |
| 150 | 31.5 | 27 | 21 | 39 | 21 | 30 | 22.5 | |
| 300 | 63 | 54 | 42 | 78 | 42 | 60 | 45 | |
| 600 | 126 | 108 | 84 | 156 | 84 | 120 | 90 | |
| 1200 | 252 | 216 | 168 | 312 | 168 | 240 | 180 | |
| 2000 | 420 | 360 | 280 | 520 | 280 | 400 | 300 | |
| 4000 | 840 | 720 | 560 | 1040 | 560 | 800 | 600 | |
| Cumulative dose | 8440 | 1172.4 | 1519.2 | 1181.6 | 2194.4 | 1181.6 | 1688 | 1266 |
| Total (N = 113) | Cashew (N = 4) | Hazelnut (N = 43) | Peanut (N = 22) | Pine Nut (N = 11) | Pistachio (N = 1) | Walnut (N = 32) | p | |
|---|---|---|---|---|---|---|---|---|
| Male (N = %) | 74; 65 | 3; 75 | 33; 77 | 12; 55 | 5; 45 | 1; 100 | 20; 62 | 0.27 |
| Age (months) (median; min; max) | 42; 8; 175 | |||||||
| AD (N = %) | 46; 41 | 0; 0 | 20; 47 | 6; 27 | 4; 36 | 1; 100 | 15; 47 | 0.22 |
| Asthma (N = %) | 33; 29 | 0; 0 | 14; 33 | 5; 23 | 5; 45 | 1; 100 | 8; 25 | 0.27 |
| Rhinitis (N = %) | 49; 43 | 1; 25 | 21; 49 | 7; 32 | 3; 27 | 1; 100 | 16; 50 | 0.38 |
| Other FA (N = %) | 34; 30 | 2; 50 | 17; 40 | 2; 9 | 2; 18 | 1; 100 | 10; 31 | 0.07 |
| Family history of allergy (N = %) | 85; 75 | 4; 100 | 33; 77 | 13; 59 | 9; 81 | 1; 100 | 25; 78 | 0.39 |
| Age at first reaction (months) (mean ± SD; min; max) | 57 ± 43; 8; 175 | 102 ± 71; 24; 172 | 45 ± 39; 8; 175 | 60 ± 41; 18; 154 | 103 ± 44; 48; 174 | - | 50 ± 33; 12; 125 | 0.00017 |
| PbP (mm) (mean ± SD; min; max) | 7 ± 3; 3; 15 | 8 ± 2; 6; 10 | 7 ± 3; 3; 15 | 6 ± 3; 3; 10 | 7 ± 2; 3; 10 | - | 7 ± 3; 3; 15 | 0.47 |
| s-IgE (KU/L) (mean ± SD; min; max) | 21 ± 32; 0.11; 100 | 3 ± 2; 1.7; 4.93 | 26 ± 35; 0.16; 100 | 31 ± 41; 0.12; 100 | 9 ± 19; 0.11; 66.3 | - | 14 ± 25; 0.3; 96.2 | 0.94 |
| Nut | Other Nuts Allergies | |||||
|---|---|---|---|---|---|---|
| Cashew (N = %) | Hazelnut (N = %) | Peanut (N = %) | Pine Nut (N = %) | Pistachio (N = %) | Walnut (N = %) | |
| Cashew (N = 4) | - | 0; 0 | 0; 0 | 0; 0 | 1; 25 | 0; 0 |
| Hazelnut (N = 43) | 2; 5 | 3; 7 | 2; 5 | 1; 2 | 3; 7 | |
| Peanut (N = 22) | 0; 0 | 2; 9 | 0; 0 | 0; 0 | 1; 5 | |
| Pine nut (N = 11) | 1; 9 | 1; 9 | 0; 0 | 0; 0 | 2; 18 | |
| Pistachio (N = 1) | 0; 0 | 1; 100 | 0; 0 | 0; 0 | 0; 0 | |
| Walnut (N = 32) | 0; 0 | 6; 19 | 1; 3 | 0; 0 | 0; 0 | |
| Severity | Cashew (N = 4) | Hazelnut (N = 43) | Peanut (N = 22) | Pine Nut (N = 11) | Walnut (N = 32) | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP (mm) (Mean ± SD) | s-IgE (KU/L) (Mean ± SD) | PbP | s-IgE | ||
| First reaction | Mild | - | - | 7 ± 3 | 26 ± 35 | 7 ± 3 | 34 ± 45 | 7 ± 3 | 1 ± 1 | 6 ± 3 | 15 ± 28 | 1.31 | 0.56 |
| Moderate | 8 ± 3 | 2 ± 1 | 9 ± 2 | 45 ± 47 | 5 ± 2 | 58 ± 60 | 8 ± 1 | 6 ± 5 | - | - | 0.20 | 0.27 | |
| Severe | - | - | 6 ± 3 | 13 ± 21 | 4 ± 2 | 26 ± 42 | - | - | 7 ± 4 | 2 ± 2 | 0.31 | 0.80 | |
| Oral food challenge | Mild | 8 ± 2 | 3 ± 2 | 7 ± 3 | 24 ± 35 | 6 ± 2 | 29 ± 41 | 7 ± 2 | 11 ± 23 | 6 ± 3 | 8 ± 11 | 0.52 | 0.78 |
| Moderate | - | - | 6 ± 1 | 45 ± 34 | 6 ± 4 | 56 ± 43 | - | - | 9 ± 5 | 54 ± 49 | 0.40 | 0.84 | |
| Severe | - | - | 6 ± 3 | - | - | - | 6 ± 1 | 3 ± 3 | 6 ± 3 | 3 ± 3 | 0.97 | 0.76 | |
