APOE ɛ4 Is Associated with Postprandial Inflammation in Older Adults with Metabolic Syndrome Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
2.3. Measurments
2.3.1. Anthropometrics, Body Composition, and Blood Pressure
2.3.2. Blood Sample Processing
2.3.3. APOE Genotype Determination
2.3.4. Serum Lipids, Non-Esterified Fatty Acids, Insulin, and Plasma Glucose
2.3.5. Biomarkers of Inflammation and Endothelial Activation
2.3.6. Biomarkers of Oxidation and the Antioxidant State
2.4. Statistical Analyses
3. Results
3.1. APOE Genotype Distribution and Baseline Characteristics
3.2. Serum Triglycerides, NEFAs, Insulin, and Plasma Glucose
3.3. Plasma IL-6, IL-1β, and Endothelial Adhesion Molecules
3.4. Plasma TEAC and oxLDL, Vitamin C, Retinol, α-Tocopherol, and β-Carotene
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Peloso, G.M.; Beiser, A.S.; Satizabal, C.L.; Xanthakis, V.; Vasan, R.S.; Pase, M.P.; Destefano, A.L.; Seshadri, S. Cardiovascular health, genetic risk, and risk of dementia in the Framingham Heart Study. Neurology 2020, 95, e1341–e1350. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Rimbach, G.; Huebbe, P. ApoE genotype: From geographic distribution to function and responsiveness to dietary factors. Proc. Nutr. Soc. 2012, 71, 410–424. [Google Scholar] [CrossRef]
- Neu, S.C.; Pa, J.; Kukull, W.; Beekly, D.; Kuzma, A.; Gangadharan, P.; Wang, L.-S.; Romero, K.; Arneric, S.P.; Redolfi, A.; et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017, 74, 1178–1189. [Google Scholar] [CrossRef]
- Huebbe, P.; Lodge, J.K.; Rimbach, G. Implications of apolipoprotein E genotype on inflammation and vitamin E status. Mol. Nutr. Food Res. 2010, 54, 623–630. [Google Scholar] [CrossRef]
- Li, H.; Dhanasekaran, P.; Alexander, E.T.; Rader, D.J.; Phillips, M.C.; Lund-Katz, S. Molecular mechanisms responsible for the differential effects of apoE3 and apoE4 on plasma lipoprotein-cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 687–693. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, L.-M.; Wu, J. Cross-talk between apolipoprotein E and cytokines. Mediat. Inflamm. 2011, 2011, 949072. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Minihane, A.M.; Jofre-Monseny, L.; Olano-Martin, E.; Rimbach, G. ApoE genotype, cardiovascular risk and responsiveness to dietary fat manipulation. Proc. Nutr. Soc. 2007, 66, 183–197. [Google Scholar] [CrossRef]
- Egert, S.; Boesch-Saadatmandi, C.; Wolffram, S.; Rimbach, G.; Müller, M.J. Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein E genotype. J. Nutr. 2010, 140, 278–284. [Google Scholar] [CrossRef]
