Early Enteral Feeding Improves Tolerance of Parenteral Nutrition in Preterm Newborns
Abstract
:1. Introduction
2. Materials and Methods
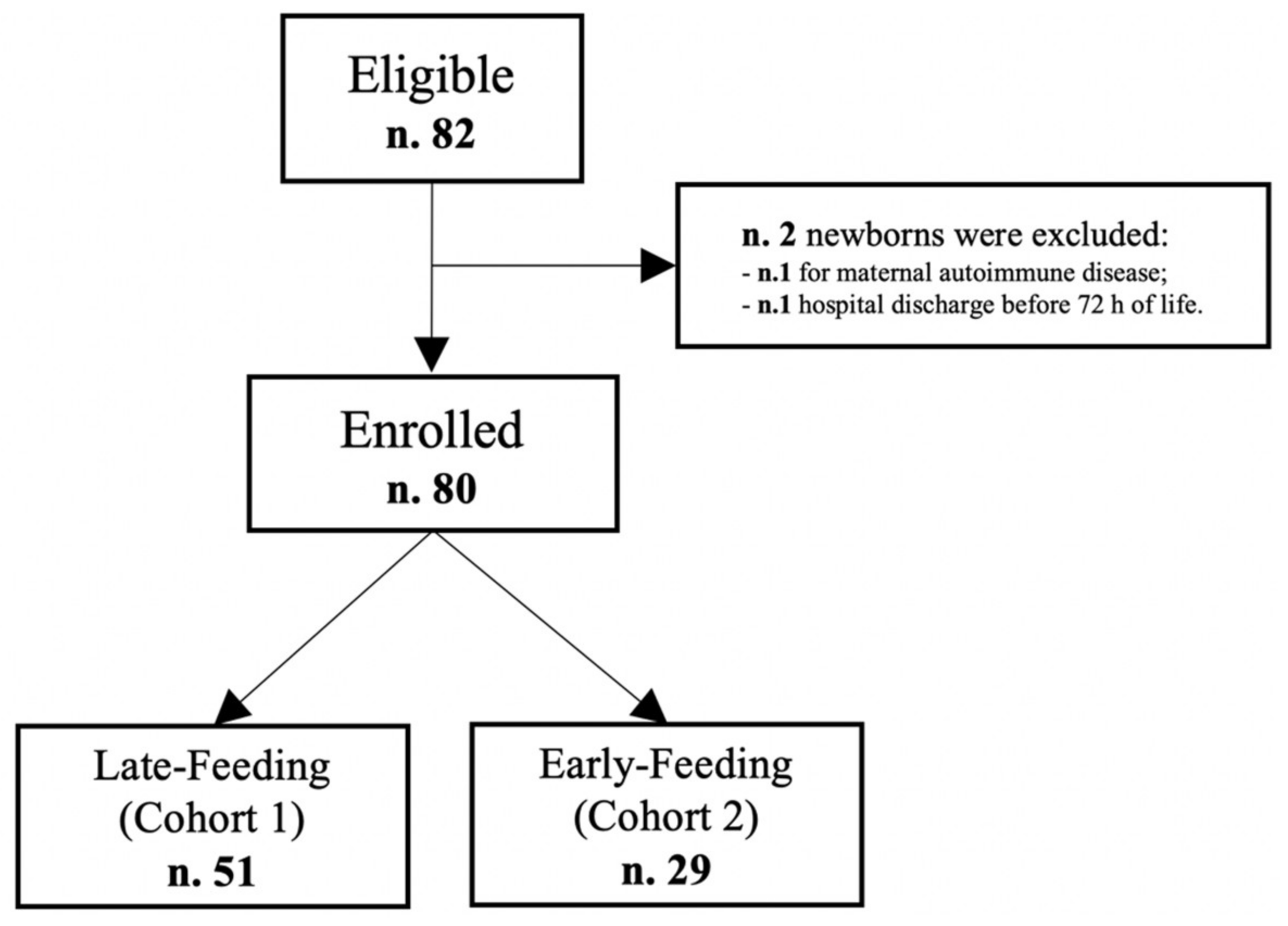
2.1. Study Design and Population
2.2. Nutritional Protocol
2.3. Outcome
2.4. Data Collection
2.5. Statistics
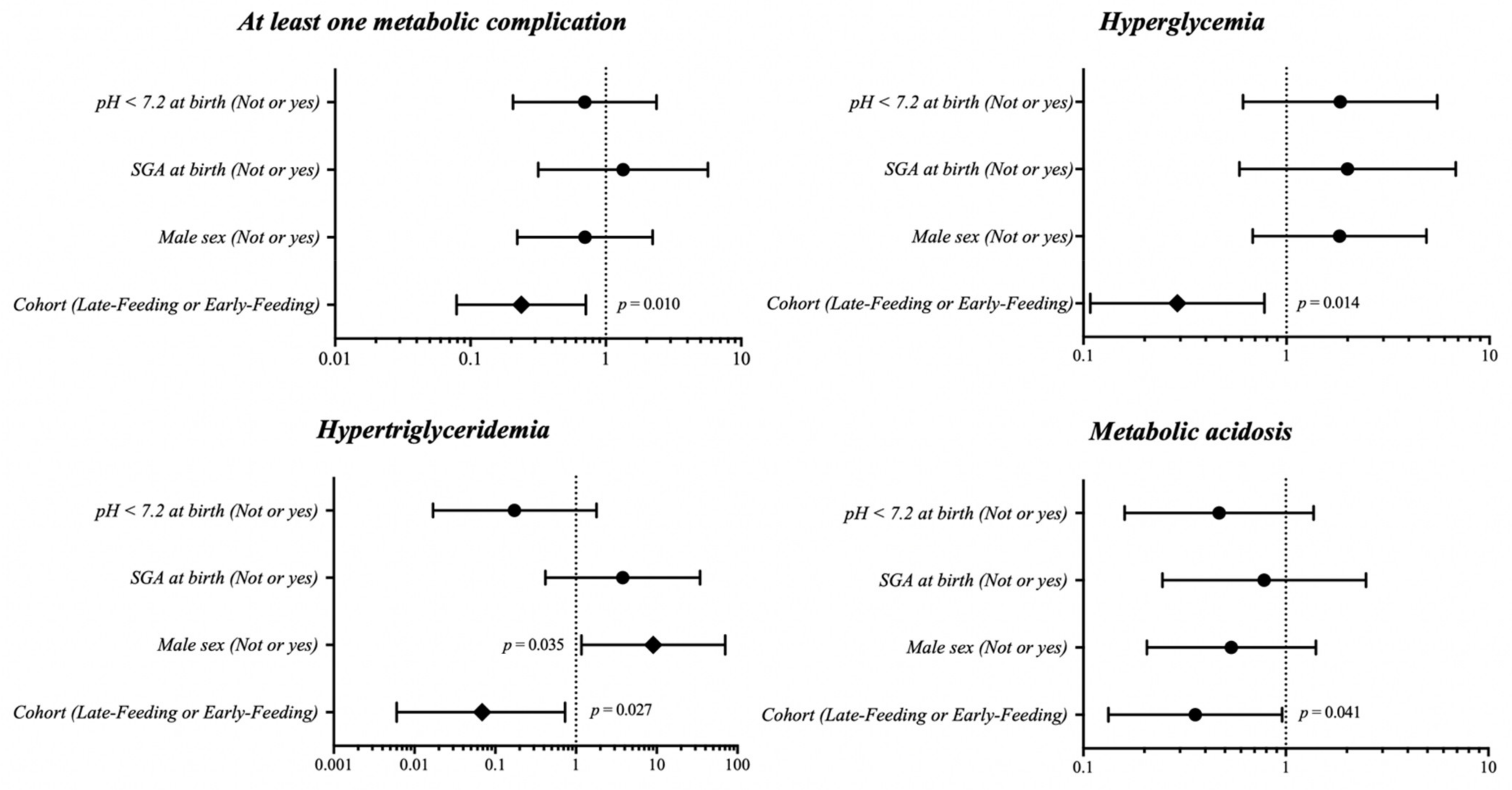
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.K.; Singhal, A.; Vaidya, U.; Banerjee, S.; Anwar, F.; Rao, S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front. Nutr. 2017, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef]
- Berni Canani, R.; Passariello, A.; Buccigrossi, V.; Terrin, G.; Guarino, A. The Nutritional Modulation of the Evolving Intestine. J. Clin. Gastroenterol. 2008, 42, S197–S200. [Google Scholar] [CrossRef]
- Moltu, S.J.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; de Koning, B.; Lapillonne, A.; Norsa, L.; Verduci, E.; et al. Nutritional Management of the Critically Ill Neonate: A Position Paper of the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 274–289. [Google Scholar] [CrossRef]
- Terrin, G.; Boscarino, G.; Gasparini, C.; Di Chiara, M.; Faccioli, F.; Onestà, E.; Parisi, P.; Spalice, A.; De Nardo, M.C.; Dito, L.; et al. Energy-Enhanced Parenteral Nutrition and Neurodevelopment of Preterm Newborns: A Cohort Study. Nutrition 2021, 89, 111219. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Coscia, A.; Boscarino, G.; Faccioli, F.; Di Chiara, M.; Greco, C.; Onestà, E.; Oliva, S.; Aloi, M.; Dito, L.; et al. Long-Term Effects on Growth of an Energy-Enhanced Parenteral Nutrition in Preterm Newborn: A Quasi-Experimental Study. PLoS ONE 2020, 15, e0235540. [Google Scholar] [CrossRef] [PubMed]
- Bonsante, F.; Gouyon, J.-B.; Robillard, P.-Y.; Gouyon, B.; Iacobelli, S. Early Optimal Parenteral Nutrition and Metabolic Acidosis in Very Preterm Infants. PLoS ONE 2017, 12, e0186936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stensvold, H.J.; Strommen, K.; Lang, A.M.; Abrahamsen, T.G.; Steen, E.K.; Pripp, A.H.; Ronnestad, A.E. Early Enhanced Parenteral Nutrition, Hyperglycemia, and Death Among Extremely Low-Birth-Weight Infants. JAMA Pediatr. 2015, 169, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscarino, G.; Di Chiara, M.; Cellitti, R.; De Nardo, M.C.; Conti, M.G.; Parisi, P.; Spalice, A.; Di Mario, C.; Ronchi, B.; Russo, A.; et al. Effects of Early Energy Intake on Neonatal Cerebral Growth of Preterm Newborn: An Observational Study. Sci. Rep. 2021, 11, 18457. [Google Scholar] [CrossRef]
- Verlinden, I.; Dulfer, K.; Vanhorebeek, I.; Güiza, F.; Hordijk, J.A.; Wouters, P.J.; Guerra, G.G.; Joosten, K.F.; Verbruggen, S.C.; Van den Berghe, G. Role of Age of Critically Ill Children at Time of Exposure to Early or Late Parenteral Nutrition in Determining the Impact Hereof on Long-Term Neurocognitive Development: A Secondary Analysis of the PEPaNIC-RCT. Clin. Nutr. 2021, 40, 1005–1012. [Google Scholar] [CrossRef]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus Late Parenteral Nutrition in Critically Ill Adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [Green Version]
- Vanhorebeek, I.; Verbruggen, S.; Casaer, M.P.; Gunst, J.; Wouters, P.J.; Hanot, J.; Guerra, G.G.; Vlasselaers, D.; Joosten, K.; Van den Berghe, G. Effect of Early Supplemental Parenteral Nutrition in the Paediatric ICU: A Preplanned Observational Study of Post-Randomisation Treatments in the PEPaNIC Trial. Lancet Respir. Med. 2017, 5, 475–483. [Google Scholar] [CrossRef]
- Agostoni, C.; Buonocore, G.; Carnielli, V.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral Nutrient Supply for Preterm Infants: Commentary From the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatric Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Terrin, G.; Passariello, A.; Canani, R.B.; Manguso, F.; Paludetto, R.; Cascioli, C. Minimal Enteral Feeding Reduces the Risk of Sepsis in Feed-Intolerant Very Low Birth Weight Newborns. Acta Paediatr. 2009, 98, 31–35. [Google Scholar] [CrossRef]
- Romijn, J.A.; Corssmit, E.P.; Havekes, L.M.; Pijl, H. Gut–Brain Axis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 518–521. [Google Scholar] [CrossRef]
- Meessen, E.C.E.; Bakker, G.J.; Nieuwdorp, M.; Dallinga-Thie, G.M.; Kemper, E.M.; Olde Damink, S.W.; Romijn, J.A.; Hartmann, B.; Holst, J.J.; Knop, F.K.; et al. Parenteral Nutrition Impairs Plasma Bile Acid and Gut Hormone Responses to Mixed Meal Testing in Lean Healthy Men. Clin. Nutr. 2021, 40, 1013–1021. [Google Scholar] [CrossRef]
- Dutta, S.; Singh, B.; Chessell, L.; Wilson, J.; Janes, M.; McDonald, K.; Shahid, S.; Gardner, V.; Hjartarson, A.; Purcha, M.; et al. Guidelines for Feeding Very Low Birth Weight Infants. Nutrients 2015, 7, 423–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passariello, A. Diarrhea in Neonatal Intensive Care Unit. WJG 2010, 16, 2664. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Terrin, G. Recent Progress in Congenital Diarrheal Disorders. Curr. Gastroenterol. Rep. 2011, 13, 257–264. [Google Scholar] [CrossRef]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s Milk and Rice Fermented with Lactobacillus Paracasei CBA L74 Prevent Infectious Diseases in Children: A Randomized Controlled Trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Salvia, G.; Cascioli, C.F.; Ciccimarra, F.; Terrin, G.; Cucchiara, S. A Case of Protein-Losing Enteropathy Caused by Intestinal Lymphangiectasia in a Preterm Infant. Pediatrics 2001, 107, 416–417. [Google Scholar] [CrossRef]
- Passariello, A.; Terrin, G.; Cecere, G.; Micillo, M.; Marco, G.; Di Costanzo, M.; Cosenza, L.; Leone, L.; Nocerino, R.; Berni Canani, R. Randomised Clinical Trial: Efficacy of a New Synbiotic Formulation Containing Lactobacillus Paracasei B21060 plus Arabinogalactan and Xilooligosaccharides in Children with Acute Diarrhoea. Aliment. Pharmacol. Ther. 2012, 35, 782–788. [Google Scholar] [CrossRef]
- Ferreira, C.R.; van Karnebeek, C.D.M. Inborn Errors of Metabolism. Handb. Clin. Neurol. 2019, 162, 449–481. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; De Nardo, M.C.; Boscarino, G.; Di Chiara, M.; Cellitti, R.; Ciccarelli, S.; Gasparini, C.; Parisi, P.; Urna, M.; Ronchi, B.; et al. Early Protein Intake Influences Neonatal Brain Measurements in Preterms: An Observational Study. Front. Neurol. 2020, 11, 885. [Google Scholar] [CrossRef] [PubMed]
- Gates, A.; Marin, T.; Leo, G.D.; Stansfield, B.K. Review of Preterm Human-Milk Nutrient Composition. Nutr. Clin. Pract. 2020. early view. [Google Scholar] [CrossRef]
- Boscarino, G.; Conti, M.G.; Gasparini, C.; Onestà, E.; Faccioli, F.; Dito, L.; Regoli, D.; Spalice, A.; Parisi, P.; Terrin, G. Neonatal Hyperglycemia Related to Parenteral Nutrition Affects Long-Term Neurodevelopment in Preterm Newborn: A Prospective Cohort Study. Nutrients 2021, 13, 1930. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Ahmed, I.; Silveyra, P. Bronchopulmonary Dysplasia: An Update on Experimental Therapeutics. Eur. Med. J. 2019, 4, 20–29. [Google Scholar]
