Prevalence of Micronutrient Deficiencies and Relationship with Clinical and Patient-Related Outcomes in Pulmonary Hypertension Types I and IV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Clinical Data Collection
2.3. Clinical Follow-Up
2.4. Venous Blood Sampling
2.5. Laboratory Analyses
2.6. Statistics
3. Results
3.1. Characteristics of Participants
3.2. Prevalence of Abnormal Micronutrient and Mineral Blood Concentrations
3.2.1. PAH Patients
3.2.2. CTEPH Patients
3.2.3. Newly Diagnosed versus Treated Patients
3.2.4. Female Patients
3.2.5. Male Patients
3.2.6. Summary
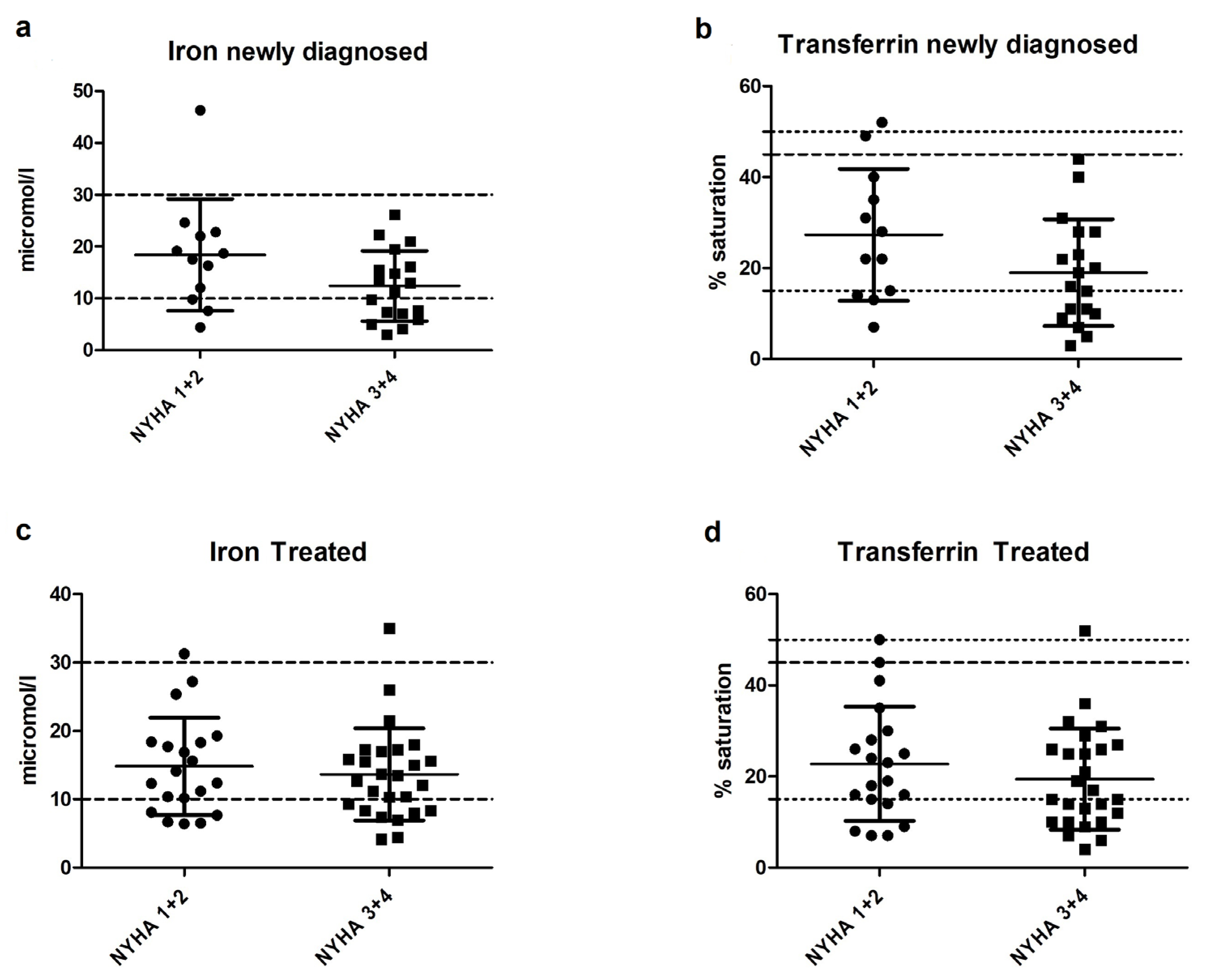
3.3. Iron Status in Newly Diagnosed and Treated PH Patients
3.4. Correlations between Micronutrient Deficiencies and Clinical Outcome
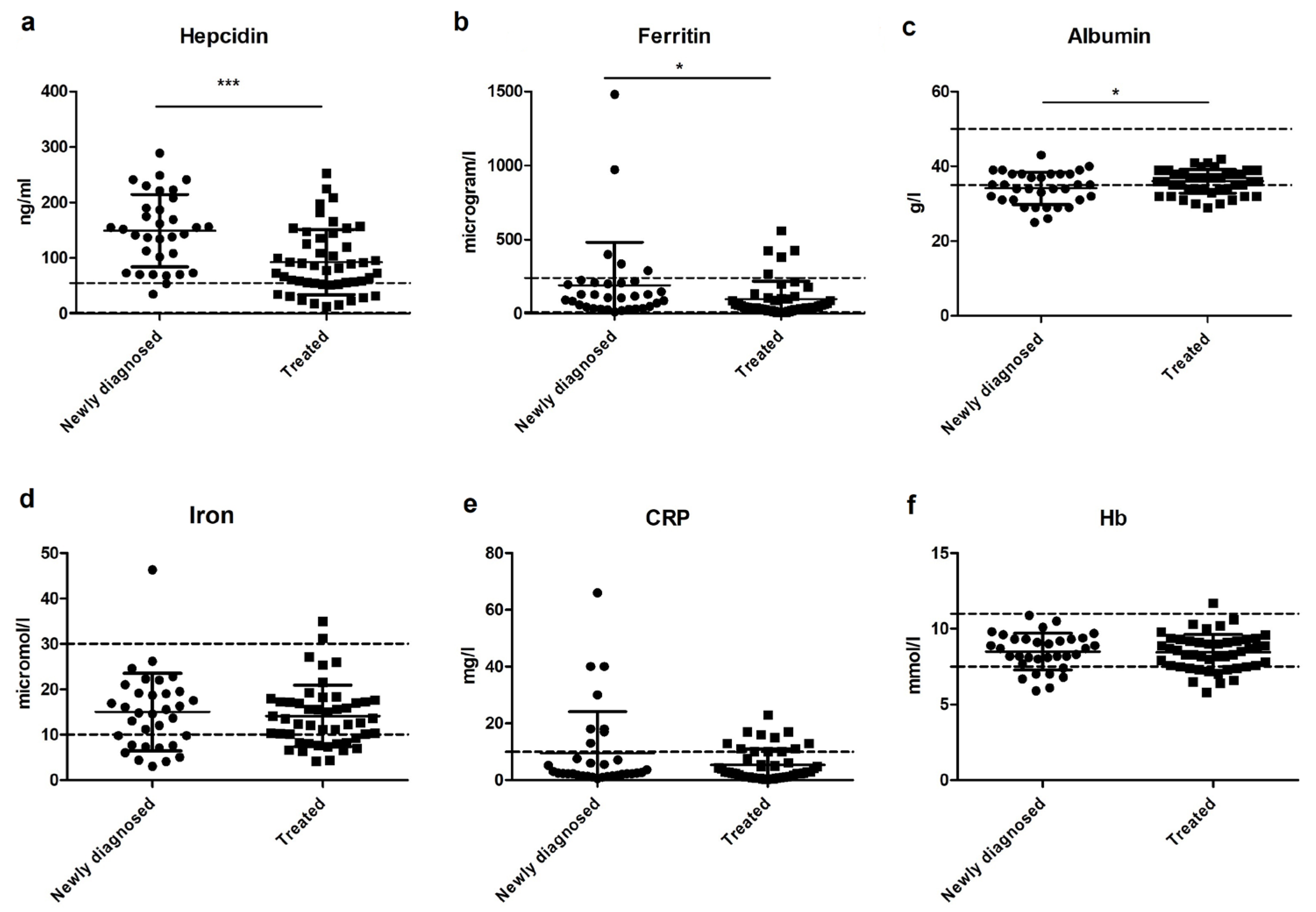
3.5. The Relation between Iron, Hepcidin, and Inflammation
3.6. Vitamin-D-Binding Protein and 25(OH)D
4. Discussion
4.1. Iron Deficiency
4.2. Vitamin D Deficiency
4.3. Summary and Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D42–D50. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Vinke, P.; Jansen, S.M.; Witkamp, R.F.; van Norren, K. Increasing quality of life in pulmonary arterial hypertension: Is there a role for nutrition? Heart Fail. Rev. 2018, 23, 711–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, D.L.; Lau, E.M.T.; Celermajer, D.S.; Davis, G.M.; Cordina, R. Pathophysiology of exercise intolerance in pulmonary arterial hypertension. Respirology 2018, 23, 148–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heukels, P.; Corneth, O.B.J.; Van Uden, D.; Van Hulst, J.A.C.; Van Den Toorn, L.M.; Van Den Bosch, A.E.; Wijsenbeek, M.S.; Boomars, K.A.; Kool, M.; Hendriks, R.W. Loss of immune homeostasis in patients with idiopathic pulmonary arterial hypertension. Thorax 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [Green Version]
- Quarck, R.; Wynants, M.; Verbeken, E.; Meyns, B.; Delcroix, M. Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2015, 46, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yan, D.; Qiu, A. The role of macrophages in pulmonary hypertension: Pathogenesis and targeting. Int. Immunopharmacol. 2020, 88, 106934. [Google Scholar] [CrossRef] [PubMed]