- Griffin, B.A.; Walker, C.G.; Jebb, S.A.; Moore, C.; Frost, G.S.; Goff, L.; Sanders, T.A.B.; Lewis, F.; Griffin, M.; Gitau, R.; et al. APOE4 Genotype Exerts Greater Benefit in Lowering Plasma Cholesterol and Apolipoprotein B than Wild Type (E3/E3), after Replacement of Dietary Saturated Fats with Low Glycaemic Index Carbohydrates. Nutrients 2018, 10, 1524. [Google Scholar] [CrossRef]
- Minihane, A.M.; Khan, S.; Leigh-Firbank, E.C.; Talmud, P.; Wright, J.W.; Murphy, M.C.; Griffin, B.A.; Williams, C.M. ApoE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1990–1997. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Anil, E.; Caslake, M.J.; Packard, C.J.; Bedford, D.; Stewart, G.; Peiris, D.; Williams, C.M.; Minihane, A.M. Contribution of apolipoprotein E genotype and docosahexaenoic acid to the LDL-cholesterol response to fish oil. Atherosclerosis 2010, 209, 104–110. [Google Scholar] [CrossRef]
- Caslake, M.J.; Miles, E.A.; Kofler, B.M.; Lietz, G.; Curtis, P.; Armah, C.K.; Kimber, A.C.; Grew, J.P.; Farrell, L.; Stannard, J.; et al. Effect of sex and genotype on cardiovascular biomarker response to fish oils: The FINGEN Study. Am. J. Clin. Nutr. 2008, 88, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Chouinard-Watkins, R.; Conway, V.; Minihane, A.M.; Jackson, K.G.; Lovegrove, J.A.; Plourde, M. Interaction between BMI and APOE genotype is associated with changes in the plasma long-chain-PUFA response to a fish-oil supplement in healthy participants. Am. J. Clin. Nutr. 2015, 102, 505–513. [Google Scholar] [CrossRef]
- Jackson, K.G.; Poppitt, S.D.; Minihane, A.M. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012, 220, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv. Nutr. Int. Rev. J. 2017, 8, 213–225. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Des Rosiers, C.; Tardif, J.-C.; Nigam, A. The role of oxidative stress in postprandial endothelial dysfunction. Nutr. Res. Rev. 2012, 25, 288–301. [Google Scholar] [CrossRef]
- Calabuig-Navarro, M.V.; Jackson, K.G.; Walden, C.M.; Minihane, A.-M.; Lovegrove, J.A. Apolipoprotein E genotype has a modest impact on the postprandial plasma response to meals of varying fat composition in healthy men in a randomized controlled trial. J. Nutr. 2014, 144, 1775–1780. [Google Scholar] [CrossRef]
- Cardona, F.; Morcillo, S.; Gonzalo-Marin, M.; Tinahones, F.J. The apolipoprotein E genotype predicts postprandial hypertriglyceridemia in patients with the metabolic syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 2972–2975. [Google Scholar] [CrossRef][Green Version]
- Schönknecht, Y.B.; Crommen, S.; Stoffel-Wagner, B.; Coenen, M.; Fimmers, R.; Holst, J.J.; Simon, M.-C.; Stehle, P.; Egert, S. Acute Effects of Three Different Meal Patterns on Postprandial Metabolism in Older Individuals with a Risk Phenotype for Cardiometabolic Diseases: A Randomized Controlled Crossover Trial. Mol. Nutr. Food Res. 2020, 64, e1901035. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br. J. Nutr. 2004, 91, 300–301. [Google Scholar] [CrossRef]
- Mészáros, Á.; Molnár, K.; Nógrádi, B.; Hernádi, Z.; Nyúl-Tóth, Á.; Wilhelm, I.; Krizbai, I.A. Neurovascular Inflammaging in Health and Disease. Cells 2020, 9, 1614. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Dose, J.; Huebbe, P.; Nebel, A.; Rimbach, G. APOE genotype and stress response—A mini review. Lipids Health Dis. 2016, 15, 121. [Google Scholar] [CrossRef]
- Muñoz, A.; Costa, M. Nutritionally mediated oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2013, 2013, 610950. [Google Scholar] [CrossRef]
- Lynch, J.R.; Tang, W.; Wang, H.; Vitek, M.P.; Bennett, E.R.; Sullivan, P.M.; Warner, D.S.; Laskowitz, D.T. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J. Biol. Chem. 2003, 278, 48529–48533. [Google Scholar] [CrossRef] [PubMed]
- Jofre-Monseny, L.; Loboda, A.; Wagner, A.E.; Huebbe, P.; Boesch-Saadatmandi, C.; Jozkowicz, A.; Minihane, A.-M.; Dulak, J.; Rimbach, G. Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem. Biophys. Res. Commun. 2007, 357, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Reznik, Y.; Pousse, P.; Herrou, M.; Morello, R.; Mahoudeau, J.; Drosdowsky, M.A.; Fradin, S. Postprandial lipoprotein metabolism in normotriglyceridemic non-insulin-dependent diabetic patients: Influence of apolipoprotein E polymorphism. Metab. Clin. Exp. 1996, 45, 63–71. [Google Scholar] [CrossRef]
- Carvalho-Wells, A.L.; Jackson, K.G.; Gill, R.; Olano-Martin, E.; Lovegrove, J.A.; Williams, C.M.; Minihane, A.M. Interactions between age and apoE genotype on fasting and postprandial triglycerides levels. Atherosclerosis 2010, 212, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Lockyer, S.; Carvalho-Wells, A.L.; Williams, C.M.; Minihane, A.M.; Lovegrove, J.A. Dietary fat manipulation has a greater impact on postprandial lipid metabolism than the apolipoprotein E (epsilon) genotype-insights from the SATgenε study. Mol. Nutr. Food Res. 2012, 56, 1761–1770. [Google Scholar] [CrossRef]

| Energy/Nutrient | MED Meal | WDHF Meal | WDHC Meal |
|---|---|---|---|
| Energy, kJ | 4238 | 4230 | 4241 |
| Energy density, kJ/g | 5.6 | 7.2 | 9.8 |
| Carbohydrates, g | 133 | 94 | 145 |
| Carbohydrates, EN % | 53 | 37 | 58 |
| Mono- and disaccharides, g | 51 | 45 | 87 |
