Dietary Fat Effect on the Gut Microbiome, and Its Role in the Modulation of Gastrointestinal Disorders in Children with Autism Spectrum Disorder
Abstract
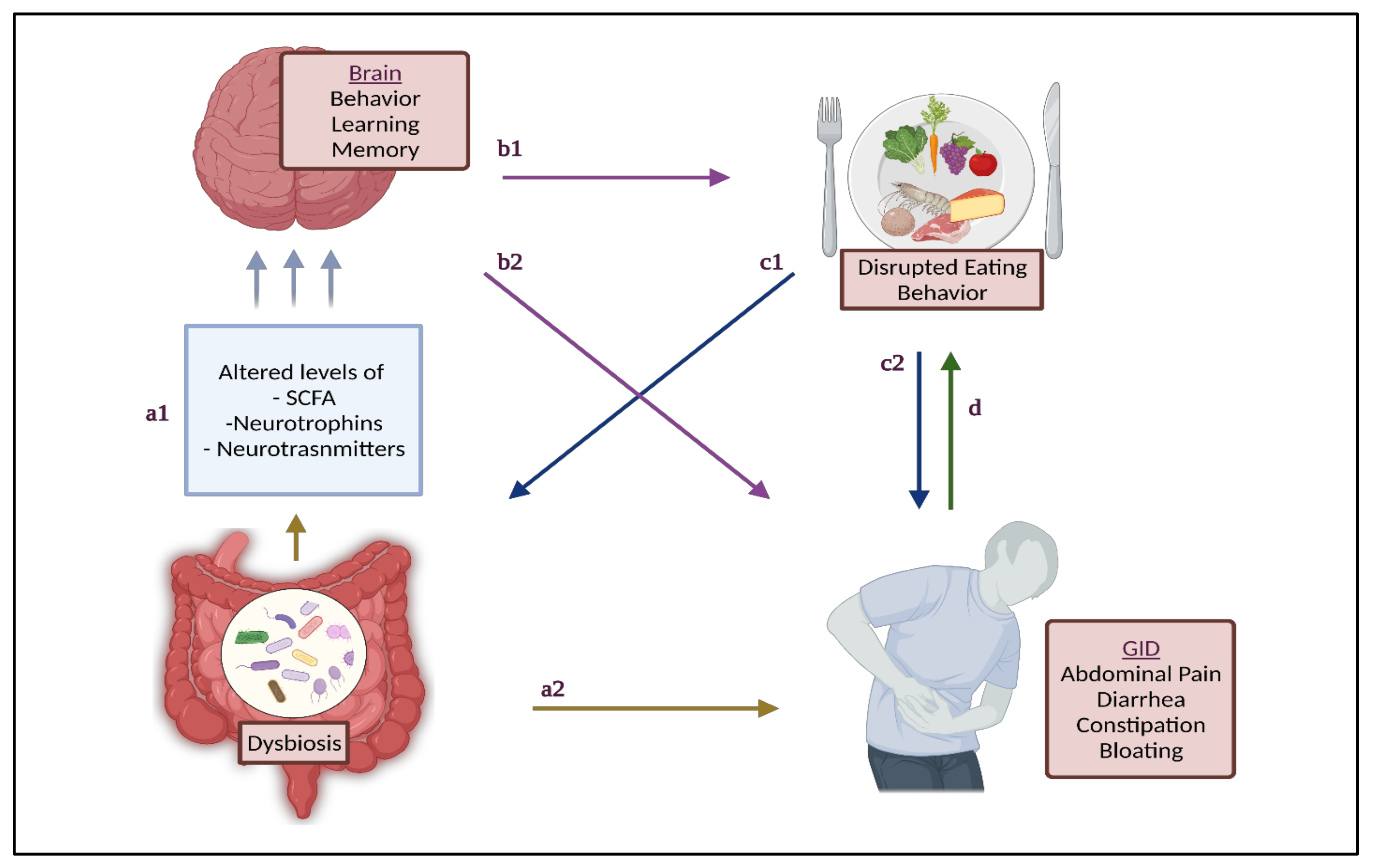
:1. Introduction
2. Methods
3. GM and GID in Children with ASD
4. Dietary Fat and Gut Health
4.1. Saturated Fatty Acids
4.2. Unsaturated Fatty Acids
4.3. High-Fat Diets
5. Limitations and Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shedlock, K.; Susi, A.; Gorman, G.H.; Hisle-Gorman, E.; Erdie-Lalena, C.R.; Nylund, C.M. Autism Spectrum Disorders and Metabolic Complications of Obesity. J. Pediatr. 2016, 178, 183–187.e1. [Google Scholar] [CrossRef]
- Murphy, C.M.; Wilson, C.E.; Robertson, D.M.; Ecker, C.; Daly, E.M.; Hammond, N.; Galanopoulos, A.; Dud, I.; Murphy, D.G.; McAlonan, G.M. Autism Spectrum Disorder in Adults: Diagnosis, Management, and Health Services Development. Neuropsychiatr. Dis. Treat. 2016, 12, 1669–1686. [Google Scholar] [CrossRef] [Green Version]
- Bauman, M.L. Medical Comorbidities in Autism: Challenges to Diagnosis and Treatment. Neurotherapeutics 2010, 7, 320–327. [Google Scholar] [CrossRef]
- Chiarotti, F.; Venerosi, A. Epidemiology of Autism Spectrum Disorders: A Review of Worldwide Prevalence Estimates Since 2014. Brain Sci. 2020, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, A.E.; Andreo-Martínez, P. The Role of Gut Microbiota in Gastrointestinal Symptoms of Children with ASD. Medicina 2019, 55, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal Problems in Children with Autism, Developmental Delays or Typical Development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuddo, T.; Nelson, K.B. How Common Are Gastrointestinal Disorders in Children with Autism? Curr. Opin. Pediatrics 2003, 15, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Sparks, B.; Cooper, J.; Hayes, C.; Williams, K. Constipation in Children with Autism Spectrum Disorder Associated with Increased Emergency Department Visits and Inpatient Admissions. J. Pediatrics 2018, 202, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Furuta, G.T.; Williams, K.; Kooros, K.; Kaul, A.; Panzer, R.; Coury, D.L.; Fuchs, G. Management of Constipation in Children and Adolescents With Autism Spectrum Disorders. Pediatrics 2012, 130, S98–S105. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal Flora and Gastrointestinal Status in Children with Autism--Comparisons to Typical Children and Correlation with Autism Severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Holingue, C.; Newill, C.; Lee, L.-C.; Pasricha, P.J.; Fallin, M.D. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Review of the Literature on Ascertainment and Prevalence. Autism Res. 2018, 11, 24–36. [Google Scholar] [CrossRef]
- Yang, X.-L.; Liang, S.; Zou, M.-Y.; Sun, C.-H.; Han, P.-P.; Jiang, X.-T.; Xia, W.; Wu, L.-J. Are Gastrointestinal and Sleep Problems Associated with Behavioral Symptoms of Autism Spectrum Disorder? Psychiatry Res. 2018, 259, 229–235. [Google Scholar] [CrossRef]
- Masi, A.; Breen, E.J.; Alvares, G.A.; Glozier, N.; Hickie, I.B.; Hunt, A.; Hui, J.; Beilby, J.; Ravine, D.; Wray, J.; et al. Cytokine Levels and Associations with Symptom Severity in Male and Female Children with Autism Spectrum Disorder. Mol. Autism 2017, 8, 63. [Google Scholar] [CrossRef]
- Niehus, R.; Lord, C. Early Medical History of Children with Autism Spectrum Disorders. J. Dev. Behav. Pediatrics 2006, 27, S120. [Google Scholar] [CrossRef]
- Bresnahan, M.; Hornig, M.; Schultz, A.F.; Gunnes, N.; Hirtz, D.; Lie, K.K.; Magnus, P.; Reichborn-Kjennerud, T.; Roth, C.; Schjølberg, S.; et al. Association of Maternal Report of Infant and Toddler Gastrointestinal Symptoms with Autism: Evidence from a Prospective Birth Cohort. JAMA Psychiatry 2015, 72, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.H.; Voigt, R.G.; Katusic, S.K.; Weaver, A.L.; Barbaresi, W.J. Incidence of Gastrointestinal Symptoms in Children With Autism: A Population-Based Study. Pediatrics 2009, 124, 680–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, K.H.; Croaker, G.D.H. Constipation in Children with Autism and Autistic Spectrum Disorder. Pediatr. Surg. Int. 2011, 27, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Gorrindo, P.; Williams, K.C.; Lee, E.B.; Walker, L.S.; McGrew, S.G.; Levitt, P. Gastrointestinal Dysfunction in Autism: Parental Report, Clinical Evaluation, and Associated Factors. Autism Res. 2012, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Brimacombe, M.; Chaaban, J.; Zimmerman-Bier, B.; Wagner, G.C. Autism Spectrum Disorders: Concurrent Clinical Disorders. J. Child. Neurol. 