- van Uden, D.; Koudstaal, T.; van Hulst, J.A.C.; Bergen, I.M.; Gootjes, C.; Morrell, N.W.; van Loo, G.; von der Thüsen, J.H.; van den Bosch, T.P.P.; Ghigna, M.R.; et al. Central role of dendritic cells in pulmonary arterial hypertension in human and mice. Int. J. Mol. Sci. 2021, 22, 1756. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk-Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2015, 46, 903–975. [Google Scholar]
- Benza, R.L.; Miller, D.P.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the reveal registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Rådegran, G.; Kjellström, B.; Ekmehag, B.; Larsen, F.; Rundqvist, B.; Blomquist, S.B.; Gustafsson, C.; Hesselstrand, R.; Karlsson, M.; Kornhall, B.; et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014. Scand. Cardiovasc. J. 2016, 50, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Quadery, S.R.; Swift, A.J.; Billings, C.G.; Thompson, A.A.R.; Elliot, C.A.; Hurdman, J.; Charalampopoulos, A.; Sabroe, I.; Armstrong, I.J.; Hamilton, N.; et al. The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2018, 52, 1800589. [Google Scholar] [CrossRef]
- Boucly, A.; Weatherald, J.; Savale, L.; Jaïs, X.; Cottin, V.; Prevot, G.; Picard, F.; De Groote, P.; Jevnikar, M.; Bergot, E.; et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1700889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandqvist, A.; Kylhammar, D.; Bartfay, S.E.; Hesselstrand, R.; Hjalmarsson, C.; Kavianipour, M.; Nisell, M.; Rådegran, G.; Wikström, G.; Kjellström, B.; et al. Risk stratification in chronic thromboembolic pulmonary hypertension predicts survival. Scand. Cardiovasc. J. 2021, 55, 43–49. [Google Scholar] [CrossRef]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11. [Google Scholar] [CrossRef]
- Zittermann, A. Vitamin D in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef] [PubMed]
- Soon, E.; Treacy, C.M.; Toshner, M.R.; MacKenzie-Ross, R.; Manglam, V.; Busbridge, M.; Sinclair-McGarvie, M.; Arnold, J.; Sheares, K.K.; Morrell, N.W.; et al. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax 2011, 66, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiter, G.; Lankhorst, S.; Boonstra, A.; Postmus, P.E.; Zweegman, S.; Westerhof, N.; Van Der Laarse, W.J.; Vonk-Noordegraaf, A. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2011, 37, 1386–1391. [Google Scholar] [CrossRef] [Green Version]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Barbagallo, M.; Lauretani, F.; Bandinelli, S.; Bos, A.; Corsi, A.M.; Simonsick, E.M.; Ferrucci, L. Magnesium and muscle performance in older persons: The InCHIANTI study. Am. J. Clin. Nutr. 2006, 84, 419–426. [Google Scholar] [CrossRef]
- Shahi, A.; Aslani, S.; Ataollahi, M.R.; Mahmoudi, M. The role of magnesium in different inflammatory diseases. Inflammopharmacology 2019, 27, 649–661. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Waldron, J.L.; Ashby, H.L.; Cornes, M.P.; Bechervaise, J.; Razavi, C.; Thomas, O.L.; Chugh, S.; Deshpande, S.; Ford, C.; Gama, R. Vitamin D: A negative acute phase reactant. J. Clin. Pathol. 2013, 66, 620–622. [Google Scholar] [CrossRef]
- Balvers, M.G.J.; Brouwer-Brolsma, E.M.; Endenburg, S.; De Groot, L.C.P.G.M.; Kok, F.J.; Gunnewiek, J.K. Recommended intakes of Vitamin D to optimise health, associated circulating 25-hydroxyVitamin D concentrations, and dosing regimens to treat deficiency: Workshop report and overview of current literature. J. Nutr. Sci. 2015, 4, e23. [Google Scholar] [CrossRef] [Green Version]
- Semen, K.O.; Bast, A. Towards improved pharmacotherapy in pulmonary arterial hypertension. Can diet play a role? Clin. Nutr. ESPEN 2019, 30, 159–169. [Google Scholar] [CrossRef]
- Yan, L.; Luo, Q.; Zhao, Z.; Zhao, Q.; Jin, Q.; Zhang, Y.; Liu, Z. Nocturnal hypoxia in patients with idiopathic pulmonary arterial hypertension. Pulm. Circ. 2020, 10, 2045894019885364. [Google Scholar] [CrossRef]
- Puente-Maestu, L.; Pérez-Parra, J.; Godoy, R.; Moreno, N.; Tejedor, A.; Gonzaiez-Aragoneses, F.; Bravo, J.L.; Alvarez, F.V.; Camano, S.; Agusti, A. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur. Respir. J. 2009, 33, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Leermakers, P.A.; Schols, A.M.W.J.; Kneppers, A.E.M.; Kelders, M.C.J.M.; de Theije, C.C.; Lainscak, M.; Gosker, H.R. Molecular signalling towards mitochondrial breakdown is enhanced in skeletal muscle of patients with chronic obstructive pulmonary disease (COPD). Sci. Rep. 2018, 8, 15007. [Google Scholar] [CrossRef] [Green Version]
- Ruiter, G.; Manders, E.; Happé, C.M.; Schalij, I.; Groepenhoff, H.; Howard, L.S.; Wilkins, M.R.; Bogaard, H.J.; Westerhof, N.; van der Laarse, W.J.; et al. Intravenous iron therapy in patients with idiopathic pulmonary arterial hypertension and iron deficiency. Pulm. Circ. 2015, 5, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.J.; Wharton, J.; Howard, L.; Gibbs, J.S.R.; Vonk-Noordegraaf, A.; Wilkins, M.R. Iron deficiency in pulmonary arterial hypertension: A potential therapeutic target. Eur. Respir. J. 2011, 38, 1453–1460. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, L.; Pedersen, S.L.; Toe, Q.K.; Quinlan, G.J.; Wort, S.J. Pulmonary arterial hypertension: Iron matters. Front. Physiol. 2018, 9, 641. [Google Scholar] [CrossRef] [Green Version]
- Kanamori, Y.; Murakami, M.; Sugiyama, M.; Hashimoto, O.; Matsui, T.; Funaba, M. Interleukin-1β (IL-1β) transcriptionally activates hepcidin by inducing CCAAT enhancer-binding protein δ (C/EBPδ) expression in hepatocytes. J. Biol. Chem. 2017, 292, 10275–10287. [Google Scholar] [CrossRef] [Green Version]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Olsson, K.M.; Fuge, J.; Brod, T.; Kamp, J.C.; Schmitto, J.; Kempf, T.; Bauersachs, J.; Hoeper, M.M. Oral iron supplementation with ferric maltol in patients with pulmonary hypertension. Eur. Respir. J. 2020, 56, 2000616. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’amelio, P. Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [Green Version]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Casey, C.; Woodside, J.V.; McGinty, A.; Young, I.S.; McPeake, J.; Chakravarthy, U.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; et al. Factors associated with serum 25-hydroxyvitamin D concentrations in older people in Europe: The EUREYE study. Eur. J. Clin. Nutr. 2019, 73, 319–328. [Google Scholar] [CrossRef] [Green Version]
- ten Haaf, D.S.M.; Balvers, M.G.J.; Timmers, S.; Eijsvogels, T.M.H.; Hopman, M.T.E.; Klein Gunnewiek, J.M.T. Determinants of vitamin D status in physically active elderly in the Netherlands. Eur. J. Nutr. 2019, 58, 3121–3128. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, R.M.; Snijder, M.B.; Dekker, J.M.; Stehouwer, C.D.A.; Bouter, L.M.; Heine, R.J.; Lips, P. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: The Hoorn Study. Am. J. Clin. Nutr. 2007, 85, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Santos, A.R.; Afonso, C.; Santos, A.; Borges, N.; Moreira, P.; Padrão, P.; Fonseca, I.; Amaral, T.F. The association between 25(OH)D levels, frailty status and obesity indices in older adults. PLoS ONE 2018, 13, e0198650. [Google Scholar] [CrossRef]
