Continuous Protein Supplementation Reduces Acute Exercise-Induced Stress Markers in Athletes Performing Marathon
Abstract
:1. Introduction
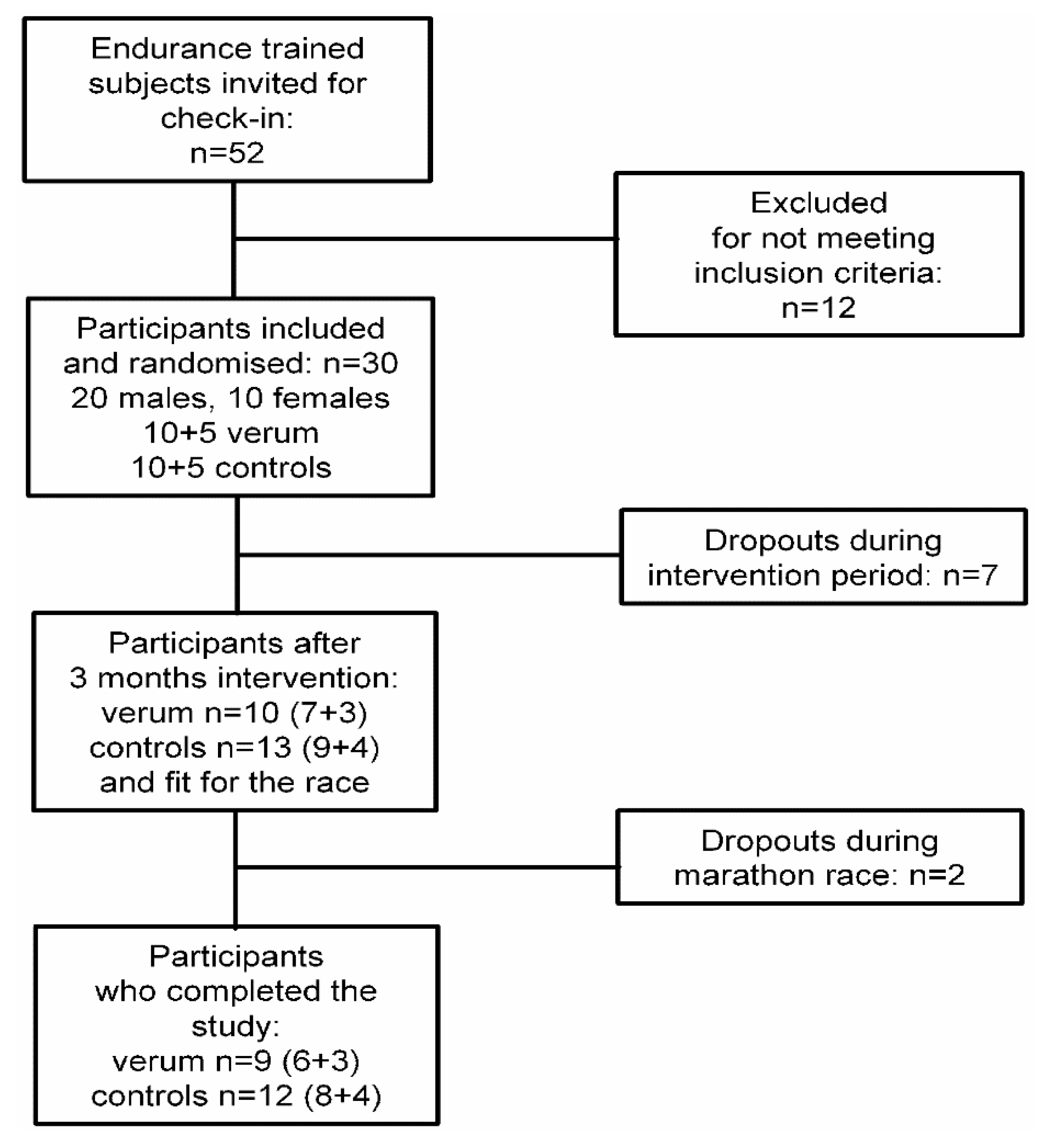
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Protein Supplementation
2.4. Measures
2.5. Training Phase
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, N.R.; Di Marco, N.M.; Langley, S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Kraut, H.; Muller, E.A.; Muller-Wecker, H. The effect of dietary protein content on nitrogen balance and on muscle training. Int. Z. Angew. Physiol. Einschl. Arb. 1958, 17, 378–390. [Google Scholar]
- Stearns, R.L.; Emmanuel, H.; Volek, J.S.; Casa, D.J. Effects of ingesting protein in combination with carbohydrate during exercise on endurance performance: A systematic review with meta-analysis. J. Strength Cond. Res. 2010, 24, 2192–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, J.A.; Toone, R.J.; Stokes, K.A.; Thompson, D. Systemic indices of skeletal muscle damage and recovery of muscle function after exercise: Effect of combined carbohydrate-protein ingestion. Appl. Physiol. Nutr. Metab. 2009, 34, 773–784. [Google Scholar] [CrossRef]
- Flakoll, P.J.; Judy, T.; Flinn, K.; Carr, C.; Flinn, S. Postexercise protein supplementation improves health and muscle soreness during basic military training in Marine recruits. J. Appl. Physiol. 2004, 96, 951–956. [Google Scholar] [CrossRef]
- Nosaka, K.; Sacco, P.; Mawatari, K. Effects of amino acid supplementation on muscle soreness and damage. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 620–635. [Google Scholar] [CrossRef] [Green Version]
- Betts, J.A.; Williams, C. Short-term recovery from prolonged exercise: Exploring the potential for protein ingestion to accentuate the benefits of carbohydrate supplements. Sports Med. 2010, 40, 941–959. [Google Scholar] [CrossRef] [Green Version]
- Negro, M.; Giardina, S.; Marzani, B.; Marzatico, F. Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J. Sports Med. Phys. Fit. 2008, 48, 347–351. [Google Scholar]