2008, 23, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Oh, D.; Cheon, K.-A. Alteration of Gut Microbiota in Autism Spectrum Disorder: An Overview. Soa Chongsonyon Chongsin Uihak 2020, 31, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Rosario, D.; Boren, J.; Uhlen, M.; Proctor, G.; Aarsland, D.; Mardinoglu, A.; Shoaie, S. Systems Biology Approaches to Understand the Host–Microbiome Interactions in Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 716. [Google Scholar] [CrossRef] [PubMed]
- Suganya, K.; Koo, B.-S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Lai, J.-B.; Du, Y.-L.; Xu, Y.; Ruan, L.-M.; Hu, S.-H. Current Understanding of Gut Microbiota in Mood Disorders: An Update of Human Studies. Front. Genet. 2019, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gutierrez, E.; Narbad, A.; Rodríguez, J.M. Autism Spectrum Disorder Associated With Gut Microbiota at Immune, Metabolomic, and Neuroactive Level. Front. Neurosci. 2020, 14, 578666. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Gao, X.; Wang, Z.; Cao, S.; Liang, G.; He, D.; Lv, Z.; Wang, L.; Xu, P.; Zhang, Q. Comparison of Gut Microbiota in Autism Spectrum Disorders and Neurotypical Boys in China: A Case-Control Study. Synth. Syst. Biotechnol. 2021, 6, 120–126. [Google Scholar] [CrossRef]
- Sun, H.; You, Z.; Jia, L.; Wang, F. Autism Spectrum Disorder Is Associated with Gut Microbiota Disorder in Children. BMC Pediatr. 2019, 19, 516. [Google Scholar] [CrossRef] [Green Version]
- Patusco, R.; Ziegler, J. Role of Probiotics in Managing Gastrointestinal Dysfunction in Children with Autism Spectrum Disorder: An Update for Practitioners. Adv. Nutr. 2018, 9, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.A.; Fontecilla, N.M.; Janelsins, B.M.; Vithayathil, P.J.; Segre, J.A.; Datta, S.K. Parental Dietary Fat Intake Alters Offspring Microbiome and Immunity. J. Immunol. 2013, 191, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Magnani, M.; Saunders, N.; Harms, C.; Bruce, I.J. Current Molecular Techniques for the Detection of Microbial Pathogens. Sci. Progress 2007, 90, 29–50. [Google Scholar] [CrossRef]
- Bharti, R.; Grimm, D.G. Current Challenges and Best-Practice Protocols for Microbiome Analysis. Brief. Bioinform. 2021, 22, 178–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuPont, A.W.; DuPont, H.L. The Intestinal Microbiota and Chronic Disorders of the Gut. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Isolauri, E.; Onnela, T. Gut Flora in Normal and Disordered States. CHE 1995, 41, 5–15. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Ranucci, G.; Buccigrossi, V.; de Freitas, M.B.; Guarino, A.; Giannattasio, A. Early-Life Intestine Microbiota and Lung Health in Children. J. Immunol. Res. 2017, 2017, e8450496. [Google Scholar] [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of Short-Chain Fatty Acid Production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Nogay, N.H.; Nahikian-Nelms, M. Can We Reduce Autism-Related Gastrointestinal and Behavior Problems by Gut Microbiota Based Dietary Modulation? A Review. Nutr. Neurosci. 2021, 24, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Coelho, O.G.L.; Cândido, F.G.; Alfenas, R.d.C.G. Dietary Fat and Gut Microbiota: Mechanisms Involved in Obesity Control. Crit. Rev. Food Sci. Nutr. 2019, 59, 3045–3053. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.L.; Yap, Y.A.; McLeod, K.H.; Mackay, C.R.; Mariño, E. Dietary Metabolites and the Gut Microbiota: An Alternative Approach to Control Inflammatory and Autoimmune Diseases. Clin. Transl. Immunol. 2016, 5, e82. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faruqui, N.A.; Prium, D.H.; Mowna, S.A.; Ullah, M.A.; Araf, Y.; Sarkar, B.; Zohora, U.S.; Rahman, M.S. Gut Microorganisms and Neurological Disease Perspectives. Future Neurol. 2021. [Google Scholar] [CrossRef]
- Tiffany, C.R.; Bäumler, A.J. Dysbiosis: From Fiction to Function. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G602–G608. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, R.A.; Savidge, T.C.; Williams, K.C. The Brain-Gut-Microbiome Axis: What Role Does It Play in Autism Spectrum Disorder? Curr. Dev. Disord. Rep. 2016, 3, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Zogg, H.; Wei, L.; Bartlett, A.; Ghoshal, U.C.; Rajender, S.; Ro, S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J. Neurogastroenterol. Motil. 2021, 27, 19–34. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [Green Version]
- Sandler, R.H.; Finegold, S.M.; Bolte, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.L.; Nelson, M.N.; Wexler, H.M. Short-Term Benefit from Oral Vancomycin Treatment of Regressive-Onset Autism. J. Child. Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulikkan, J.; Mazumder, A.; Grace, T. Role of the Gut Microbiome in Autism Spectrum Disorders. In Reviews on Biomarker Studies in Psychiatric and Neurodegenerative Disorders; Guest, P.C., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; pp. 253–269. ISBN 978-3-030-05542-4. [Google Scholar]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics Function Mechanistically as Delivery Vehicles for Neuroactive Compounds: Microbial Endocrinology in the Design and Use of Probiotics. Bioessays 2011, 33, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut Microbiota Depletion from Early Adolescence in Mice: Implications for Brain and Behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell Dev. Biol. 2021, 9, 472. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suliman, S.; Hemmings, S.; Seedat, S. Brain-Derived Neurotrophic Factor (BDNF) Protein Levels in Anxiety Disorders: Systematic Review and Meta-Regression Analysis. Front. Integr. Neurosci. 2013, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallisch, A.; Nowell, S.; Little, L. Picky Eating in Children with Autism Spectrum Disorder (ASD): An Examination of Behavioral Profiles. Am. J. Occup. 2020, 74, 7411510297p1. [Google Scholar] [CrossRef]
- Meguid, N.A.; Anwar, M.; Bjørklund, G.; Hashish, A.; Chirumbolo, S.; Hemimi, M.; Sultan, E. Dietary Adequacy of Egyptian Children with Autism Spectrum Disorder Compared to Healthy Developing Children. Metab. Brain Dis. 2017, 32, 607–615. [Google Scholar] [CrossRef]
- Al-Kindi, N.M.; Al-Farsi, Y.M.; Waly, M.I.; A-Shafaee, M.S.; Bakheit, C.S. Dietary Intake and Food Preferences of Autistic Children Versus Children with Typical Development: A Comparative Cross-Sectional Study. EC Nutr. 2016, 6, 72–85. [Google Scholar]
- El Khatib, A.A.; El Tekeya, M.M.; El Tantawi, M.A.; Omar, T. Oral Health Status and Behaviours of Children with Autism Spectrum Disorder: A Case-Control Study. Int. J. Paediatr. Dent. 2014, 24, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Murshid, E.Z. Diet, Oral Hygiene Practices and Dental Health in Autistic Children in Riyadh, Saudi Arabia. Oral Health Dent. Manag. 2014, 13, 91–96. [Google Scholar] [PubMed]
- Hammouda, S.A.I.; Farghal, S.; Al-Harbi, G.; Abduallah, M.; Al-Rehaly, R.; Al-Johani, G. Assessment of Nutritional Risk Factors Predisposing to Autism among Saudi Children. Int. J. Nutraceuticals Funct. Foods Nov. Foods. 2018, 6. [Google Scholar] [CrossRef]
- Tharner, A.; Jansen, P.W.; Kiefte-de Jong, J.C.; Moll, H.A.; Hofman, A.; Jaddoe, V.W.V.; Tiemeier, H.; Franco, O.H. Bidirectional Associations between Fussy Eating and Functional Constipation in Preschool Children. J. Pediatrics 2015, 166, 91–96.e1. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.A.S.; Ramli, N.S.; Hamzaid, N.H.; Hassan, N.I. Exploring Eating and Nutritional Challenges for Children with Autism Spectrum Disorder: Parents’ and Special Educators’ Perceptions. Nutrients 2020, 12, 2530. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.-H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef] [Green Version]
- Seong, C.N.; Kang, J.W.; Lee, J.H.; Seo, S.Y.; Woo, J.J.; Park, C.; Bae, K.S.; Kim, M.S. Taxonomic Hierarchy of the Phylum Firmicutes and Novel Firmicutes Species Originated from Various Environments in Korea. J. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered Composition and Function of Intestinal Microbiota in Autism Spectrum Disorders: A Systematic Review. Transl. Psychiatry 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing Study of Fecal Microflora of Autistic and Control Children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen Sulfide Toxicity in the Gut Environment: Meta-Analysis of Sulfate-Reducing and Lactic Acid Bacteria in Inflammatory Processes. J. Adv. Res. 2021, 27, 55–69. [Google Scholar] [CrossRef]
- Finegold, S.M. Desulfovibrio Species Are Potentially Important in Regressive Autism. Med. Hypotheses 2011, 77, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G.; Allain, T.; Motta, J.-P.; Wallace, J.L. Effects of Hydrogen Sulfide on the Microbiome: From Toxicity to Therapy. Antioxid. Redox Signal. 2021. [Google Scholar] [CrossRef]
- Kumbhare, S.V.; Francis-Lyon, P.A.; Kachru, D.; Uday, T.; Irudayanathan, C.; Muthukumar, K.M.; Ricchetti, R.R.; Singh-Rambiritch, S.; Ugalde, J.A.; Dulai, P.S.; et al. Digital Therapeutics Care Utilizing Genetic and Gut Microbiome Signals for the Management of Functional Gastrointestinal Disorders: Results from a Preliminary Retrospective Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Finegold, S.M.; Downes, J.; Summanen, P.H. Microbiology of Regressive Autism. Anaerobe 2012, 18, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Heberling, C.; Dhurjati, P. Novel Systems Modeling Methodology in Comparative Microbial Metabolomics: Identifying Key Enzymes and Metabolites Implicated in Autism Spectrum Disorders. Int. J. Mol. Sci. 2015, 16, 8949–8967. [Google Scholar] [CrossRef] [Green Version]
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.-P. Neurobiological Effects of Intraventricular Propionic Acid in Rats: Possible Role of Short Chain Fatty Acids on the Pathogenesis and Characteristics of Autism Spectrum Disorders. Behav. Brain Res. 2007, 176, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.A.; MacFabe, D.F.; Vaz, A.; Ossenkopp, K.; Kavaliers, M. Sexually Dimorphic Effects of Prenatal Exposure to Propionic Acid and Lipopolysaccharide on Social Behavior in Neonatal, Adolescent, and Adult Rats: Implications for Autism Spectrum Disorders. Int. J. Dev. Neurosci. 2014, 39, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal Dysbiosis and Yeast Isolation in Stool of Subjects with Autism Spectrum Disorders. Mycopathologia 2017, 182, 349–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabit, H.; Tombuloglu, H.; Rehman, S.; Almandil, N.B.; Cevik, E.; Abdel-Ghany, S.; Rashwan, S.; Abasiyanik, M.F.; Yee Waye, M.M. Gut Microbiota Metabolites in Autistic Children: An Epigenetic Perspective. Heliyon 2021, 7, e06105. [Google Scholar] [CrossRef]
- Madore, C.; Leyrolle, Q.; Lacabanne, C.; Benmamar-Badel, A.; Joffre, C.; Nadjar, A.; Layé, S. Neuroinflammation in Autism: Plausible Role of Maternal Inflammation, Dietary Omega 3, and Microbiota. Neural Plast. 2016, 2016, 3597209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the Gut Microflora of Children with Autistic Spectrum Disorders and That of Healthy Children. J. Med Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Oezguen, N.; Balderas, M.; Venkatachalam, A.; Runge, J.K.; Versalovic, J.; Veenstra-VanderWeele, J.; Anderson, G.M.; Savidge, T.; Williams, K.C. Distinct Microbiome-Neuroimmune Signatures Correlate with Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 218–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalif, I.L.; Quigley, E.M.M.; Konovitch, E.A.; Maximova, I.D. Alterations in the Colonic Flora and Intestinal Permeability and Evidence of Immune Activation in Chronic Constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, J.G.; Motta, M.E.F.d.A.; Beltrão, M.F.d.S.; Salviano, T.L.; da Silva, G.A.P. Fecal Microbiota and Diet of Children with Chronic Constipation. Int. J. Pediatrics 2016, 2016, e6787269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional Dysbiosis within the Gut Microbiota of Patients with Constipated-Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.-B. Intestinal Microbiota and Chronic Constipation. Springerplus 2016, 5, 1130. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Ma, W.; Zhang, J.; He, Y.; Wang, J. Analysis of Gut Microbiota Profiles and Microbe-Disease Associations in Children with Autism Spectrum Disorders in China. Sci. Rep. 2018, 8, 13981. [Google Scholar] [CrossRef] [Green Version]
- Tana, C.; Umesaki, Y.; Imaoka, A.; Handa, T.; Kanazawa, M.; Fukudo, S. Altered Profiles of Intestinal Microbiota and Organic Acids May Be the Origin of Symptoms in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2010, 22, 512–519, e114. [Google Scholar] [CrossRef] [PubMed]
- De Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the Intestinal Barrier in Patients with Autism Spectrum Disorders and in Their First-Degree Relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nankova, B.B.; Agarwal, R.; MacFabe, D.F.; La Gamma, E.F. Enteric Bacterial Metabolites Propionic and Butyric Acid Modulate Gene Expression, Including CREB-Dependent Catecholaminergic Neurotransmission, in PC12 Cells--Possible Relevance to Autism Spectrum Disorders. PLoS ONE 2014, 9, e103740. [Google Scholar] [CrossRef]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of Functional Constipation in Children and Adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y. Altered Gut Microbiota and Short Chain Fatty Acids in Chinese Children with Autism Spectrum Disorder. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-C.; Lee, C.-H.; Wang, H. Exploring the Association of Autism Spectrum Disorders and Constipation through Analysis of the Gut Microbiome. Int. J. Environ. Res. Public Health 2021, 18, 667. [Google Scholar] [CrossRef] [PubMed]
- Dan, Z.; Mao, X.; Liu, Q.; Guo, M.; Zhuang, Y.; Liu, Z.; Chen, K.; Chen, J.; Xu, R.; Tang, J.; et al. Altered Gut Microbial Profile Is Associated with Abnormal Metabolism Activity of Autism Spectrum Disorder. Gut Microbes 2020, 11, 1246–1267. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased Abundance of Sutterella Spp. and Ruminococcus Torques in Feces of Children with Autism Spectrum Disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low Relative Abundances of the Mucolytic Bacterium Akkermansia Muciniphila and Bifidobacterium Spp. in Feces of Children with Autism. Appl. Env. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Liu, J.; Cetinbas, M.; Sadreyev, R.; Koh, M.; Huang, H.; Adeseye, A.; He, P.; Zhu, J.; Russell, H.; et al. New and Preliminary Evidence on Altered Oral and Gut Microbiota in Individuals with Autism Spectrum Disorder (ASD): Implications for ASD Diagnosis and Subtyping Based on Microbial Biomarkers. Nutrients 2019, 11, 2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, D.R.; Yang, H.; Serena, G.; Sturgeon, C.; Ma, B.; Careaga, M.; Hughes, H.K.; Angkustsiri, K.; Rose, M.; Hertz-Picciotto, I.; et al. Differential Immune Responses and Microbiota Profiles in Children with Autism Spectrum Disorders and Co-Morbid Gastrointestinal Symptoms. Brain Behav. Immun. 2018, 70, 354–368. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.K.H.; Tong, V.J.W.; Syn, N.; Nagarajan, N.; Tham, E.H.; Tay, S.K.; Shorey, S.; Tambyah, P.A.; Law, E.C.N. Gut Microbiota Changes in Children with Autism Spectrum Disorder: A Systematic Review. Gut Pathog. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Yang, J.; Zhang, J.; Liang, C.; Wang, Y.; Chen, B.; Zhao, C.; Wang, J.; Zhang, G.; Zhao, D.; et al. Correlation of Gut Microbiome Between ASD Children and Mothers and Potential Biomarkers for Risk Assessment. Genom. Proteom. Bioinform. 2019, 17, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Gondalia, S.V.; Palombo, E.A.; Knowles, S.R.; Cox, S.B.; Meyer, D.; Austin, D.W. Molecular Characterisation of Gastrointestinal Microbiota of Children with Autism (with and without Gastrointestinal Dysfunction) and Their Neurotypical Siblings. Autism Res. 2012, 5, 419–427. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between Diet, Gut Microbiota Composition and Gut Metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of Environmental Pollutants on Gut Microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Clifford, J.; Kozil, A. Dietary Fat and Cholesterol. Available online: https://extension.colostate.edu/topic-areas/nutrition-food-safety-health/dietary-fat-and-cholesterol-9-319/ (accessed on 14 April 2021).
- APUS: An Introduction to Nutrition (Byerley). Available online: https://med.libretexts.org/Courses/American_Public_University/APUS%3A_An_Introduction_to_Nutrition_(Byerley) (accessed on 14 April 2021).
- Lang, J.M.; Pan, C.; Cantor, R.M.; Tang, W.H.W.; Garcia-Garcia, J.C.; Kurtz, I.; Hazen, S.L.; Bergeron, N.; Krauss, R.M.; Lusis, A.J. Impact of Individual Traits, Saturated Fat, and Protein Source on the Gut Microbiome. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cândido, F.G.; Valente, F.X.; Grześkowiak, Ł.M.; Moreira, A.P.B.; Rocha, D.M.U.P.; Alfenas, R.d.C.G. Impact of Dietary Fat on Gut Microbiota and Low-Grade Systemic Inflammation: Mechanisms and Clinical Implications on Obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.A.; Holscher, H.D. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 193–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvard—School of Public Health Types of Fat. Available online: https://www.hsph.harvard.edu/nutritionsource/what-should-you-eat/fats-and-cholesterol/types-of-fat/ (accessed on 14 April 2021).
- Christie, W. Sterols: 5. Bile Acids and Alcohols. 2021. Available online: https://www.lipidmaps.org/resources/lipidweb/lipidweb_html/lipids/simple/bileacids/index.htm (accessed on 27 April 2021).
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.E.; Guyton, A.C. Guyton and Hall Textbook of Medical Physiology, 12th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2011; ISBN 978-1-4160-4574-8. [Google Scholar]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary Bile Acids: An Underrecognized Cause of Colon Cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlidis, P.; Powell, N.; Vincent, R.P.; Ehrlich, D.; Bjarnason, I.; Hayee, B. Systematic Review: Bile Acids and Intestinal Inflammation-Luminal Aggressors or Regulators of Mucosal Defence? Aliment. Pharmacol. Ther. 2015, 42, 802–817. [Google Scholar] [CrossRef]
- Winston, J.A.; Theriot, C.M. Diversification of Host Bile Acids by Members of the Gut Microbiota. Gut Microbes 2020, 11, 158–171. [Google Scholar] [CrossRef]
- Wasilewska, J.; Klukowski, M. Gastrointestinal Symptoms and Autism Spectrum Disorder: Links and Risks—A Possible New Overlap Syndrome. Pediatric Health Med. 2015, 6, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.-L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila Wadsworthia Aggravates High Fat Diet Induced Metabolic Dysfunctions in Mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zhang, B.; Verne, G.N. Intestinal Membrane Permeability and Hypersensitivity In the Irritable Bowel Syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J. Fat, Bile and Gut Microbes. Nature 2012, 487, 47–48. [Google Scholar] [CrossRef]
- De Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; van der Meer, R. Saturated Fat Stimulates Obesity and Hepatic Steatosis and Affects Gut Microbiota Composition by an Enhanced Overflow of Dietary Fat to the Distal Intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G589–G599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, D.; Gong, X.; Wang, L.; Yu, X.; Dong, Q. Involvement of Reduced Microbial Diversity in Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, e6951091. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. A High-Fat Diet Differentially Affects the Gut Metabolism and Blood Lipids of Rats Depending on the Type of Dietary Fat and Carbohydrate. Nutrients 2014, 6, 616–626. [Google Scholar] [CrossRef] [Green Version]
- De Filippo, C.; Cavalieri, D.; Paola, M.D.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Devkota, S.; Wang, Y.; Musch, M.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary Fat-Induced Taurocholic Acid Production Promotes Pathobiont and Colitis in IL-10−/− Mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentges, D.J.; Maier, B.R.; Burton, G.C.; Flynn, M.A.; Tsutakawa, R.K. Effect of a High-Beef Diet on the Fecal Bacterial Flora of Humans. Cancer Res. 1977, 37, 568–571. [Google Scholar]
- Wang, G.; Jiao, T.; Xu, Y.; Li, D.; Si, Q.; Hao, J.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium Adolescentis and Lactobacillus Rhamnosus Alleviate Non-Alcoholic Fatty Liver Disease Induced by a High-Fat, High-Cholesterol Diet through Modulation of Different Gut Microbiota-Dependent Pathways. Food Funct. 2020, 11, 6115–6127. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kikuchi, E.; Tanaka, N.; Kosaka, T.; Suzuki, E.; Mizuno, R.; Shinojima, T.; Miyajima, A.; Umezawa, K.; Oya, M. Down-Regulation of NF Kappa B Activation Is an Effective Therapeutic Modality in Acquired Platinum-Resistant Bladder Cancer. BMC Cancer 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahola, A.J.; Lassenius, M.I.; Forsblom, C.; Harjutsalo, V.; Lehto, M.; Groop, P.-H. Dietary Patterns Reflecting Healthy Food Choices Are Associated with Lower Serum LPS Activity. Sci. Rep. 2017, 7, 6511. [Google Scholar] [CrossRef] [Green Version]
- Pendyala, S.; Walker, J.M.; Holt, P.R. A High-Fat Diet Is Associated With Endotoxemia That Originates From the Gut. Gastroenterology 2012, 142, 1100–1101.e2. [Google Scholar] [CrossRef] [Green Version]
- Nozu, T.; Miyagishi, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Lipopolysaccharide Induces Visceral Hypersensitivity: Role of Interleukin-1, Interleukin-6, and Peripheral Corticotropin-Releasing Factor in Rats. J. Gastroenterol. 2017, 52, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delvaux, M. Role of Visceral Sensitivity in the Pathophysiology of Irritable Bowel Syndrome. Gut 2002, 51, i67–i71. [Google Scholar] [CrossRef] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, D.M.; Riehle, K.; Mistretta, T.-A.; Diaz, M.-A.; Mandal, D.; Raza, S.; Weidler, E.M.; Qin, X.; Coarfa, C.; Milosavljevic, A.; et al. Gastrointestinal Microbiome Signatures of Pediatric Patients with Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1782–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Sha, L.; Li, K.; Wang, Z.; Wang, T.; Li, Y.; Liu, P.; Dong, X.; Dong, Y.; Zhang, X.; et al. Dietary Flaxseed Oil Rich in Omega-3 Suppresses Severity of Type 2 Diabetes Mellitus via Anti-Inflammation and Modulating Gut Microbiota in Rats. Lipids Health Dis. 2020, 19, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. Int. J. Env. Res. Public Health 2020, 17, 2647. [Google Scholar] [CrossRef] [Green Version]
- Parellada, M.; Llorente, C.; Calvo, R.; Gutierrez, S.; Lázaro, L.; Graell, M.; Guisasola, M.; Dorado, M.L.; Boada, L.; Romo, J.; et al. Randomized Trial of Omega-3 for Autism Spectrum Disorders: Effect on Cell Membrane Composition and Behavior. Eur. Neuropsychopharmacol. 2017, 27, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Tabbaa, M.; Golubic, M.; Roizen, M.F.; Bernstein, A.M. Docosahexaenoic Acid, Inflammation, and Bacterial Dysbiosis in Relation to Periodontal Disease, Inflammatory Bowel Disease, and the Metabolic Syndrome. Nutrients 2013, 5, 3299–3310. [Google Scholar] [CrossRef] [Green Version]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 Fatty Acids Correlate with Gut Microbiome Diversity and Production of N-Carbamylglutamate in Middle Aged and Elderly Women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. Autism as a Disorder of Deficiency of Brain-Derived Neurotrophic Factor and Altered Metabolism of Polyunsaturated Fatty Acids. Nutrition 2013, 29, 1175–1185. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A Randomised Trial of the Effect of Omega-3 Polyunsaturated Fatty Acid Supplements on the Human Intestinal Microbiota. BMJ 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia Spp.: A Marker of Health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; Gobbetti, M. Autism Spectrum Disorders and Intestinal Microbiota. Gut Microbes 2015, 6, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, B.J.; Dovgan, K.; Severns, D.; Martin, S.; Marler, S.; Gross Margolis, K.; Bauman, M.L.; Veenstra-VanderWeele, J.; Sohl, K.; Beversdorf, D.Q. Lack of Associations Between Dietary Intake and Gastrointestinal Symptoms in Autism Spectrum Disorder. Front. Psychiatry 2019, 10, 528. [Google Scholar] [CrossRef] [Green Version]
- Mankad, D.; Dupuis, A.; Smile, S.; Roberts, W.; Brian, J.; Lui, T.; Genore, L.; Zaghloul, D.; Iaboni, A.; Marcon, P.; et al. A Randomized, Placebo Controlled Trial of Omega-3 Fatty Acids in the Treatment of Young Children with Autism. Mol. Autism 2015, 6, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J. Almond Consumption and Processing Affects the Composition of the Gastrointestinal Microbiota of Healthy Adult Men and Women: A Randomized Controlled Trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holscher, H.D.; Guetterman, H.M.; Swanson, K.S.; An, R.; Matthan, N.R.; Lichtenstein, A.H.; Novotny, J.A.; Baer, D.J. Walnut Consumption Alters the Gastrointestinal Microbiota, Microbially Derived Secondary Bile Acids, and Health Markers in Healthy Adults: A Randomized Controlled Trial. J. Nutr. 2018, 148, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Khazanehei, H.; Jones, P.J.; Khafipour, E. Interactions between Obesity Status and Dietary Intake of Monounsaturated and Polyunsaturated Oils on Human Gut Microbiome Profiles in the Canola Oil Multicenter Intervention Trial (COMIT). Front. Microbiol. 2016, 7, 1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.; Godfrey, K.; McDonald, D.; Treuren, W.; Bjørnholt, J.; Midtvedt, T.; Moen, B.; Rudi, K.; Knight, R.; Brantsaeter, A.L.; et al. Fat and Vitamin Intakes during Pregnancy Have Stronger Relations with a Pro-Inflammatory Maternal Microbiota than Does Carbohydrate Intake. Microbiome 2016, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Berding, K.; Donovan, S.M. Microbiome and Nutrition in Autism Spectrum Disorder: Current Knowledge and Research Needs. Nutr. Rev. 2016, 74, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Academy of Nutrition and Dietetics What Is the Ketogenic Diet. Available online: https://www.eatright.org/health/weight-loss/fad-diets/what-is-the-ketogenic-diet (accessed on 28 April 2021).
- Castro, K.; Faccioli, L.S.; Baronio, D.; Gottfried, C.; Perry, I.S.; dos Santos Riesgo, R. Effect of a Ketogenic Diet on Autism Spectrum Disorder: A Systematic Review. Res. Autism Spectr. Disord. 2015, 20, 31–38. [Google Scholar] [CrossRef]
- Varesio, C.; Grumi, S.; Zanaboni, M.P.; Mensi, M.M.; Chiappedi, M.; Pasca, L.; Ferraris, C.; Tagliabue, A.; Borgatti, R.; De Giorgis, V. Ketogenic Dietary Therapies in Patients with Autism Spectrum Disorder: Facts or Fads? A Scoping Review and a Proposal for a Shared Protocol. Nutrients 2021, 13, 2057. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Bonfiglio, F.; Belheouane, M.; Vallier, M.; Sauer, S.; Bang, C.; Bujanda, L.; Andreasson, A.; Agreus, L.; Engstrand, L.; et al. Faecal Microbiota Composition Associates with Abdominal Pain in the General Population. Gut 2018, 67, 778–779. [Google Scholar] [CrossRef]
- Islam, K.B.M.S.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile Acid Is a Host Factor That Regulates the Composition of the Cecal Microbiota in Rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Meng, Y.; Li, X.; Zhang, J.; Wang, C.; Lu, F. Effects of Different Diets on Microbiota in The Small Intestine Mucus and Weight Regulation in Rats. Sci. Rep. 2019, 9, 8500. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Possemiers, S.; Verstraete, W.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Dietary Modulation of Clostridial Cluster XIVa Gut Bacteria (Roseburia Spp.) by Chitin–Glucan Fiber Improves Host Metabolic Alterations Induced by High-Fat Diet in Mice. J. Nutr. Biochem. 2012, 23, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, X.; Zhang, R.; Si, D.; Petitte, J.N.; Ahmad, B.; Zhang, M. A Novel Peptide Ameliorates LPS-Induced Intestinal Inflammation and Mucosal Barrier Damage via Its Antioxidant and Antiendotoxin Effects. Int. J. Mol. Sci. 2019, 20, 3974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emanuele, E.; Orsi, P.; Boso, M.; Broglia, D.; Brondino, N.; Barale, F.; di Nemi, S.U.; Politi, P. Low-Grade Endotoxemia in Patients with Severe Autism. Neurosci. Lett. 2010, 471, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The Type and Quantity of Dietary Fat and Carbohydrate Alter Faecal Microbiome and Short-Chain Fatty Acid Excretion in a Metabolic Syndrome “at-Risk” Population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodes, L.; Khan, A.; Paul, A.; Coussa-Charley, M.; Marinescu, D.; Tomaro-Duchesneau, C.; Shao, W.; Kahouli, I.; Prakash, S. Effect of Probiotics Lactobacillus and Bifidobacterium on Gut-Derived Lipopolysaccharides and Inflammatory Cytokines: An in Vitro Study Using a Human Colonic Microbiota Model. J. Microbiol. Biotechnol. 2013, 23, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| GID | Disorder Definition | Increase in: | Decrease in: | Ref. |
|---|---|---|---|---|
| Functional Constipation | Hard, infrequent bowel movements without an organic etiology [98] | Escherichia/Shigella and Clostridium cluster XVIII | -- | [99] |
| Fusobacterium, Barnesiella, Coprobacter and Actinomycetaceae | Butyrate-producing taxa | [100] | ||
| -- | Turicibacter, Roseburia, Dialister, Staphylococcus, Butyricicoccus, Faecalibacterium, Gemmiger | [101] | ||
| Lachnospiraceae NK4A136, Subdoligranulum, Ruminococcus, Barnesiella, Butyricicoccus, and Ruminiclostridium | Fusobacterium, Acidaminococcus, and Veillonella | [102] | ||
| Abdominal Pain | Abdominal pain accompanying various GID | Turicibacter sanguinis, Clostridium aldenense, Clostridium lituseburense, Flavonifractor plautii, Clostridium disporicum, Clostridium tertium, Tyzzerella species and Parasutterella excrementihominis | -- | [89] |
| Ruminococcus torques | -- | [103] | ||
| Bacteroides fragilis | -- | [104] | ||
| Dorea, Prevotella | Bacteroides, Roseburia | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittana, M.; Ahmadani, A.; Al Marzooq, F.; Attlee, A. Dietary Fat Effect on the Gut Microbiome, and Its Role in the Modulation of Gastrointestinal Disorders in Children with Autism Spectrum Disorder. Nutrients 2021, 13, 3818. https://doi.org/10.3390/nu13113818
Kittana M, Ahmadani A, Al Marzooq F, Attlee A. Dietary Fat Effect on the Gut Microbiome, and Its Role in the Modulation of Gastrointestinal Disorders in Children with Autism Spectrum Disorder. Nutrients. 2021; 13(11):3818. https://doi.org/10.3390/nu13113818
Chicago/Turabian StyleKittana, Monia, Asma Ahmadani, Farah Al Marzooq, and Amita Attlee. 2021. "Dietary Fat Effect on the Gut Microbiome, and Its Role in the Modulation of Gastrointestinal Disorders in Children with Autism Spectrum Disorder" Nutrients 13, no. 11: 3818. https://doi.org/10.3390/nu13113818






