Bariatric Surgery and Liver Disease: General Considerations and Role of the Gut–Liver Axis
Abstract
1. Introduction
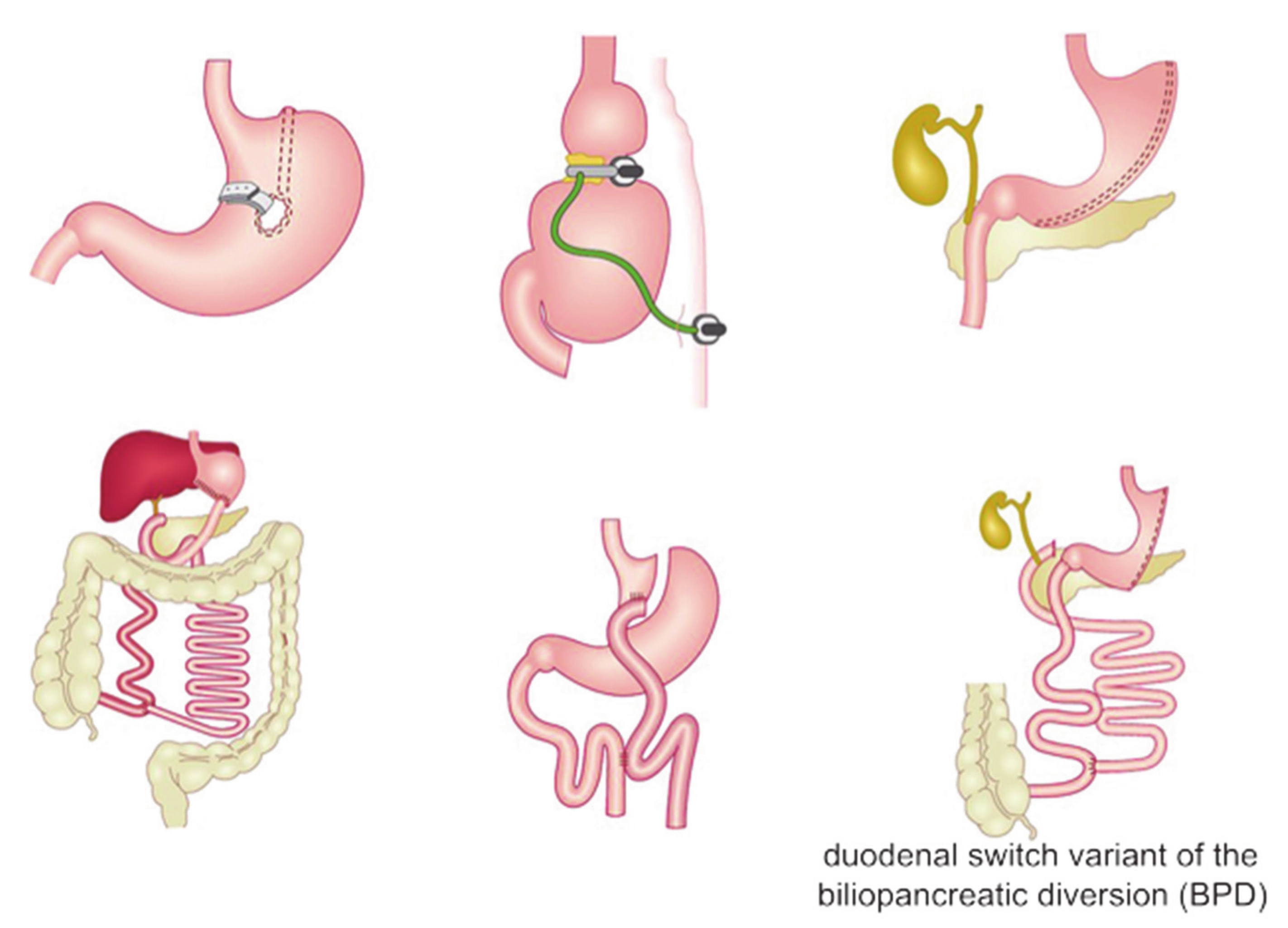
2. Bariatric Surgery
2.1. Restrictive Procedures
2.2. Malabsorptive Procedures
2.3. Combined Procedures
3. Bariatric Surgery in Cirrhotic Patients
4. Bariatric Surgery and Liver Transplant
5. Effects of Bariatric Surgery on the Liver
6. Effects of Bariatric Surgery on the Gut Microbiota
6.1. Gut-Liver Axis and Liver Disease
6.2. Bariatric Surgery and Microbiota
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Brien, P. Surgical Treatment of Obesity. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef]
- Wang, Y.C.; McPherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011, 378, 815–825. [Google Scholar] [CrossRef]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.L.; Michielsen, P.P.; van den Eynden, G.G.; Pelckmand, P.A.; Francque, S.M. Rapidly evolving liver decompensation with some remarkable features 14 years after biliopancreatic derivation; a case-report and literature review. Acta Gastroenterol. Belg. 2010, 73, 46–51. [Google Scholar]
- Neuschwander-Tetri, B. Fatty liver and nonalcoholic steatohepatitis. In Handbook of Liver Disease, 3rd ed.; Friedman, L.S., Keeffe, E.B., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012; pp. 106–114. [Google Scholar]
- Sasaki, A.; Nitta, H.; Otsuka, K.; Umemura, A.; Baba, S.; Obuchi, T.; Wakabayashi, G. Bariatric surgery and non-alcoholic fatty liver disease: Current and potential future treatments. Front. Endocrinol. 2014, 5, 164. [Google Scholar] [CrossRef]
- Subichin, M.; Clanton, J.; Makuszewski, M.; Zografakis, J.G.; Dan, A. Liver disease in the morbidly obese: A review of 1000 consecutive patients undergoing weight loss surgery. Surg. Obes. Relat. Dis. 2015, 11, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease–metaanalytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Koehler, E.M.; Schouten, J.N.; Hansen, B.E.; van Rooij, F.J.; Hofman, A.; Stricker, B.H.; Janssen, H.L. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: Results from the Rotterdam study. J. Hepatol. 2012, 57, 1305–1311. [Google Scholar] [CrossRef]