- Sousa, M.; Teixeira, V.H.; Soares, J. Dietary strategies to recover from exercise-induced muscle damage. Int. J. Food Sci. Nutr. 2014, 65, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, F.; Verdi, M.; Schlemper, B.R., Jr.; Caponi, S. 50th anniversary of the Declaration of Helsinki: The double standard was introduced. Arch. Med. Res. 2014, 45, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Siri, W.E. The gross composition of the body. Adv. Biol. Med. Phys. 1956, 4, 239–280. [Google Scholar] [CrossRef] [PubMed]
- Roecker, K.; Schotte, O.; Niess, A.M.; Horstmann, T.; Dickhuth, H.H. Predicting competition performance in long-distance running by means of a treadmill test. Med. Sci. Sports Exerc. 1998, 30, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Exercise immunology: Practical applications. Int. J. Sports Med. 1997, 18 (Suppl. 1), S91–S100. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Haralambie, G. Changes in serum creatine kinase and hexose phosphate isomerase activity with exercise duration. Eur. J. Appl. Physiol. Occup. Physiol. 1978, 39, 191–201. [Google Scholar] [CrossRef]
- MacIntyre, D.L.; Sorichter, S.; Mair, J.; Berg, A.; McKenzie, D.C. Markers of inflammation and myofibrillar proteins following eccentric exercise in humans. Eur. J. Appl. Physiol. 2001, 84, 180–186. [Google Scholar] [CrossRef]
- Northoff, H.; Berg, A. Immunologic mediators as parameters of the reaction to strenuous exercise. Int. J. Sports Med. 1991, 12 (Suppl. 1), S9–S15. [Google Scholar] [CrossRef]
- Kuoppasalmi, K.; Näveri, H.; Härkönen, M.; Adlercreutz, H. Plasma cortisol, androstenedione, testosterone and luteinizing hormone in running exercise of different intensities. Scand. J. Clin. Lab. Investig. 1980, 40, 403–409. [Google Scholar] [CrossRef]
- Berg, A.; Jakob, E.; Lehmann, M.; Dickhuth, H.H.; Huber, G.; Keul, J. Current aspects of modern ergometry. Pneumologie 1990, 44, 2–13. [Google Scholar]
- Brooks, G.A. Anaerobic threshold: Review of the concept and directions for future research. Med. Sci. Sports Exerc. 1985, 17, 22–34. [Google Scholar] [CrossRef]
- Rosenbaum, N. Ernährungssoftware im Vergleich. Ernährungs-Umschau 2006, 53, 150–151. [Google Scholar]
- Kerksick, C.M.; Jagim, A. Plant Proteins and Exercise: What Role Can Plant Proteins Have in Promoting Adaptations to Exercise? Nutrients 2021, 13, 1962. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Lieberman, H.R.; McLellan, T.M. Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: A systematic review. Sports Med. 2014, 44, 655–670. [Google Scholar] [CrossRef]
- Ten Haaf, D.S.M.; Flipsen, M.A. The Effect of Protein Supplementation versus Carbohydrate Supplementation on Muscle Damage Markers and Soreness Following a 15-km Road Race: A Double-Blind Randomized Controlled Trial. Nutrients 2021, 13, 858. [Google Scholar] [CrossRef]
- Ten Haaf, D.S.M.; Bongers, C.; Hulshof, H.G.; Eijsvogels, T.M.H.; Hopman, M.T.E. The Impact of Protein Supplementation on Exercise-Induced Muscle Damage, Soreness and Fatigue Following Prolonged Walking Exercise in Vital Older Adults: A Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2020, 12, 1806. [Google Scholar] [CrossRef]
- Köhne, J.L.; Ormsbee, M.J.; McKune, A.J. The effects of a multi-ingredient supplement on markers of muscle damage and inflammation following downhill running in females. J. Int. Soc. Sports Nutr. 2016, 13, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cintineo, H.P.; Arent, M.A.; Antonio, J.; Arent, S.M. Effects of Protein Supplementation on Performance and Recovery in Resistance and Endurance Training. Front. Nutr. 2018, 5, 83. [Google Scholar] [CrossRef]
- Kanda, K.; Sugama, K.; Hayashida, H.; Sakuma, J.; Kawakami, Y.; Miura, S.; Yoshioka, H.; Mori, Y.; Suzuki, K. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc. Immunol. Rev. 2013, 19, 72–85. [Google Scholar] [PubMed]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, G.P.; Siddiqui, R.A. Biologically active dietary peptides. Mini Rev. Med. Chem. 2004, 4, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yamaguchi, M.; Sobue, T.; Takahashi, T.; Miura, T.; Arai, Y.; Mazur, W.; Wähälä, K.; Adlercreutz, H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). J. Nutr. 1998, 128, 1710–1715. [Google Scholar] [CrossRef] [Green Version]
- Sorichter, S.; Martin, M.; Julius, P.; Schwirtz, A.; Huonker, M.; Luttmann, W.; Walterspacher, S.; Berg, A. Effects of unaccustomed and accustomed exercise on the immune response in runners. Med. Sci. sports Exerc. 2006, 38, 1739–1745. [Google Scholar] [CrossRef]
- Bergström, J.; Hermansen, L.; Hultman, E.; Saltin, B. Diet, muscle glycogen and physical performance. Acta Physiol. Scand. 1967, 71, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Viribay, A.; Arribalzaga, S.; Mielgo-Ayuso, J. Effects of 120 g/h of Carbohydrates Intake during a Mountain Marathon on Exercise-Induced Muscle Damage in Elite Runners. Nutrients 2020, 12, 1367. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Calleja-González, J.; Refoyo, I.; León-Guereño, P.; Cordova, A.; Del Coso, J. Exercise-Induced Muscle Damage and Cardiac Stress During a Marathon Could be Associated with Dietary Intake During the Week Before the Race. Nutrients 2020, 12, 316. [Google Scholar] [CrossRef] [Green Version]
- Arribalzaga, S.; Viribay, A.; Calleja-González, J. Relationship of Carbohydrate Intake during a Single-Stage One-Day Ultra-Trail Race with Fatigue Outcomes and Gastrointestinal Problems: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 5737. [Google Scholar] [CrossRef]
| Variable | Verum (n = 10) | Control (n = 13) | ||
|---|---|---|---|---|
| a | b | a | b | |
| Sex (m/f) | 7/3 | 9/4 | ||
| Age (y) | 29.0 ± 11.0 | 28.6 ± 8.7 | ||
| Body mass (kg) | 73.7 ± 6.7 | 74.5 ± 8.2 | 71.1 ± 71.5 | 71.5 ± 8.8 |
| BMI (kg/m2) | 23.5 ± 1.2 | 23.3 ± 1.5 | 22.4 ± 2.1 | 22.5 ± 1.9 |
| BFM (%BW) | 22.9 ± 9.9 | 21.8 ± 8.9 | 22.2 ± 7.7 | 20.9 ± 7.8 |
| Variable | Verum (n = 10) | Control (n = 13) | ||
|---|---|---|---|---|
| a | b | a | b | |
| Max. blood lactate concentration (mmol/L) | 8.80 ± 1.20 | 9.60 ± 1.50 | 9.20 ± 2.30 | 10.20 ± 3.60 |
| Running speed max (km/h) | 16.4 ± 2.0 | 16.8 ± 1.9 | 15.9 ± 1.3 * | 16.4 ± 1.5 |
| 1000 m-time at the IAT (mm:ss) | 04:59 ± 00:42 | 04:44 ± 00:52 | 05:12 ±00:31 | 04:41 ± 00:24 |
| VO2max (mL/min/kg) | 51.8 ± 6.3 * | 53.3 ± 5.9 | 50.4 ± 4.1 * | 51.9 ± 4.9 |
| CK (U/L) | 140 ± 120 | 190 ± 150 | 170 ± 97 | 165 ± 95 |