| Polysaccharides, g | 79 | 47 | 57 |
| Ratio of polysaccharides to mono- and disaccharides | 0.6 | 1.0 | 1.5 |
| Dietary fiber, g | 14 | 4 | 5 |
| Protein, g | 26 | 26 | 26 |
| Protein, EN % | 10 | 10 | 10 |
| Total fat, g | 40 | 59 | 34 |
| Total fat, EN % | 36 | 53 | 31 |
| SFA, g | 6 | 32 | 19 |
| MUFA, g | 24 | 20 | 11 |
| PUFA, g | 9 | 4 | 2 |
| β-carotene, mg | 4.8 | 0.2 | 2.3 |
| Retinol, mg RE | 832 | 365 | 522 |
| Vitamin E, mg TE | 10.8 | 2.3 | 2.9 |
| Vitamin C, mg | 102 | 9 | 16 |
| All | E3 | E4 | p-Value E3 vs. E4 4 | |
|---|---|---|---|---|
| n | 49 | 39 | 10 | |
| Age (years) | 69.8 ± 5.4 | 69.7 ± 5.4 | 70.4 ± 5.8 | 0.715 |
| Body weight (kg) | 89.7 ± 10.6 | 89.9 ± 10.7 | 88.8 ± 10.7 | 0.773 |
| BMI (kg/m2) | 31.3 ± 3.0 | 31.3 ± 3.1 | 31.2 ± 3.1 | 0.915 |
| Waist circumference (cm) | 106.9 ± 8.2 | 106.4 ± 7.6 | 108.7 ± 10.4 | 0.534 |
| Waist-to-height ratio | 0.63 ± 0.05 | 0.63 ± 0.05 | 0.64 ± 0.05 | 0.371 |
| Fat mass (%) | 33.9 ± 7.2 | 34.2 ± 7.3 | 32.6 ± 7.6 | 0.558 |
| Systolic BP (mmHg) | 148 ± 16 | 149 ± 17.9 | 145.5 ± 14.7 | 0.599 |
| Diastolic BP (mmHg) | 88.0 ± 9.3 | 87.9 ± 9.5 | 88.7 ± 9.1 | 0.820 |
| Pulse (min−1) | 64 ± 11 | 64 ± 11 | 64 ± 13 | 0.881 |
| Plasma glucose (mmol/L) | 5.61 ± 1.00 | 5.71 ± 1.06 | 5.22 ± 0.60 | 0.154 |
| Serum insulin (pmol/L) | 88.2 ± 35.0 | 88.4 ± 36.2 | 87.4 ± 31.6 | 0.992 |
| Serum triglycerides (mmol/L) | 1.87 ± 0.85 | 1.90 ± 0.85 | 1.79 ± 0.88 | 0.739 |
| Serum total cholesterol (mmol/L) | 5.28 ± 0.92 | 5.27 ± 0.90 | 5.30 ± 1.02 | 0.928 |
| Serum HDL cholesterol (mmol/L) | 1.40 ± 0.32 | 1.41 ± 0.34 | 1.33 ± 0.23 | 0.498 |
| Serum LDL cholesterol (mmol/L) | 3.31 ± 0.81 | 3.26 ± 0.78 | 3.50 ± 0.94 | 0.417 |
| Serum hs-CRP (mg/L) | 4.2 ± 7.5 | 3.9 ± 7.7 | 5.4 ± 2.1 | 0.608 |
| E3 (n = 39) | E4 (n = 10) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MED | WDHF | WDHC | MED | WDHF | WDHC | Test Meal | Genotype | Genotype × Meal | |
| Glucose iAUC (mmol/L × h) | 4.7 ± 1.1 | 3.0 ± 0.5 | 5.1 ± 1.0 | 3.8 ± 1.4 | 3.1 ± 0.8 | 6.8 ± 1.9 | 0.006 | 0.810 | 0.800 |
| Insulin iAUC (pmol/L × h) | 1752 ± 187 | 1489 ± 134 | 2103 ± 194 | 2018 ± 498 | 1380 ± 147 | 2933 ± 567 | <0.001 | 0.926 | 0.096 |
| Triglycerides iAUC (mmol/L × h) | 3.1 ± 0.3 | 4.0 ± 0.3 | 2.9 ± 0.3 | 2.6 ± 0.5 | 3.9 ± 0.4 | 2.9 ± 0.5 | <0.001 | 0.680 | 0.574 |
| NEFA iAUC (mmol/L × h) | −1.44 ± 0.11 | −0.92 ± 0.10 | −1.48 ± 0.10 | −1.18 ± 0.20 | −0.87 ± 0.17 | −1.77 ± 0.2 | <0.001 | 0.921 | 0.161 |
| IL-1β iAUC (pg/mL × h) | 0.02 ± 0.02 | 0.00 ± 0.02 | 0.03 ± 0.03 | −0.03 ± 0.04 | 0.05 ± 0.04 | −0.02 ± 0.04 | 0.952 | 0.708 | 0.270 |
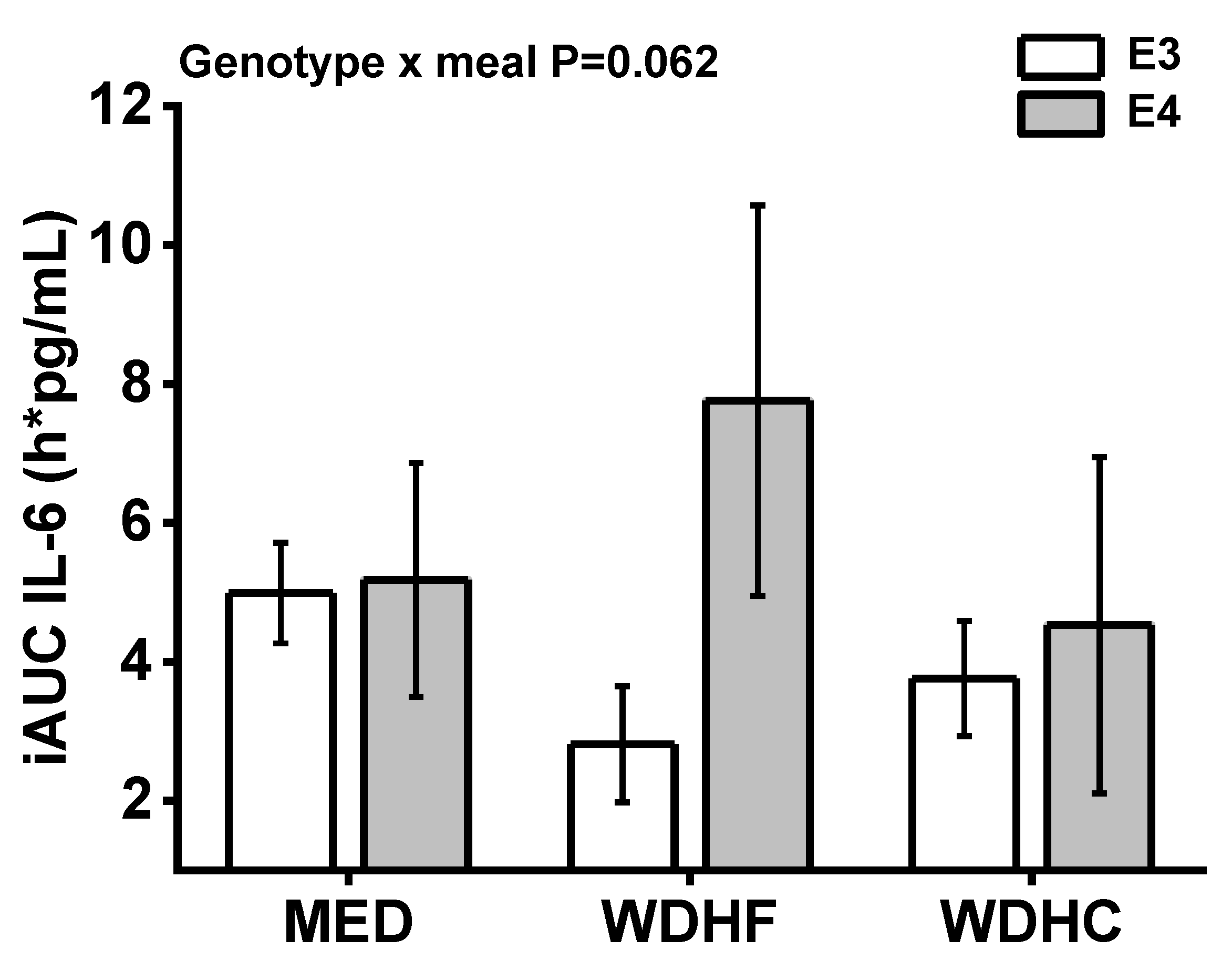
| IL-6 iAUC (pg/mL × h) | 4.99 ± 0.72 | 2.81 ± 0.84 | 3.76 ± 0.83 | 5.18 ± 1.69 | 7.76 ± 2.81 | 4.53 ± 2.42 | 0.161 | 0.289 | 0.062 |
| sE-selectin iAUC (ng/mL × h) | −3.9 ± 1.2 | −4.2 ± 1.4 | −3.6 ± 1.9 | 3.3 ± 5.3 | −4.9 ± 2.1 | −9.3 ± 2.4 | 0.728 | 0.839 | 0.179 |
| sICAM-1 iAUC (ng/mL × h) | 3.3 ± 10.6 | −32.7 ± 14.0 | −19.5 ± 18.7 | −42.5 ± 38.7 | −87.6 ± 77.1 | −20.7 ± 20.3 | 0.227 | 0.090 | 0.581 |
| sVCAM-1 iAUC (ng/mL × h) | −81.8 ± 40.6 | −84.8 ± 46.6 | −157.2 ± 90.7 | 182.8 ± 247.8 | −45.8 ± 95.7 | −156.1 ± 45.7 | 0.371 | 0.277 | 0.349 |
| oxLDL iAUC (ng/mL × h) | 0.9 ± 11.3 | 14.1 ± 9.5 | −1.1 ± 12.8 | −23.5 ± 17.3 | −0.8 ± 20.9 | 9.6 ± 27.6 | 0.474 | 0.579 | 0.261 |
| Vitamin C iAUC (mg/L × h) | 3.1 ± 1.0 | −4.9 ± 0.8 | −4.7 ± 1.0 | 1.4 ± 2.2 | −3.1 ± 1.0 | −1.3 ± 2.7 | <0.001 | 0.298 | 0.271 |
| Tocopherol iAUC (µg/mL × h) | −1.8 ± 0.6 | −1.3 ± 0.4 | −0.5 ± 1.0 | −4.3 ± 4.2 | −2.0 ± 1.0 | −2.2 ± 1.7 | 0.531 | 0.279 | 0.732 |
| β-carotene iAUC (ng/mL × h) | −17.4 ± 13.5 | −29.4 ± 14.8 | −14.9 ± 26.6 | −3.0 ± 33.8 | −83.0 ± 30.1 | −3.2 ± 36.6 | 0.334 | 0.752 | 0.321 |
| Retinol iAUC (ng/mL × h) | −9.6 ± 22.0 | −23.9 ± 19.3 | 7.8 ± 15.0 | −10.6 ± 47.3 | −3.7 ± 24.9 | 8.3 ± 28.2 | 0.288 | 0.922 | 0.988 |
| TEAC iAUC (mmol/L × h) | −0.11 ± −0.05 | −0.14 ± 0.005 | −0.13 ± 0.05 | −0.01 ± 0.09 | −0.10 ± 0.18 | −0.08 ± 0.12 | 0.868 | 0.265 | 0.921 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönknecht, Y.B.; Crommen, S.; Stoffel-Wagner, B.; Coenen, M.; Fimmers, R.; Stehle, P.; Ramirez, A.; Egert, S. APOE ɛ4 Is Associated with Postprandial Inflammation in Older Adults with Metabolic Syndrome Traits. Nutrients 2021, 13, 3924. https://doi.org/10.3390/nu13113924
Schönknecht YB, Crommen S, Stoffel-Wagner B, Coenen M, Fimmers R, Stehle P, Ramirez A, Egert S. APOE ɛ4 Is Associated with Postprandial Inflammation in Older Adults with Metabolic Syndrome Traits. Nutrients. 2021; 13(11):3924. https://doi.org/10.3390/nu13113924
Chicago/Turabian StyleSchönknecht, Yannik Bernd, Silke Crommen, Birgit Stoffel-Wagner, Martin Coenen, Rolf Fimmers, Peter Stehle, Alfredo Ramirez, and Sarah Egert. 2021. "APOE ɛ4 Is Associated with Postprandial Inflammation in Older Adults with Metabolic Syndrome Traits" Nutrients 13, no. 11: 3924. https://doi.org/10.3390/nu13113924
APA StyleSchönknecht, Y. B., Crommen, S., Stoffel-Wagner, B., Coenen, M., Fimmers, R., Stehle, P., Ramirez, A., & Egert, S. (2021). APOE ɛ4 Is Associated with Postprandial Inflammation in Older Adults with Metabolic Syndrome Traits. Nutrients, 13(11), 3924. https://doi.org/10.3390/nu13113924