- Kagansky, N.; Levy, S.; Keter, D.; Rimon, E.; Taiba, Z.; Fridman, Z.; Berger, D.; Knobler, H.; Malnick, S. Non-alcoholic fatty liver disease–A common and benign finding in octogenarian patients. Liver. Int. 2004, 24, 588–594. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Halpern, Z.; Oren, R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver. Int. 2006, 26, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, G.C.; Tan, H.Y.; Hao, F.B.; Hu, J.J. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: A meta-analysis. Sci. Rep. 2019, 9, 11124. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Hashimoto, Y.; Obora, A.; Kojima, T.; Fukui, M. Non-alcoholic fatty liver disease with obesity as an independent predictor for incident gastric and colorectal cancer: A population-based longitudinal study. BMJ Open Gastroenterol. 2019, 6, e000295. [Google Scholar] [CrossRef]
- Cho, Y.; Lim, S.K.; Joo, S.K.; Jeong, D.H.; Kim, J.H.; Bae, J.M.; Park, J.H.; Chang, M.S.; Lee, D.H.; Jung, Y.J.; et al. Nonalcoholic steatohepatitis is associated with a higher risk of advanced colorectal neoplasm. Liver Int. 2019, 39, 1722–1731. [Google Scholar] [CrossRef]
- Mohamad, B.; Shah, V.; Onyshchenko, M.; Elshamy, M.; Aucejo, F.; Lopez, R.; Hanouneh, I.A.; Alhaddad, R.; Alkhouri, N. Characterization of hepatocellular carcinoma (HCC) in nonalcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol. Int. 2016, 10, 632–639. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Rinella, M.E. Extrahepatic manifestations of nonalcoholic fatty liver disease. Curr. Hepatol. Rep. 2016, 15, 75–85. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. Nonalcoholic fatty liver disease. Gastroenterology 2002, 122, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.G.; Wadden, T.A. Systematic Review: An Evaluation of Major Commercial Weight Loss Programs in The United States. Ann. Intern. Med. 2005, 142, 56–66. [Google Scholar] [CrossRef]
- Thompson, W.G.; Cook, D.A.; Clark, M.M.; Bardia, A.; Levine, J.A. Treatment of obesity. Mayo Clin. Proc. 2007, 82, 93–101. [Google Scholar] [CrossRef]
- Haddock, C.K.; Poston, W.S.; Dill, P.L.; Foreyt, J.P.; Ericsson, M. Pharmacotherapy for obesity: A quantitative analysis of four decades of published randomized clinical trials. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 262–273. [Google Scholar] [CrossRef] [PubMed]
- DeWald, T.; Khaodhiar, L.; Donahue, M.P.; Blackburn, G. Pharmacological and surgical treatments for obesity. Am. Heart J. 2006, 151, 604–624. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Berkowitz, R.I.; Womble, L.G.; Sarwer, D.B.; Phelan, S.; Cato, R.K.; Hesson, L.A.; Osei, S.Y.; Kaplan, R.; Stunkard, A.J. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N. Engl. J. Med. 2005, 353, 2111–2120. [Google Scholar] [CrossRef]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjöström, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161, Erratum in Diabetes Care 2004, 27, 856. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Kessler, A.; Brazowsky, E.; Webb, M.; Lurie, Y.; Santo, M.; Leshno, M.; Blendis, L.; Halpern, Z.; Oren, R. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2006, 4, 6392013644. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Maglione, M.; Tu, W.; Mojica, W.; Arterburn, D.; Shugarman, L.R.; Hilton, L.; Suttorp, M.; Solomon, V.; Shekelle, P.G.; et al. Meta-analysis: Pharmacologic treatment of obesity. Ann. Intern. Med. 2005, 142, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Maggard, M.A.; Shugarman, L.R.; Suttorp, M.; Maglione, M.; Sugerman, H.J.; Livingston, E.H.; Nguyen, N.T.; Li, Z.; Mojica, W.A.; Hilton, L.; et al. Metaanalysis: Surgical Treatment of Obesity. Ann. Intern. Med. 2005, 142, 547–559. [Google Scholar] [CrossRef]
- Colquitt, J.L.; Picot, J.; Loveman, E.; Clegg, A.J. Surgery for Obesity. Cochrane Database Syst. Rev. 2009, 2, Cd003641. [Google Scholar] [CrossRef]
- Scopinaro, N.; Adami, G.F.; Marinari, G.M.; Gianetta, E.; Traverso, E.; Friedman, D.; Camerini, G.; Baschieri, G.; Simonelli, A. Biliopancreatic Diversion. World J. Surg. 1998, 22, 936–946. [Google Scholar] [CrossRef]
- Sjöström, L.; Narbro, K.; Sjöström, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef]
- El Ansari, W.; Elhag, W. Weight Regain and Insufficient Weight Loss after Bariatric Surgery: Definitions, Prevalence, Mechanisms, Predictors, Prevention and Management Strategies, and Knowledge Gaps-a Scoping Review. Obes. Surg. 2021, 4, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Karmali, S.; Brar, B.; Shi, X.; Sharma, A.M.; de Gara, C.; Birch, D.W. Weight recidivism post-bariatric surgery: A systematic review. Obes. Surg. 2013, 11, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- Romagna, E.C.; Lopes, K.G.; Mattos, D.M.F.; Farinatti, P.; Kraemer-Aguiar, L.G. Physical Activity Level, Sedentary Time, and Weight Regain After Bariatric Surgery in Patients Without Regular Medical Follow-up: A Cross-Sectional Study. Obes. Surg. 2021, 4, 1705–1713. [Google Scholar] [CrossRef]
- Athanasiadis, D.I.; Martin, A.; Kapsampelis, P.; Monfared, S.; Stefanidis, D. Factors associated with weight regain post-bariatric surgery: A systematic review. Surg. Endosc. 2021, 8, 4069–4084. [Google Scholar] [CrossRef] [PubMed]
- Santo, M.A.; Riccioppo, D.; