The Effect of Saffron Supplementation on Blood Pressure in Adults: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Inclusion Criteria
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Synthesis and Statistical Analysis
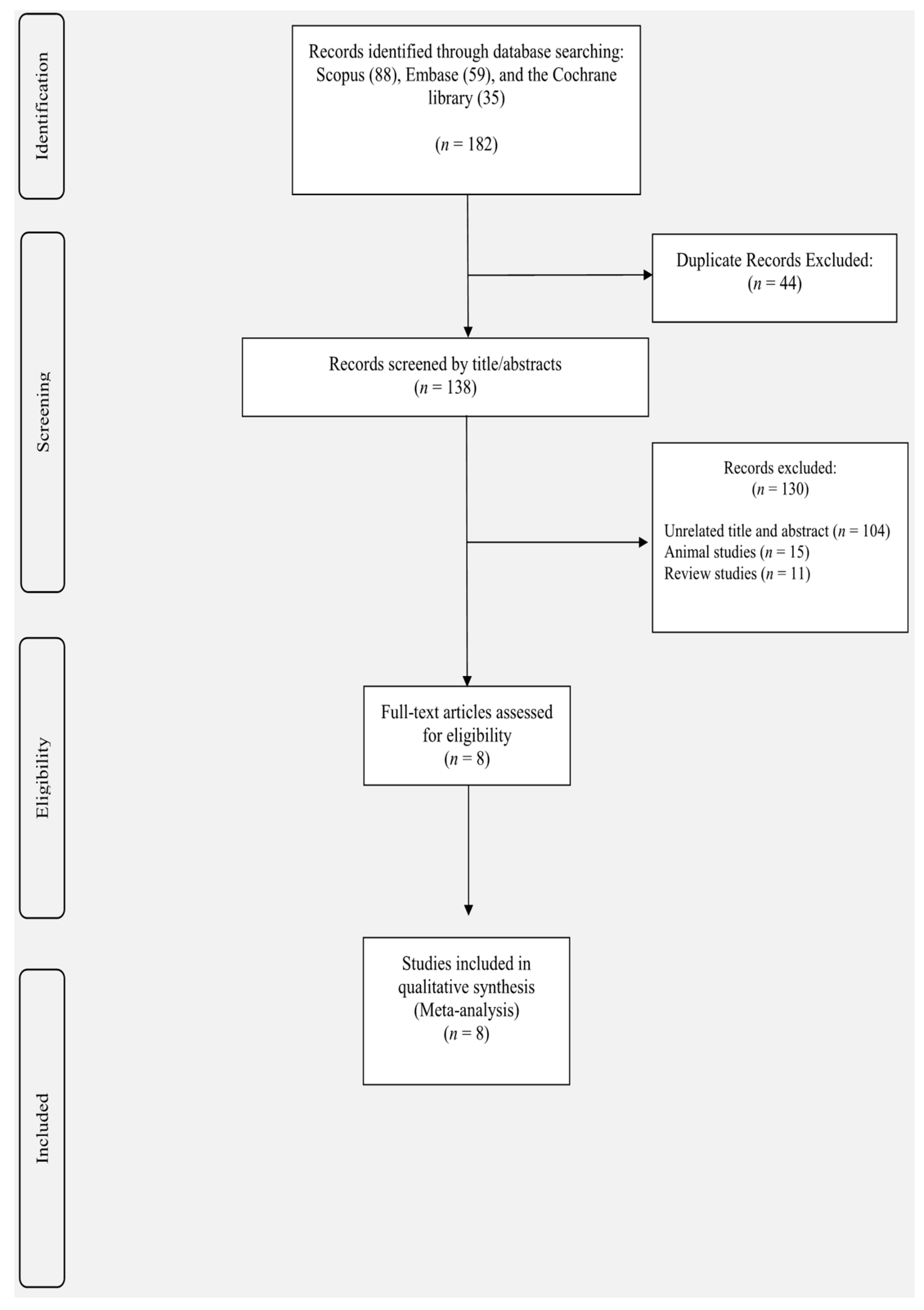
3. Results
3.1. Study Selection
3.2. Study Characteristics
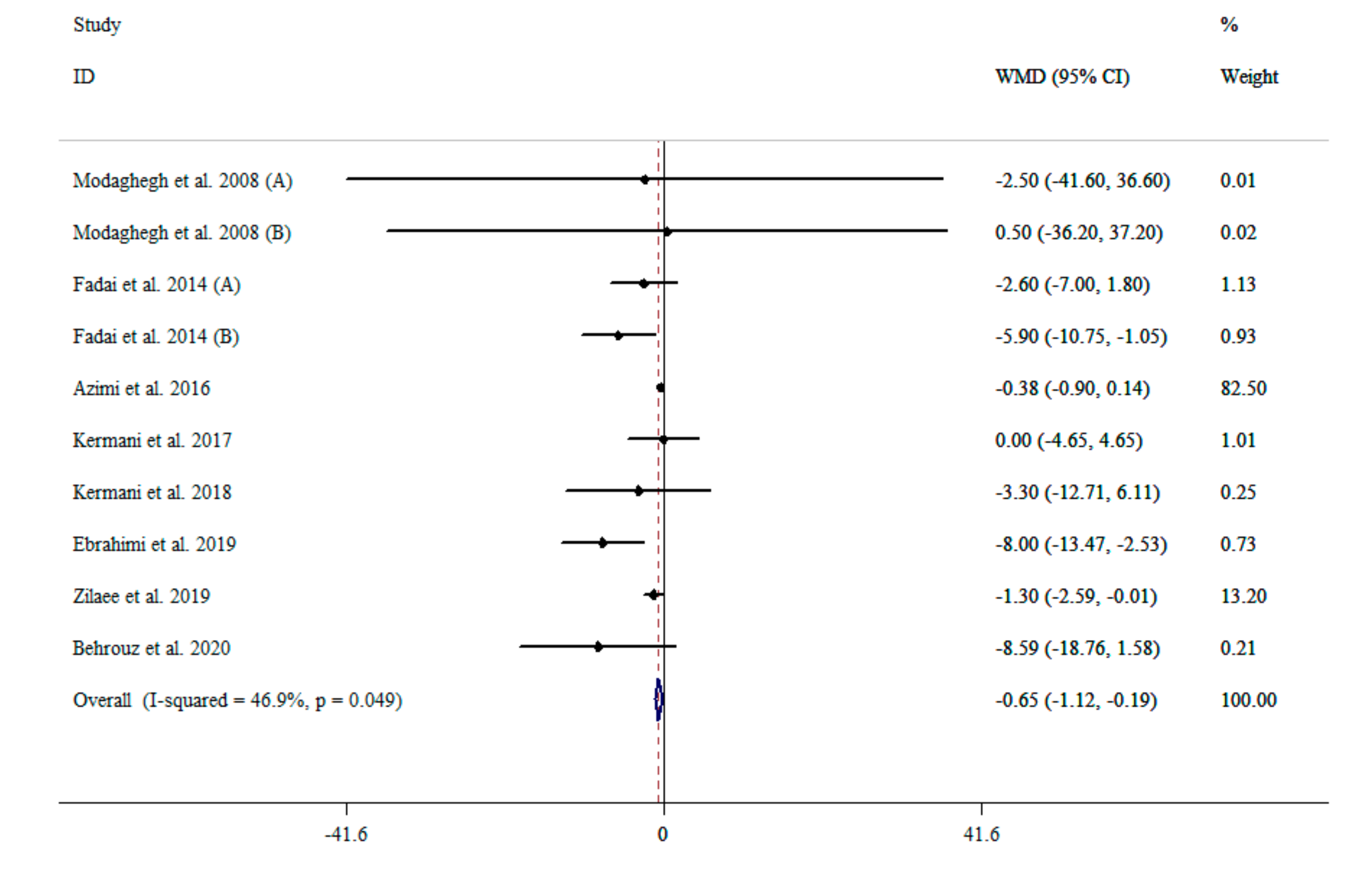
3.3. The Effect of Saffron Supplementation on SBP
3.4. The Effect of Saffron Supplementation on DBP
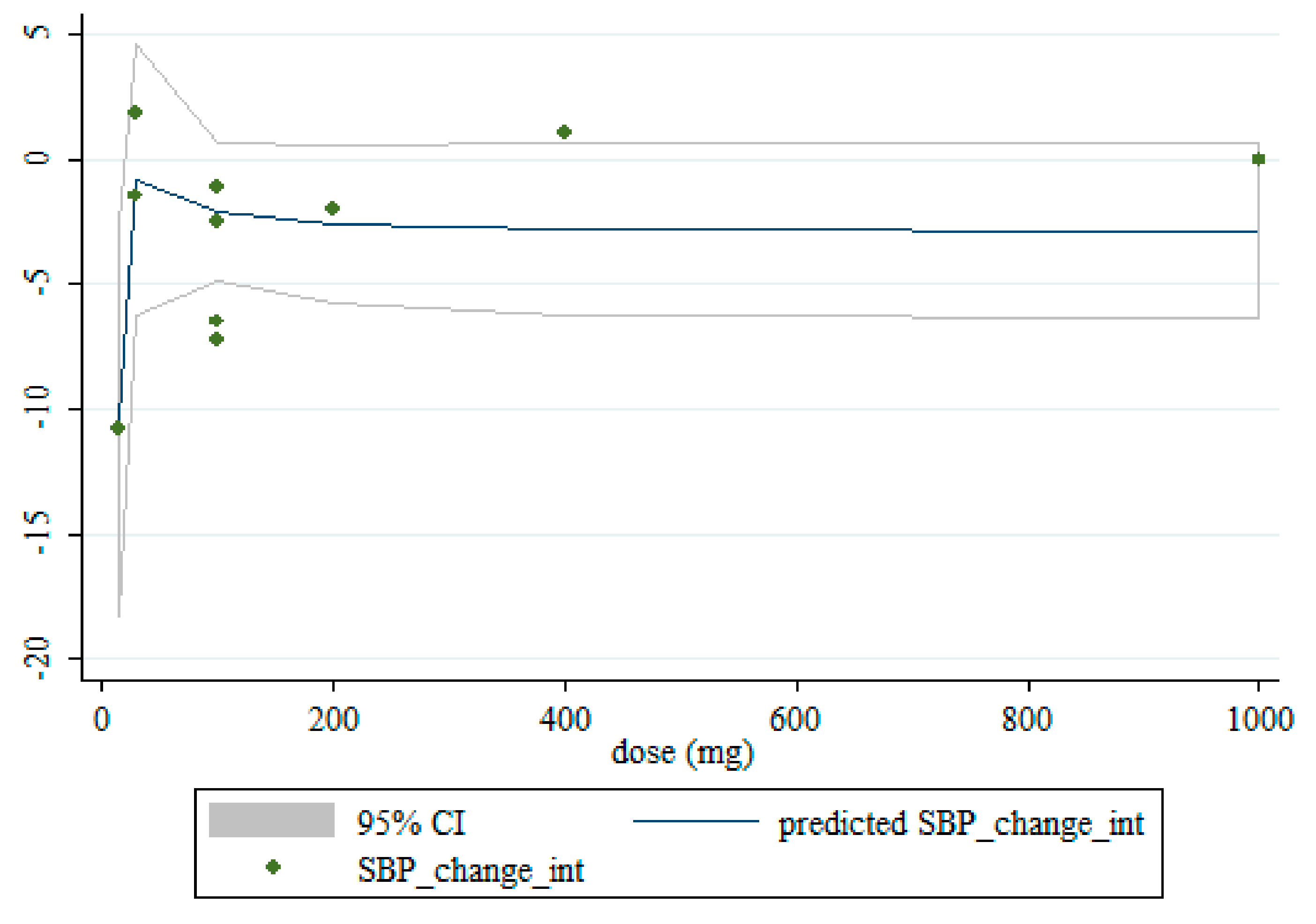
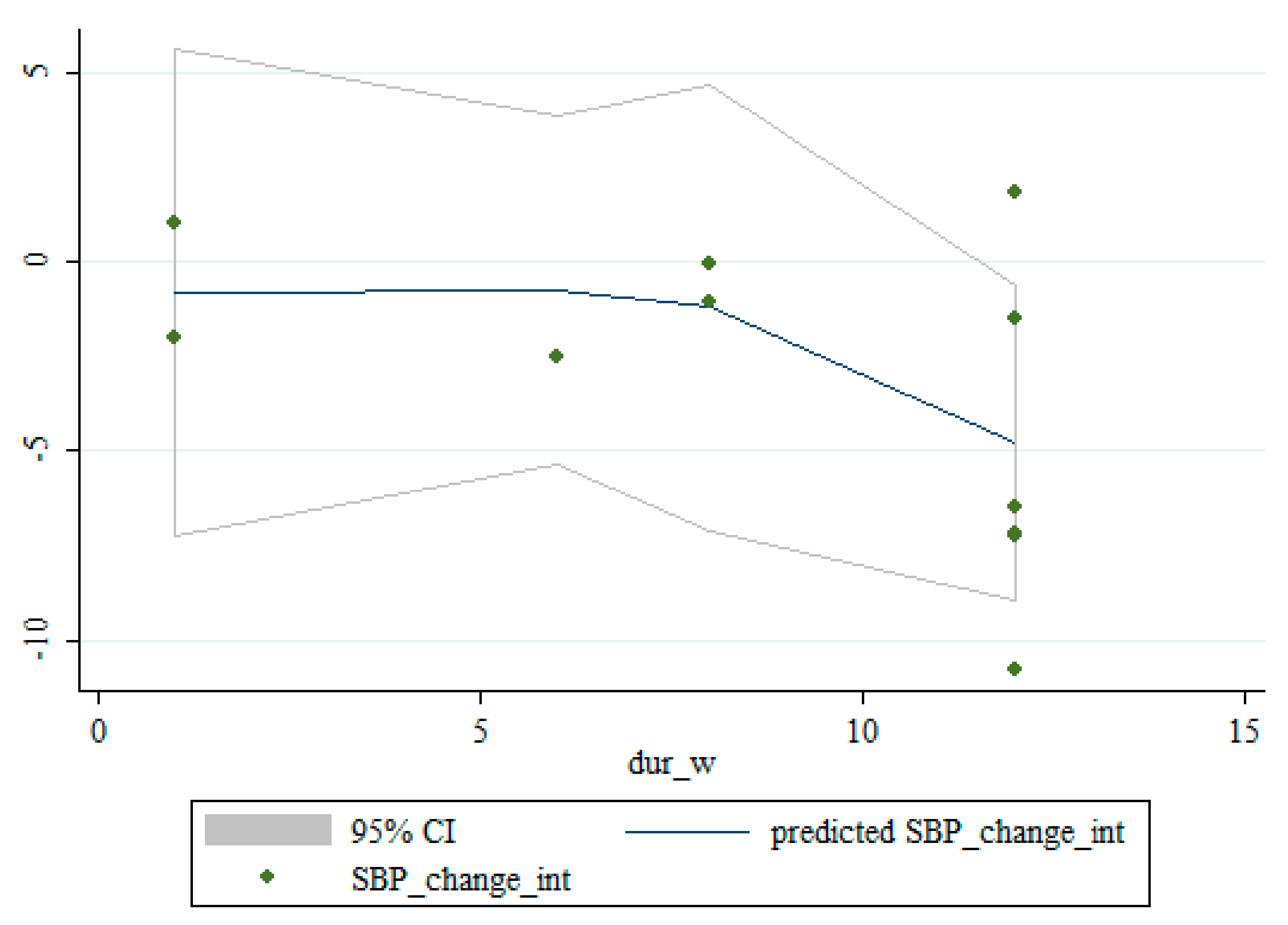
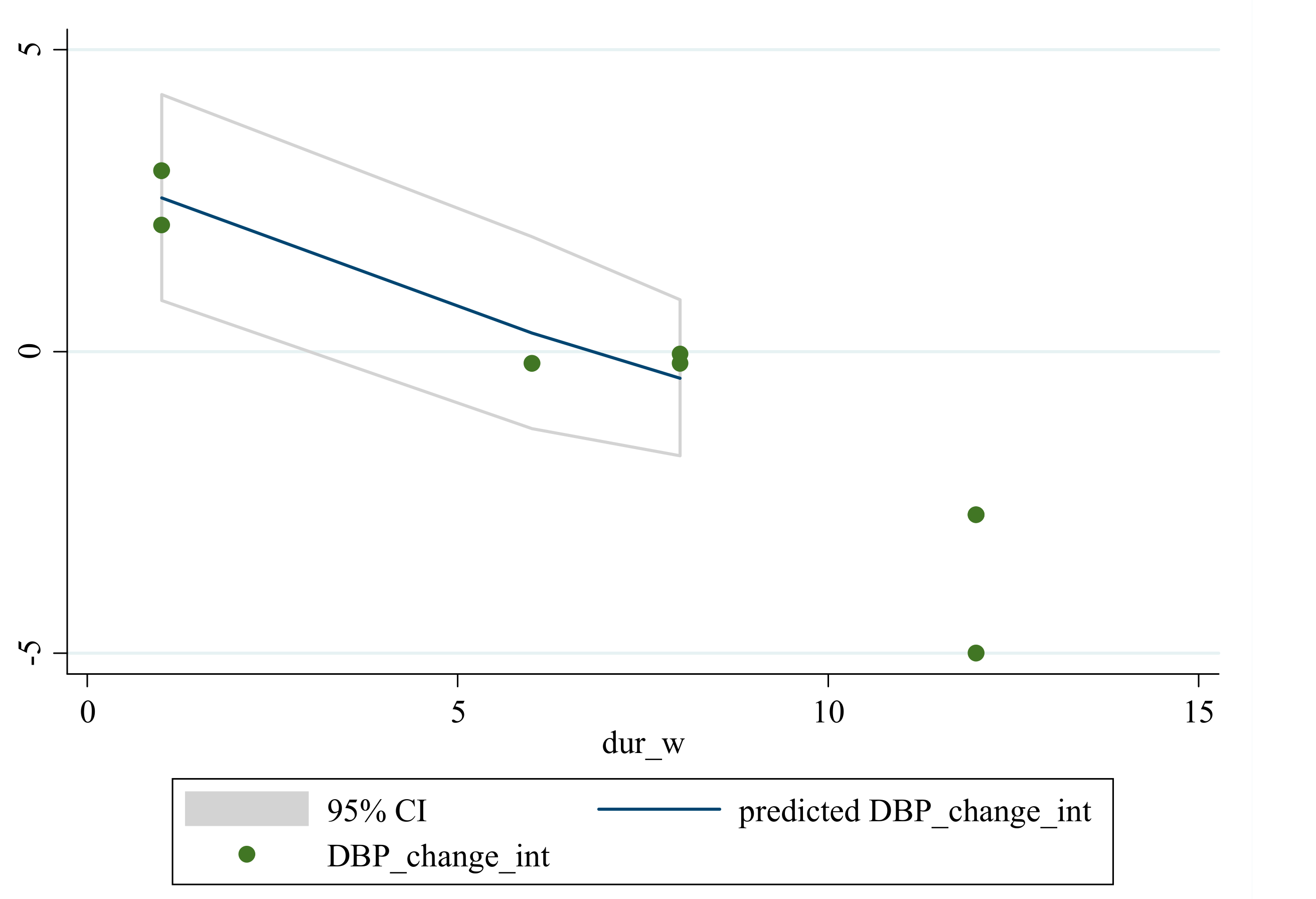
3.5. Non-Linear Dose-Response between the Doses and Duration of Saffron Supplementation and Blood Pressure
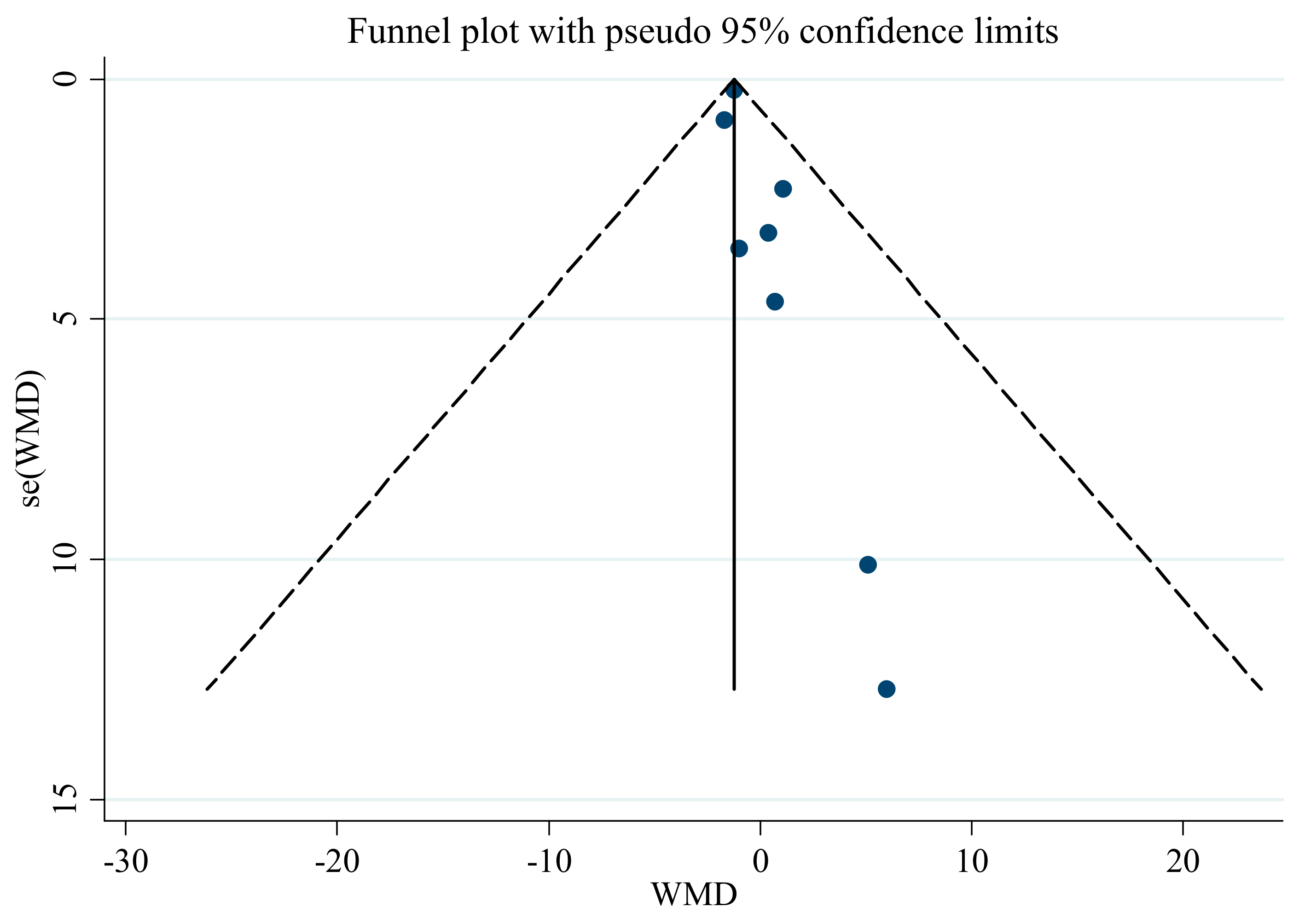
3.6. Publication Bias
3.7. Sensitivity Analysis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Campbell, N.R.; Lackland, D.T.; Niebylski, M.L. World Hypertension League; International Society of Hypertension Executive Committees. High Blood Pressure: Why Prevention and Control Are Urgent and Important-A 2014 Fact Sheet From the World Hypertension League and the International Society of Hypertension. J. Clin. Hypertens. 2014, 16, 551–553. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Pistoia, F.; Sacco, S.; Degan, D.; Tiseo, C.; Ornello, R.; Carolei, A. Hypertension and Stroke: Epidemiological Aspects and Clinical Evaluation. High Blood Press. Cardiovasc. Prev. 2015, 23, 9–18. [Google Scholar] [CrossRef]
- El Nahas, A.M.; Bello, A.K. Chronic kidney disease: The global challenge. Lancet 2005, 365, 331–340. [Google Scholar] [CrossRef]
- Ravisankar, P.; Shajeeya Amren, S.; Devadasu, C.; Devala Rao, G. Controlling hypertension: A brief review. J. Chem. Pharm. Sci. 2014, 7, 122–136. [Google Scholar]
- Dickinson, H.O.; Mason, J.M.; Nicolson, D.J.; Campbell, F.; Beyer, F.R.; Cook, J.V.; Cook, J.; Williams, B.; Ford, G.A. Lifestyle interventions to reduce raised blood pressure: A systematic review of randomized controlled trials. J. Hypertens. 2006, 24, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Musini, V.M.; Gueyffier, F.; Puil, L.; Salzwedel, D.M.; Wright, J.M. Pharmacotherapy for hypertension in adults aged 18 to 59 years. Cochrane Database Syst. Rev. 2017, 8. [Google Scholar] [CrossRef]
- Curb, J.D.; Borhani, N.O.; Blaszkowski, T.P.; Zimbaldi, N.; Fotiu, S.; Williams, W. Long-term surveillance for adverse effects of antihypertensive drugs. JAMA 1985, 253, 3263–3268. [Google Scholar] [CrossRef]
- Woolf, K.J.; Bisognano, J.D. Nondrug Interventions for Treatment of Hypertension. J. Clin. Hypertens. 2011, 13, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, K.S.; Appathurai, A.; Jois, M.; Radcliffe, J.E. Effects of herbs and spices on blood pressure: A systematic literature review of randomised controlled trials. J. Hypertens. 2019, 37, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Qian, Z.; Xu, G.; Zheng, S.; Sun, S.; Wen, N.; Sheng, L.; Shi, Y.; Zhang, Y. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J. Nutr. Biochem. 2007, 18, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Imenshahidi, M.; Hosseinzadeh, H.; Javadpour, Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 2010, 24, 990–994. [Google Scholar] [CrossRef]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Liu, J.; Chen, N.; Yang, J.; Yang, B.; Ouyang, Z.; Wu, C.; Yuan, Y.; Wang, W.; Chen, M. An integrated approach combining HPLC, GC/MS, NIRS, and chemometrics for the geographical discrimination and commercial categorization of saffron. Food Chem. 2018, 253, 284–292. [Google Scholar] [CrossRef]
- Parray, J.A.; Kamili, A.N.; Hamid, R.; Reshi, Z.A.; Qadri, R.A. Antibacterial and antioxidant activity of methanol extracts of Crocus sativus L. cv. Kashmirianus. Front. Life Sci. 2015, 8, 40–46. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Mokhtari, P.; Asbaghi, O.; Rigi, S.; Persad, E.; Jayedi, A.; Rezvani, H.; Mahamat-Saleh, Y.; Sadeghi, O. Does saffron supplementation have favorable effects on liver function indicators? A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 1–13. [Google Scholar] [CrossRef]
- Asbaghi, O.; Sadeghian, M.; Sadeghi, O.; Rigi, S.; Tan, S.C.; Shokri, A.; Mousavi, S.M. Effects of saffron (Crocus sativus L.) supplementation on inflammatory biomarkers: A systematic review and meta-analysis. Phytother. Res. 2021, 35, 20–32. [Google Scholar] [CrossRef]
- Ghaderi, A.; Asbaghi, O.; Reiner, Ž.; Kolahdooz, F.; Amirani, E.; Mirzaei, H.; Banafshe, H.R.; Dana, P.M.; Asemi, Z. The effects of saffron (Crocus sativus L.) on mental health parameters and C-reactive protein: A meta-analysis of randomized clinical trials. Complement. Ther. Med. 2020, 48, 102250. [Google Scholar] [CrossRef]
- Rao, S.V.; Muralidhara; Yenisetti, S.; Rajini, P.S. Evidence of neuroprotective effects of saffron and crocin in a Drosophila model of parkinsonism. Neurotoxicology 2016, 52, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Inamdar, M.N. Potential of Crocus sativus (saffron) and its Constituent, Crocin, as Hypolipidemic and Antioxidant in Rats. Appl. Biochem. Biotechnol. 2010, 162, 358–372. [Google Scholar] [CrossRef]
- Asbaghi, O.; Soltani, S.; Norouzi, N.; Milajerdi, A.; Choobkar, S.; Asemi, Z. The effect of saffron supplementation on blood glucose and lipid profile: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 47, 102158. [Google Scholar] [CrossRef]
- Naserizadeh, S.K.; Taherifard, M.H.; Shekari, M.; Mesrkanlou, H.A.; Asbaghi, O.; Nazarian, B.; Khosroshahi, M.Z.; Heydarpour, F. The effect of crocin supplementation on lipid concentrations and fasting blood glucose: A systematic review and meta-analysis and meta-regression of randomized controlled trials. Complement. Ther. Med. 2020, 52, 102500. [Google Scholar] [CrossRef]
- Wang, Y.; Han, T.; Zhu, Y.; Zheng, C.-J.; Ming, Q.-L.; Rahman, K.; Qin, L.-P. Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J. Nat. Med. 2010, 64, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, B.H.; Bagheri, R.; Roozbeh, B.; Ashtary-Larky, D.; Gaeini, A.A.; Dutheil, F.; Wong, A. Impact of saffron (Crocus Sativus Linn) supplementation and resistance training on markers implicated in depression and happiness levels in untrained young males. Physiol. Behav. 2021, 233, 113352. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.R. Crocus sativus L. (saffron) for cancer chemoprevention: A mini review. J. Tradit. Complement. Med. 2015, 5, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Farkhondeh, T. Antiinflammatory, antioxidant, and immunomodulatory effects of Crocus sativus L. and its main constituents. Phytother. Res. 2016, 30, 1072–1094. [Google Scholar] [CrossRef]
- Schmidt, M.; Betti, G.; Hensel, A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien. Med. Wochenschr. 2007, 157, 315–319. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ziaee, T.; Sadeghi, A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine 2008, 15, 491–495. [Google Scholar] [CrossRef]
- Rahaiee, S.; Moini, S.; Hashemi, M.; Shojaosadati, S.A. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): A review. J. Food Sci. Technol. 2015, 52, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Sheng, L.; Qian, Z.; Zheng, S.; Xi, L. Mechanism of hypolipidemic effect of crocin in rats: Crocin inhibits pancreatic lipase. Eur. J. Pharmacol. 2006, 543, 116–122. [Google Scholar] [CrossRef]
- He, S.-Y.; Qian, Z.-Y.; Wen, N.; Tang, F.-T.; Xu, G.-L.; Zhou, C.-H. Influence of crocetin on experimental atherosclerosis in hyperlipidamic-diet quails. Eur. J. Pharmacol. 2007, 554, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Modaghegh, M.-H.; Shahabian, M.; Esmaeili, H.-A.; Rajbai, O.; Hosseinzadeh, H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine 2008, 15, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Fadai, F.; Mousavi, S.B.; Ashtari, Z.; Beigi, N.A.; Farhang, S.; Hashempour, S.; Shahhamzei, N.; Bathaie, S.Z. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: A randomized triple blind placebo controlled study. Pharmacopsychiatry 2014, 47, 156–161. [Google Scholar] [CrossRef]
- Kermani, T.; Zebarjadi, M.; Mehrad-Majd, H.; Mirhafez, S.-R.; Shemshian, M.; Ghasemi, F.; Mohammadzadeh, E.; Mousavi, S.H.; Norouzy, A.; Moghiman, T.; et al. Anti-inflammatory effect of Crocus sativus on serum cytokine levels in subjects with metabolic syndrome: A randomized, double-blind, placebo- controlled trial. Curr. Clin. Pharmacol. 2017, 12, 122–126. [Google Scholar] [CrossRef]
- Kermani, T.; Kazemi, T.; Molki, S.; Ilkhani, K.; Sharifzadeh, G.; Rajabi, O. The efficacy of crocin of saffron (Crocus sativus L.) on the components of metabolic syndrome: A randomized controlled clinical trial. J. Res. Pharm. Pract. 2017, 6, 228–232. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Aryaeian, N.; Pahlavani, N.; Abbasi, D.; Hosseini, A.F.; Fallah, S.; Moradi, N.; Heydari, I. The effect of saffron (Crocus sativus L.) supplementation on blood pressure, and renal and liver function in patients with type 2 diabetes mellitus: A double-blinded, randomized clinical trial. Avicenna J. Phytomed. 2019, 9, 322–333. [Google Scholar] [PubMed]
- Zilaee, M.; Hosseini, S.A.; Jafarirad, S.; Abolnezhadian, F.; Cheraghian, B.; Namjoyan, F.; Ghadiri, A. An evaluation of the effects of saffron supplementation on the asthma clinical symptoms and asthma severity in patients with mild and moderate persistent allergic asthma: A double-blind, randomized placebo-controlled trial. Respir. Res. 2019, 20, 39. [Google Scholar] [CrossRef] [Green Version]
- Behrouz, V.; Dastkhosh, A.; Hedayati, M.; Sedaghat, M.; Sharafkhah, M.; Sohrab, G. The Effect of Crocin Supplementation on Glycemic Control, Insulin Resistance and Active AMPK Levels in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Diabetol. Metab. Syndr. 2020, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hosseinzadeh, J.; Bahreynian, M.; Hariri, M.; Khosravi-Boroujeni, H. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Blood Press. 2016, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Sahebkar, A.; Aryaeian, N.; Pahlavani, N.; Fallah, S.; Moradi, N.; Abbasi, D.; Hosseini, A.F. Effects Of Saffron Supplementation On Inflammation And Metabolic Responses In Type 2 Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2107–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef]
- Asbaghi, O.; Fouladvand, F.; Moradi, S.; Ashtary-Larky, D.; Choghakhori, R.; Abbasnezhad, A. Effect of green tea extract on lipid profile in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 293–301. [Google Scholar] [CrossRef]
- Javandoost, A.; Afshari, A.; Nikbakht-Jam, I.; Khademi, M.; Eslami, S.; Nosrati, M.; Foroutan-Tanha, M.; Sahebkar, A.; Tavalaie, S.; Ghayour-Mobarhan, M.; et al. Effect of crocin, a carotenoid from saffron, on plasma cholesteryl ester transfer protein and lipid profile in subjects with metabolic syndrome: A double blind randomized clinical trial. ARYA Atheroscler. 2017, 13, 245. [Google Scholar]
- Nikbakht-Jam, I.; Khademi, M.; Nosrati, M.; Eslami, S.; Foroutan-Tanha, M.; Sahebkar, A.; Tavalaie, S.; Ghayour-Mobarhan, M.; Ferns, G.A.A.; Hadizadeh, F.; et al. Effect of crocin extracted from saffron on pro-oxidant–anti-oxidant balance in subjects with metabolic syndrome: A randomized, placebo-controlled clinical trial. Eur. J. Integr. Med. 2016, 8, 307–312. [Google Scholar] [CrossRef]
- Ghaderi, A.; Rasouli-Azad, M.; Vahed, N.; Banafshe, H.R.; Soleimani, A.; Omidi, A.; Ghoreishi, F.S.; Asemi, Z. Clinical and metabolic responses to crocin in patients under methadone maintenance treatment: A randomized clinical trial. Phytother. Res. 2019, 33, 2714–2725. [Google Scholar] [CrossRef]
- Rahmani, J.; Bazmi, E.; Clark, C.; Nazari, S.S.H. The effect of Saffron supplementation on waist circumference, HA1C, and glucose metabolism: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2020, 49, 102298. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Khanna, N. Pathophysiology and risk factors related to hypertension and its cure using herbal drugs. Spatula DD 2012, 2, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Asadi-Samani, M.; Farkhondeh, T.; Bahmani, M. Assessment the effect of saffron ethanolic extract (Crocus sativus L.) on oxidative damages in aged male rat liver. Pharm. Lett. 2016, 8, 283–290. [Google Scholar]
- Nabavizadeh, F.; Salimi, E.; Sadroleslami, Z.; Karimian, S.M.; Vahedian, J. Pharmacology. Saffron (Crocus sativus) increases gastric acid and pepsin secretions in rats: Role of nitric oxide (NO). Afr. J. Pharm. Pharmacol. 2009, 3, 181–184. [Google Scholar]
- Tang, F.; Qian, Z.; Liu, P.; Zheng, S.; He, S.; Bao, L.; Huang, H. Crocetin improves endothelium-dependent relaxation of thoracic aorta in hypercholesterolemic rabbit by increasing eNOS activity. Biochem. Pharmacol. 2006, 72, 558–565. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Tayarani, N.Z.; Parsaee, H. Protective Effect of Saffron Extract and Crocin on Reactive Oxygen Species-Mediated High Glucose-Induced Toxicity in PC12 Cells. Cell. Mol. Neurobiol. 2010, 30, 185–191. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Parsaee, H.; Mousavi, S.H. Study of high glucose-induced toxicity and reactive oxygen species production and the protective effect of saffron extract in PC12 cells. J. Adv. Med. Biomed. Res. 2010, 18, 42–51. [Google Scholar]
- El-Beshbishy, H.A.; Hassan, M.; Aly, H.A.; Doghish, A.S.; Alghaithy, A.A. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol. Environ. Saf. 2012, 83, 47–54. [Google Scholar] [CrossRef]
- Das, I.; Chakrabarty, R.N.; Das, S. Saffron can prevent chemically induced skin carcinogenesis in Swiss albino mice. Asian Pac. J. Cancer Prev. 2004, 5, 70–76. [Google Scholar]
- Razavi, B.M.; Hosseinzadeh, H. Saffron as an antidote or a protective agent against natural or chemical toxicities. DARU J. Pharm. Sci. 2015, 23, 31. [Google Scholar]
- Ahmadi, N.; Razzaghi, M.; Hamzeloo-Moghadam, M.; Rostami-Nejad, M.; Rezaei-Tavirani, M.; Heidari, M.H.; Safari, S.; Rezaei-Tavirani, M. Assessment of saffron neuroprotective properties in rat retina versus light damage. Res. J. Pharmacogn. 2020, 7, 33–42. [Google Scholar] [CrossRef]
- Elsherbiny, N.; Salama, M.F.; Said, E.; El-Sherbiny, M.; Al-Gayyar, M. Crocin protects against doxorubicin-induced myocardial toxicity in rats through down-regulation of inflammatory and apoptic pathways. Chem. Interact. 2016, 247, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Qian, Z.-Y.; Zhou, C.-H.; Liu, J.; Li, W.-N. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J. Ethnopharmacol. 2006, 107, 25–31. [Google Scholar] [CrossRef]
- Cottone, S.; Mule, G.; Nardi, E.; Vadalà, A.; Lorito, M.C.; Guarneri, M.; Arsena, R.; Palermo, A.; Cerasola, G. C-reactive protein and intercellular adhesion molecule-1 are stronger predictors of oxidant stress than blood pressure in established hypertension. J. Hypertens. 2007, 25, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qian, Z.; Ji, H.; Yang, R.; Wang, Y.; Xi, L.; Sheng, L.; Zhao, B.; Zhang, X. Inhibitory effect on protein kinase Cθ by Crocetin attenuates palmitate-induced insulin insensitivity in 3T3-L1 adipocytes. Eur. J. Pharmacol. 2010, 642, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.; Asghari, S.; Zohoori, E.; Karamian, M. Hypoglycemic effects of three Iranian edible plants; jujube, barberry and saffron: Correlation with serum adiponectin level. Pak. J. Pharm. Sci. 2015, 28, 2095–2099. [Google Scholar]
- Yiannikouris, F.; Gupte, M.; Putnam, K.; Cassis, L. Adipokines and blood pressure control. Curr. Opin. Nephrol. Hypertens. 2010, 19, 195–200. [Google Scholar] [CrossRef]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W., Jr.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef]
- Stamler, J.; Rose, G.; Stamler, R.; Elliott, P.; Dyer, A.; Marmot, M. Special feature INTERSALT study findings. Hypertension 1989, 14, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Cook, N.R.; Cohen, J.; Hebert, P.R.; O Taylor, J.; Hennekens, C.H. Implications of small reductions in diastolic blood pressure for primary prevention. Arch. Intern. Med. 1995, 155, 701–709. [Google Scholar] [CrossRef]
- Stamler, J. The INTERSALT Study: Background, methods, findings, and implications. Am. J. Clin. Nutr. 1997, 65, 626S–642S. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Authors | Publication Year | Country | Study Design | Participant’s Sex | Sample Size | Participants | Duration (Week) | BMI | Age (Years) | Intervention/Control (Type and Dosage) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IG | CG | IG | CG | IG | CG | IG | Dose (mg) | CG | |||||||
| Modaghegh et al., 2008 (A) [37] | 2008 | IRAN | R/DB/PL | F/M | 10 | 5 | healthy volunteers | 1 | 27.6 | 28.7 | NR | NR | saffron | 200 | placebo |
| Modaghegh et al., 2008 (B) [37] | 2008 | IRAN | R/DB/PL | F/M | 10 | 5 | healthy volunteers | 1 | 28.7 | 28.7 | NR | NR | saffron | 400 | placebo |
| Fadai et al., 2014 (A) [38] | 2014 | IRAN | R/TB/PL | M | 20 | 21 | patients with schizophrenia | 12 | 49.3 ± 7.1 | 48.1 ± 6.1 | NR | NR | saffron | 30 | placebo |
| Fadai et al., 2014 (B) [38] | 2014 | IRAN | R/TB/PL | M | 20 | 21 | patients with schizophrenia | 12 | 48.1 ± 7.7 | 48.1 ± 6.1 | NR | NR | crocin | 30 | placebo |
| Kermani et al., 2017 [39] | 2017 | IRAN | R/DB/PL | F/M | 22 | 22 | metabolic syndrome | 12 | 43.64 ± 11.17 | 42.59 ± 8.44 | 31.02 ± 5.45 | 30.48 ± 6.26 | saffron | 100 | placebo |
| Kermani et al., 2017 [40] | 2018 | IRAN | R/DB/PL | F/M | 24 | 24 | metabolic syndrome | 6 | 53.8 ± 9.2 | 50.9 ± 8.8 | 29.9 ± 3.9 | 29.8 ± 5.3 | crocin | 100 | placebo |
| Ebrahimi et al., 2019 [45] | 2019 | IRAN | R/DB/PL | F/M | 40 | 40 | type 2 diabetic patients | 12 | 55.2 ± 7.3 | 53 ± 10.6 | 29.3 ± 4.9 | 30.5 ± 4.7 | saffron | 100 | placebo |
| Zilaee et al., 2019 [42] | 2019 | IRAN | R/DB/PL | F/M | 38 | 38 | patients with mild and moderate persistent allergic asthma | 8 | 41.27 ± 9.77 | 40.77 ± 10.07 | 26.84 | 26.84 | saffron | 100 | placebo |
| Behrouz et al., 2020 [43] | 2020 | IRAN | R/DB/PL | F/M | 23 | 22 | type 2 diabetic patients | 12 | 57.08 ± 7.41 | 59.86 ± 9.46 | 30.64 ± 4.79 | 30.85 ± 3.19 | crocin | 15 | placebo |
| Azimi et al., 2016 [44] | 2016 | IRAN | R/SB/PL | F/M | 42 | 39 | type 2 diabetic patients | 8 | 57.02 ± 1.0 | 53.64 ± 1.3 | 28.86 ± 0.2 | 28.40 ± 0.2 | saffron | 1000 | placebo |
| NO | WMD (95%CI) | p Value | P Heterogeneity | I2 (%) | |
|---|---|---|---|---|---|
| Effect of saffron supplementation on SBP | |||||
| Overall effect | 10 | −0.65 (−1.12, −0.18) | 0.006 | 0.049 | 46.9% |
| Baseline SBP (mmHg) | |||||
| <120 | 6 | −1.57 (−2.73, −0.41) | 0.008 | 0.565 | 0.0% |
| ≥120 | 4 | −0.47 (−0.98, 0.03) | 0.068 | 0.017 | 70.6% |
| Duration | |||||
| <12 | 5 | −0.51 (−0.99, −0.03) | 0.035 | 0.729 | 0.0% |
| ≥12 | 5 | −4.00 (−6.34, −1.67) | 0.001 | 0.155 | 40.0% |
| Intervention dose (mg) | |||||
| ≥100 | 7 | −0.56 (−1.03, −0.09) | 0.019 | 0.160 | 35.1% |
| <100 | 3 | −4.50 (−7.61, −1.40) | 0.004 | 0.436 | 0.0% |
| Intervention type | |||||
| Saffron | 7 | −0.58 (−1.05, −0.11) | 0.016 | 0.136 | 38.4% |
| Crocin | 3 | −5.84 (−9.81, −1.87) | 0.004 | 0.755 | 0.0% |
| Effect of saffron supplementation on DBP | |||||
| Overall effect | 8 | −1.23 (−1.64, −0.81) | <0.001 | 0.928 | 0.0% |
| Baseline DBP (mmHg) | |||||
| <80 | 5 | 0.66 (−2.50, 3.83) | 0.681 | 0.959 | 0.0% |
| ≥80 | 3 | −1.26 (−1.68, −0.84) | <0.001 | 0.800 | 0.0% |
| Duration | |||||
| <12 | 5 | −1.25 (−1.68, −0.83) | <0.001 | 0.883 | 0.0% |
| ≥12 | 3 | 0.45(−2.77, 3.69) | 0.782 | 0.882 | 0.0% |
| Intervention dose (mg) | |||||
| ≥100 | 7 | −1.23 (−1.65, −0.81) | <0.001 | 0.871 | 0.0% |
| <100 | 1 | −1.01 (−7.94, 5.92) | 0.775 | - | - |
| Intervention type | |||||
| Saffron | 6 | −1.23 (−1.65, −0.81) | <0.001 | 0.806 | 0.0% |
| Crocin | 2 | −0.38 (−5.89, 5.13) | 0.892 | 0.769 | 0.0% |
| Study (Year) | Random Sequence Generation | Allocation Concealment | Selective Outcome Reporting | Other Sources of Bias | Blinding of Participants Personnel | Blinding of Outcome Assessors | Incomplete Outcome Data |
|---|---|---|---|---|---|---|---|
| Modaghegh et al., 2008 [37] | L | U | L | H | L | U | L |
| Fadai et al., 2014 [38] | L | U | H | H | L | L | L |
| Azimi et al., 2016 [44] | L | U | L | L | H | H | L |
| Kermani et al., 2017 [39] | L | U | L | H | L | U | L |
| Kermani et al., 2018 [40] | L | U | L | H | L | U | L |
| Ebrahimi et al., 2019 [45] | L | U | L | L | L | U | L |
| Zilaee et al., 2019 [42] | L | U | L | L | L | U | L |
| Behrouz et al., 2020 [43] | L | U | L | L | L | U | L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setayesh, L.; Ashtary-Larky, D.; Clark, C.C.T.; Rezaei Kelishadi, M.; Khalili, P.; Bagheri, R.; Asbaghi, O.; Suzuki, K. The Effect of Saffron Supplementation on Blood Pressure in Adults: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2736. https://doi.org/10.3390/nu13082736
Setayesh L, Ashtary-Larky D, Clark CCT, Rezaei Kelishadi M, Khalili P, Bagheri R, Asbaghi O, Suzuki K. The Effect of Saffron Supplementation on Blood Pressure in Adults: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients. 2021; 13(8):2736. https://doi.org/10.3390/nu13082736
Chicago/Turabian StyleSetayesh, Leila, Damoon Ashtary-Larky, Cain C. T. Clark, Mahnaz Rezaei Kelishadi, Pardis Khalili, Reza Bagheri, Omid Asbaghi, and Katsuhiko Suzuki. 2021. "The Effect of Saffron Supplementation on Blood Pressure in Adults: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials" Nutrients 13, no. 8: 2736. https://doi.org/10.3390/nu13082736