- Vitali, R.; Terrin, G.; Palone, F.; Laudadio, I.; Cucchiara, S.; Boscarino, G.; Di Chiara, M.; Stronati, L. Fecal High-Mobility Group Box 1 as a Marker of Early Stage of Necrotizing Enterocolitis in Preterm Neonates. Front. Pediatr. 2021, 9, 672131. [Google Scholar] [CrossRef]
- Conti, M.G.; Angelidou, A.; Diray-Arce, J.; Smolen, K.K.; Lasky-Su, J.; De Curtis, M.; Levy, O. Immunometabolic Approaches to Prevent, Detect, and Treat Neonatal Sepsis. Pediatr. Res. 2020, 87, 399–405. [Google Scholar] [CrossRef]
- Terrin, G.; Di Chiara, M.; Boscarino, G.; Versacci, P.; Di Donato, V.; Giancotti, A.; Pacelli, E.; Faccioli, F.; Onestà, E.; Corso, C.; et al. Echocardiography-Guided Management of Preterms With Patent Ductus Arteriosus Influences the Outcome: A Cohort Study. Front. Pediatr. 2020, 8, 582735. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Di Chiara, M.; Boscarino, G.; Metrangolo, V.; Faccioli, F.; Onestà, E.; Giancotti, A.; Di Donato, V.; Cardilli, V.; De Curtis, M. Morbidity Associated with Patent Ductus Arteriosus in Preterm Newborns: A Retrospective Case-Control Study. Ital. J. Pediatr. 2021, 47, 9. [Google Scholar] [CrossRef]
- McClure, R.J. Trophic Feeding of the Preterm Infant. Acta Paediatr. Suppl. 2001, 90, 19–21. [Google Scholar] [CrossRef]
- Evans, R.A.; Thureen, P. Early Feeding Strategies in Preterm and Critically Ill Neonates. Neonatal. Netw. 2001, 20, 7–18. [Google Scholar] [CrossRef]
- Campbell, J.; McDowell, J.R.S. Comparative Study on the Effect of Enteral Feeding on Blood Glucose. Br. J. Nurs. 2007, 16, 344–349. [Google Scholar] [CrossRef]
- Prakash, V.; Parameswaran, N.; Biswal, N. Early versus Late Enteral Feeding in Critically Ill Children: A Randomized Controlled Trial. Intensive Care Med. 2016, 42, 481–482. [Google Scholar] [CrossRef]
- Srinivasan, V.; Hasbani, N.R.; Mehta, N.M.; Irving, S.Y.; Kandil, S.B.; Allen, H.C.; Typpo, K.V.; Cvijanovich, N.Z.; Faustino, E.V.S.; Wypij, D.; et al. Early Enteral Nutrition Is Associated With Improved Clinical Outcomes in Critically Ill Children: A Secondary Analysis of Nutrition Support in the Heart and Lung Failure-Pediatric Insulin Titration Trial. Pediatric Crit. Care Med. 2020, 21, 213–221. [Google Scholar] [CrossRef]
- Burrin, D.; Sangild, P.T.; Stoll, B.; Thymann, T.; Buddington, R.; Marini, J.; Olutoye, O.; Shulman, R.J. Translational Advances in Pediatric Nutrition and Gastroenterology: New Insights from Pig Models. Annu. Rev. Anim. Biosci. 2020, 8, 321–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niinikoski, H.; Stoll, B.; Guan, X.; Kansagra, K.; Lambert, B.D.; Stephens, J.; Hartmann, B.; Holst, J.J.; Burrin, D.G. Onset of Small Intestinal Atrophy Is Associated with Reduced Intestinal Blood Flow in TPN-Fed Neonatal Piglets. J. Nutr. 2004, 134, 1467–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrin, D.G.; Stoll, B.; Chang, X.; Van Goudoever, J.B.; Fujii, H.; Hutson, S.M.; Reeds, P.J. Parenteral Nutrition Results in Impaired Lactose Digestion and Hexose Absorption When Enteral Feeding Is Initiated in Infant Pigs. Am. J. Clin. Nutr. 2003, 78, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Meier, J.J. Incretin Hormones: Their Role in Health and Disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Boscarino, G.; Conti, M.G.; De Luca, F.; Di Chiara, M.; Deli, G.; Bianchi, M.; Favata, P.; Cardilli, V.; Di Nardo, G.; Parisi, P.; et al. Intravenous Lipid Emulsions Affect Respiratory Outcome in Preterm Newborn: A Case-Control Study. Nutrients 2021, 13, 1243. [Google Scholar] [CrossRef] [PubMed]

| Late-Feeding (Cohort 1) (n = 51) | Early-Feeding (Cohort 2) (n = 29) | p Value | |
|---|---|---|---|
| Intrauterine growth restriction, N. (%) | 7 (13.7) | 4 (13.8) | 0.629 |
| Pregnancy-induced hypertension, N. (%) | 12 (23.5) | 5 (17.2) | 0.481 |
| Antenatal corticosteroids a, N. (%) | 30 (58.8) | 18 (62.1) | 0.856 |
| Gestational age, weeks | 27 ± 2 | 27 ± 2 | 0.086 |
| Birth weight, g | 864 ± 258 | 952 ± 232 | 0.099 |
| Small for gestational age at birth, N. (%) | 11 (21.6) | 5 (17.2) | 0.612 |
| Male sex, N. (%) | 27 (52.9) | 18 (62.1) | 0.429 |
| Cesarean section, N. (%) | 40 (78.4) | 25 (86.2) | 0.392 |
| Twins, N. (%) | 12 (23.5) | 6 (20.7) | 0.770 |
| pH at birth | 7.2 ± 0.1 | 7.2 ± 0.1 | 0.892 |
| 5 min Apgar score, mean (IQR) | 7 (3) | 7 (1) | 0.055 |
| Respiratory distress syndrome, N. (%) | 45 (88.2) | 28 (96.6) | 0.278 |
| Late-Feeding (Cohort 1) (n = 51) | Early-Feeding (Cohort 2) (n = 29) | p Value | |
|---|---|---|---|
| Necrotizing enterocolitis | 0 (0) | 1 (3.4) | 0.362 |
| Bronchopulmonary dysplasia | 10 (19.6) | 2 (6.9) | 0.098 |
| Sepsis proven by positive culture | 9 (17.6) | 5 (17.2) | 0.963 |
| Retinopathy of prematurity | 20 (39.2) | 14 (48.3) | 0.431 |
| Periventricular leukomalacia | 1 (2.0) | 1 (3.4) | 0.597 |
| Intraventricular hemorrhage: | |||
| Before 7 days of age | 9 (17.6) | 1 (3.4) | 0.058 |
| After 7 days of age | 4 (7.8) | 0 (0) | 0.153 |
| Late-Feeding (Cohort 1) (n = 51) | Early-Feeding (Cohort 2) (n = 29) | p Value | |
|---|---|---|---|
| Energy intake, kcal/kg/week | 626.4 ± 191.5 | 635.9 ± 186.6 | 0.885 |
| Protein intake, g/kg/week | 24.3 ± 8.4 | 23.3 ± 7.3 | 0.635 |
| Glucose intake, g/kg/week | 84.8 ± 25.6 | 84.2 ± 23.3 | 0.885 |
| Fat intake, g/kg/week | 19.9 ± 6.3 | 21.1 ± 6.8 | 0.548 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscarino, G.; Conti, M.G.; Di Chiara, M.; Bianchi, M.; Onestà, E.; Faccioli, F.; Deli, G.; Repole, P.; Oliva, S.; Cresi, F.; et al. Early Enteral Feeding Improves Tolerance of Parenteral Nutrition in Preterm Newborns. Nutrients 2021, 13, 3886. https://doi.org/10.3390/nu13113886
Boscarino G, Conti MG, Di Chiara M, Bianchi M, Onestà E, Faccioli F, Deli G, Repole P, Oliva S, Cresi F, et al. Early Enteral Feeding Improves Tolerance of Parenteral Nutrition in Preterm Newborns. Nutrients. 2021; 13(11):3886. https://doi.org/10.3390/nu13113886
Chicago/Turabian StyleBoscarino, Giovanni, Maria Giulia Conti, Maria Di Chiara, Marco Bianchi, Elisa Onestà, Francesca Faccioli, Giorgia Deli, Paola Repole, Salvatore Oliva, Francesco Cresi, and et al. 2021. "Early Enteral Feeding Improves Tolerance of Parenteral Nutrition in Preterm Newborns" Nutrients 13, no. 11: 3886. https://doi.org/10.3390/nu13113886
APA StyleBoscarino, G., Conti, M. G., Di Chiara, M., Bianchi, M., Onestà, E., Faccioli, F., Deli, G., Repole, P., Oliva, S., Cresi, F., & Terrin, G. (2021). Early Enteral Feeding Improves Tolerance of Parenteral Nutrition in Preterm Newborns. Nutrients, 13(11), 3886. https://doi.org/10.3390/nu13113886