| First Reaction | Oral Food Challenge | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Allergens | Available Data (N=%) | Value (KU/L) (Mean ± SD; Min; Max) | Mild (KU/L) (Mean ± SD) | Moderate (KU/L) (Mean ± SD) | Severe (KU/L) (Mean ± SD) | p | Mild (KU/L) (Mean ± SD) | Moderate (KU/L) (Mean ± SD) | Severe (KU/L) (Mean ± SD) | p |
| Ara h 1 | 22; 100 | 33 ± 41; 0.15; 100 | 46 ± 51 | 28 ± 32 | 39 ± 40 | 0.80 | 34 ± 42 | 27 ± 45 | - | 0.98 |
| Ara h 2 | 22; 100 | 38 ± 39; 0.6; 100 | 41 ± 47 | 32 ± 33 | 48 ± 42 | 0.92 | 34 ± 39 | 59 ± 45 | - | 0.64 |
| Ara h 3 | 21; 95 | 19 ± 32; 0.11; 100 | 39 ± 43 | 11 ± 14 | 3 ± 3 | 0.88 | 20 ± 33 | - | - | - |
| Ara h 8 | 21; 95 | 1 ± 1; 0.15; 2.17 | 1 ±1 | - | 1 ± 1 | 0.53 | 0 ± 0 | - | - | - |
| Ara h 9 | 21; 95 | 16 ± 22; 0.88; 40.8 | 16 ± 22 | - | - | - | 3 ± 4 | - | - | - |
| Jug r 1 | 16; 50 | 11 ± 22; 0.27; 88.3 | 4 ± 4 | - | - | - | 13 ± 25 | 4 ± 5 | 4 ± 4 | 0.93 |
| Jur r 3 | 16; 50 | 3 ± 6; 0.12; 16 | 4 ± 7 | - | - | - | 3 ± 7 | - | - | - |
| Cor a 1 | 32; 74 | 8 ± 11; 0.16; 32.3 | 8 ± 13 | 12 ± 14 | 5 ± 7 | 0.42 | 7 ± 10 | - | - | - |
| Cor a 8 | 32; 74 | 5 ± 11; 0.11; 36.6 | 3 ± 4 | 0 ± 0 | 20 ± 23 | 0.51 | 6 ± 11 | - | - | - |
| Cor a 9 | 33; 77 | 22 ± 36; 0.11; 100 | 21 ± 33 | 50 ± 57 | 9 ± 18 | 0.14 | 20 ± 34 | 49 ± 20 | - | 0.92 |
| Cor a 14 | 31; 71 | 16 ± 23; 0.11; 90 | 19 ± 27 | 16 ± 17 | 7 ± 10 | 0.08 | 17 ± 24 | 12 ± 12 | - | 0.95 |
| Total (N = 113) | Cashew (N = 4) | Hazelnut (N = 43) | Peanut (N = 22) | Pine Nut (N = 11) | Pistachio (N = 1) | Walnut (N = 32) | p | |
|---|---|---|---|---|---|---|---|---|
| Mild (N = %) | 91; 81 | 4; 100 | 36; 84 | 18; 82 | 8; 73 | 1; 100 | 24; 75 | 0.77 |
| Protein ingested (mean (mg) ± SD) | 283 ± 630 | 79 ± 35 | 279 ± 518 | 472 ± 1054 | 199 ± 350 | - | 221 ± 478 | 0.65 |
| Moderate (N = %) | 13; 11 | 0; 0 | 5; 12 | 3; 14 | 1; 9 | 0; 0 | 4; 12 | 0.97 |
| Protein ingested (mean (mg) ± SD) | 376 ± 686 | - | 764 ± 1074 | 123 ± 75 | - | - | 168 ± 47 | 0.36 |
| Severe (N = %) | 9; 8 | 0; 0 | 2; 5 | 1; 5 | 2; 18 | 0; 0 | 4; 12 | 0.58 |
| Protein ingested (mean (mg) ± SD) | 69 ± 72 | - | 43 ± 32 | - | 104 ± 136 | - | 44 ± 56 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagliati, S.; Barni, S.; Giovannini, M.; Liccioli, G.; Sarti, L.; Alicandro, T.; Paladini, E.; Perferi, G.; Azzari, C.; Novembre, E.; et al. Nut Allergy: Clinical and Allergological Features in Italian Children. Nutrients 2021, 13, 4076. https://doi.org/10.3390/nu13114076
Tagliati S, Barni S, Giovannini M, Liccioli G, Sarti L, Alicandro T, Paladini E, Perferi G, Azzari C, Novembre E, et al. Nut Allergy: Clinical and Allergological Features in Italian Children. Nutrients. 2021; 13(11):4076. https://doi.org/10.3390/nu13114076
Chicago/Turabian StyleTagliati, Sylvie, Simona Barni, Mattia Giovannini, Giulia Liccioli, Lucrezia Sarti, Tatiana Alicandro, Erika Paladini, Giancarlo Perferi, Chiara Azzari, Elio Novembre, and et al. 2021. "Nut Allergy: Clinical and Allergological Features in Italian Children" Nutrients 13, no. 11: 4076. https://doi.org/10.3390/nu13114076
APA StyleTagliati, S., Barni, S., Giovannini, M., Liccioli, G., Sarti, L., Alicandro, T., Paladini, E., Perferi, G., Azzari, C., Novembre, E., & Mori, F. (2021). Nut Allergy: Clinical and Allergological Features in Italian Children. Nutrients, 13(11), 4076. https://doi.org/10.3390/nu13114076