| Myoglobin (μg/L) | 35.0 ± 50.0 | 30.0 ± 15.0 | 19.0 ± 7.1 | 18.2 ± 8.0 |
| Leukocytes (1000/μL) | 5.3 ± 0.9 | 5.0 ± 0.3 | 5.4 ± 1.3 | 5.2 ± 1.1 |
| IL-6 (pg/mL) | 2.0 ± 0.0 | 2.1 ± 0.2 | 2.0 ± 0.0 | 2.1 ± 0.2 |
| Cortisol (ng/mL) | 167.00 ± 31.00 | 206.00 ± 58.00 | 161.00 ± 36.00 * | 195.00 ± 45.00 |
| Variable | Verum (n = 9) | Control (n = 12) | ||||
|---|---|---|---|---|---|---|
| c | d | inc. F | c | d | inc. F | |
| CK (U/L) | 122 ± 41 ** | 530 ± 220 | 4 | 180 ± 110 ** | 1400 ± 1300 | 8 |
| Myoglobin (μg/L) | 26.0 ± 15.0 ** | 770 ± 540 † | 29 † | 32.0 ± 35.0 ** | 2200 ± 1800 | 68 |
| Leukocytes (1.000/μL) | 5.3 ± 0.5 ** | 15.8 ± 2.5 | 3.0 | 5.4 ± 1.1 ** | 18.0 ± 4.2 | 3.3 |
| IL-6 (pg/mL) | 2.0 ± 0.0 ** | 37.0 ± 30.0 | 18 | 2.0 ± 0.1 ** | 43.0 ± 15.0 | 21.3 |
| Cortisol (ng/mL) | 184 ± 48.0 ** | 302 ± 70.0 | 1.6 | 194 ± 48.0 ** | 335 ± 95.0 | 1.7 |
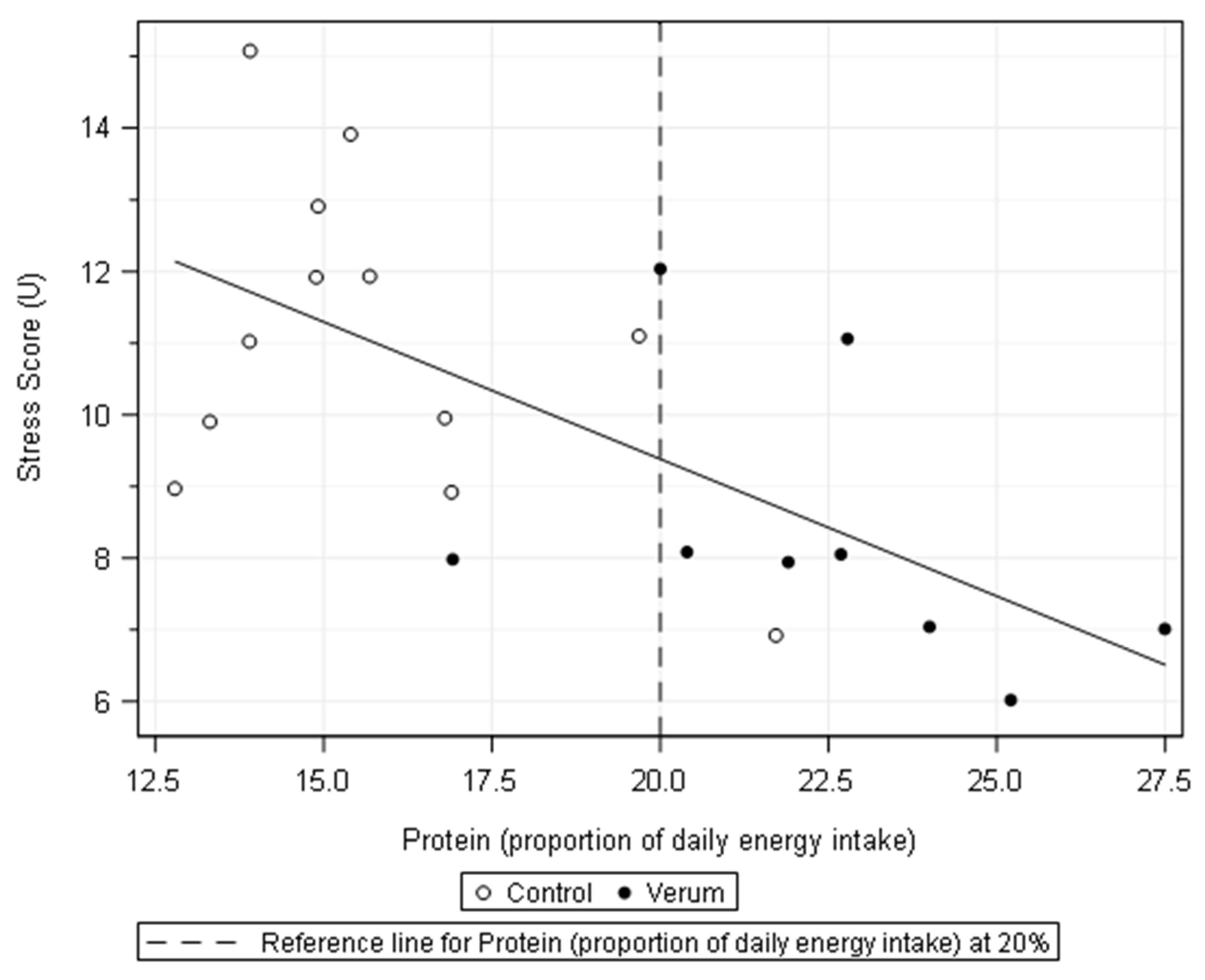
| Variable | PI < 20% (n = 12) | PI ≥ 20% (n = 9) | r (Diff.y = f(PI%)) | Sign. r |
|---|---|---|---|---|
| Δ CK (U/L) | 1.372 ± 1.242 | 521 ± 238 * | −0.387 | 0.083 |
| Δ Myoglobin (µg/L) | 2.129 ± 1.770 | 838 ± 602 * | −0.421 | 0.057 |
| Δ Leukocytes (1.000/mL) | 18.180 ± 3.920 | 15.160 ± 2.540 | −0.411 | 0.064 |
| IL−6 (pg/mL) | 40.9 ± 16.2 | 31.0 ± 17.1 | −0.476 | 0.034 |
| Cortisol (ng/mL) | 361 ± 90.4 | 278 ± 75.6 * | −0.459 | 0.036 |
| Stress score (U) | 11.2 ± 2.12 | 8.2 ± 1.99 ** | −0.667 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röhling, M.; McCarthy, D.; Berg, A. Continuous Protein Supplementation Reduces Acute Exercise-Induced Stress Markers in Athletes Performing Marathon. Nutrients 2021, 13, 2929. https://doi.org/10.3390/nu13092929
Röhling M, McCarthy D, Berg A. Continuous Protein Supplementation Reduces Acute Exercise-Induced Stress Markers in Athletes Performing Marathon. Nutrients. 2021; 13(9):2929. https://doi.org/10.3390/nu13092929
Chicago/Turabian StyleRöhling, Martin, David McCarthy, and Aloys Berg. 2021. "Continuous Protein Supplementation Reduces Acute Exercise-Induced Stress Markers in Athletes Performing Marathon" Nutrients 13, no. 9: 2929. https://doi.org/10.3390/nu13092929