- Drincic, A.; Fuller, E.; Heaney, R.P.; Armas, L.A.G. 25-Hydroxyvitamin D response to graded vitamin D3 supplementation among obese adults. J. Clin. Endocrinol. Metab. 2013, 98, 4845–4851. [Google Scholar] [CrossRef] [Green Version]
- Mirdamadi, A.; Moshkdar, P. Benefits from the correction of vitamin D deficiency in patients with pulmonary hypertension. Casp. J. Intern. Med. 2016, 7, 253–259. [Google Scholar]
- Tanaka, H.; Kataoka, M.; Isobe, S.; Yamamoto, T.; Shirakawa, K.; Endo, J.; Satoh, T.; Hakamata, Y.; Kobayashi, E.; Sano, M.; et al. Therapeutic impact of dietary vitamin D supplementation for preventing right ventricular remodeling and improving survival in pulmonary hypertension. PLoS ONE 2017, 12, e0180615. [Google Scholar] [CrossRef] [Green Version]


| Characteristic | PAH | CTEPH |
|---|---|---|
| n (%) | 19 (58) | 14 (42) |
| Gender, female (%) | 14 (74) | 6 (43) |
| Age, year | 62.5 ± 14.7 | 63.9 ± 12.9 |
| Height, cm | 164.6 ± 9.8 | 174.5 ± 10.0 * |
| Weight, kg | 72.9 ± 18.7 | 80.1 ± 17.6 |
| BMI, kg/m2 | 26.7 ± 5.6 | 26.4 ± 5.9 |
| NYHA class (1:2:3:4) | 0:6:12:1 | 2:5:6:1 |
| NT-pro-BNP, pmol/L ** | 351 ± 863 | 224 ± 232 |
| Cause of pulmonary hypertension | ||
| HPAH (%) | 2 (11) | |
| IPAH (%) | 4 (21) | |
| CTD (%) | 9 (47) | |
| CHD (%) | 0 (0) | |
| Portopulmonary (%) | 3 (16) | |
| Drugs/toxins/medication (%) | 1 (5) | |
| Other (%) | 0 (0) | |
| Type of CTD-related PAH | ||
| SSc (%) | 7 (78) | |
| SLE (%) | 1 (11) | |
| Sjogren (%) | 1 (11) | |
| 6MWD, m | 352 ± 121 | 447 ± 240 |
| Echocardiography, RVSP in mm/Hg | 76.5 ± 19.9 | 72.1 ± 21.0 |
| R-catheterization | ||
| Mean PAH, mmHg | 46.5 ± 14.0 | 42.0 ± 11.9 |
| PAWP, mmHg | 9.7 ± 3.6 | 9.3 ± 4.3 |
| PVR, WU | 7.3 ± 3.3 | 6.2 ± 3.2 |
| CI | 2.7 ± 0.7 | 2.8 ± 0.8 |
| Characteristic | PAH | CTEPH |
|---|---|---|
| n (%) | 38 (79) | 10 (21) |
| Gender, female (%) | 24 (63) | 7 (70) |
| Age, year | 48.8 ± 15.1 | 64.5 ± 12.0 ** |
| Height, cm | 168.7 ± 10.8 | 169.8 ± 6.3 |
| Weight, kg | 75.6 ± 18.4 | 83.3 ± 11.3 |
| BMI, kg/m2 | 26.7 ± 7.1 | 28.9 ± 3.9 |
| NYHA class (1:2:3:4) | 1:16:19:2 | 2:3:5:0 |
| NT-pro-BNP, pmol/L ** | 71 ± 157 | 23 ± 20 |
| Cause of pulmonary hypertension | ||
| HPAH (%) | 2 (5) | |
| IPAH (%) | 14 (37) | |
| CTD (%) | 9 (24) | |
| CHD (%) | 8 (21) | |
| Portopulmonary (%) | 4 (11) | |
| Drugs/toxins/medication (%) | 0 (0) | |
| Other (%) | 1 (3) | |
| Type of CTD-related PAH | ||
| SSc (%) | 7 (78) | |
| SLE (%) | 2 (22) | |
| Sjogren (%) | 0 (0) | |
| PAH-specific drugs | ||
| PDE-5 inhibitor (%) | 33 | 4 |
| ERA (%) | 33 | 8 |
| sGCs (%) | 1 | 1 |
| Prostacycline receptor agonist (Selexipag) (%) | 5 | 0 |
| Prostacycline (IV) (%) | 6 | 0 |
| Treprostinil (IV/SC) (%) | 2 | 0 |
| Drug combination therapy | ||
| Monotherapy (%) | 5 | 3 |
| Duo therapy (%) | 21 | 5 |
| Triple therapy (%) | 11 | 0 |
| 6MWD, m | ||
| Baseline | 374 ± 140 | 346 ± 99 |
| 18 months follow-up | 445 ± 142 | 420 ± 121 |
| Echocardiography, RVSP in mmHg | ||
| Baseline | 76.9 ± 18.6 | 61.5 ± 17.5 * |
| 18-month Follow-up | 62.7 ± 22.6 | 48.1 ± 17.9 |
| R-catheterization (baseline) | ||
| Mean PAP, mmHg | 51.3 ± 13.8 | 38.9 ± 13.5 * |
| PAWP, mmHg | 9.8 ± 3.3 | 9.8 ± 3.5 |
| PVR, WU | 9.6 ± 4.9 | 5.1 ± 2.6 * |
| CI | 2.6 ± 0.7 | 2.7 ± 0.2 |
| Newly Diagnosed | Treated | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Female | Male | PAH | CTEPH | Total | Female | Male | PAH | CTEPH | |
| (n = 33) | (n = 20) | (n = 13) | (n = 19) | (n = 14) | (n = 48) | (n = 31) | (n = 17) | (n = 38) | (n = 10) | |
| Iron (<10 µmol/L) | 33% | 40% | 23% | 42% | 21% | 27% | 36% | 12% | 29% | 20% |
| Magnesium (<0.7 mmol/L) | 15% | 20% | 8% | 21% | 7% | 4% | 3% | 6% | 3% | 10% |
| Calcium (<2.2 mmol/L) | 15% | 15% | 15% | 16% | 14% | 2% | 0% | 6% | 3% | 0% |
| Phosphate (<0.8 mmol/L) | 0% | 0% | 0% | 0% | 0% | 17% | 13% | 24% | 16% | 20% |
| Vitamin B11 (<5 nmol/L) | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| Vitamin B12 (<145 pmol/L) | 3% | 5% | 0% | 5% | 0% | 4% | 7% | 0% | 5% | 0% |
| 25(OH)D (<50 nmol/L) | 55% | 50% | 62% | 42% | 71% | 69% | 61% | 82% | 68% | 70% |
| Newly Diagnosed | Treated | |||||||
|---|---|---|---|---|---|---|---|---|
| PAH Males | PAH Females | CTEPH Males | CTEPH Females | PAH Males | PAH Females | CTEPH Males | CTEPH Females | |
| (n = 5) | (n = 14) | (n = 8) | (n = 6) | (n = 14) | (n = 24) | (n = 3) | (n = 7) | |
| Iron (<10 µmol/L) | 20% | 50% | 25% | 17% | 14% | 38% | 0% | 29% |
| Magnesium (<0.7 mmol/L) | 0% | 29% | 13% | 0% | 0 | 4% | 33% | 0% |
| Calcium (<2.2 mmol/L) | 20% | 14% | 13% | 17% | 7% | 0% | 0% | 0% |
| Phosphate (<0.8 mmol/L) | 0% | 0% | 0% | 0% | 29% | 14% | 0% | 29% |
| Vitamin B11 (<5 nmol/L) | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| Vitamin B12 (<145 pmol/L) | 0% | 7% | 0% | 0% | 0% | 8,3% | 0% | 0% |
| 25(OH)D (<50 nmol/L) | 60% | 36% | 63% | 83% | 79% | 63% | 100% | 57% |
| Newly Diagnosed | Treated | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Female | Male | PAH | CTEPH | Total | Female | Male | PAH | CTEPH | |
| (n = 33) | (n = 20) | (n = 13) | (n = 19) | (n = 14) | (n = 48) | (n = 31) | (n = 17) | (n = 38) | (n = 10) | |
| Hb (F: <7.5 mmol/L, M: <8.5 mmol/L) | 21% | 30% | 8% | 26% | 14% | 25% | 26% | 24% | 24% | 30% |
| Iron (<10 mmol/L) | 33% | 40% | 23% | 42% | 21% | 27% | 36% | 12% | 29% | 20% |
| Ferritin (F: <10 µg/L, M: <30 µg/L) | 3% | 0% | 8% | 0% | 7% | 6% | 0% | 18% | 8% | 0% |
| Transferrin sat. (F: <15%, M: <20%) | 36% | 40% | 31% | 47% | 21% | 44% | 36% | 59% | 47% | 30% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinke, P.; Koudstaal, T.; Muskens, F.; van den Bosch, A.; Balvers, M.; Poland, M.; Witkamp, R.F.; van Norren, K.; Boomars, K.A. Prevalence of Micronutrient Deficiencies and Relationship with Clinical and Patient-Related Outcomes in Pulmonary Hypertension Types I and IV. Nutrients 2021, 13, 3923. https://doi.org/10.3390/nu13113923
Vinke P, Koudstaal T, Muskens F, van den Bosch A, Balvers M, Poland M, Witkamp RF, van Norren K, Boomars KA. Prevalence of Micronutrient Deficiencies and Relationship with Clinical and Patient-Related Outcomes in Pulmonary Hypertension Types I and IV. Nutrients. 2021; 13(11):3923. https://doi.org/10.3390/nu13113923
Chicago/Turabian StyleVinke, Paulien, Thomas Koudstaal, Femke Muskens, Annemien van den Bosch, Michiel Balvers, Mieke Poland, Renger F. Witkamp, Klaske van Norren, and Karin A. Boomars. 2021. "Prevalence of Micronutrient Deficiencies and Relationship with Clinical and Patient-Related Outcomes in Pulmonary Hypertension Types I and IV" Nutrients 13, no. 11: 3923. https://doi.org/10.3390/nu13113923






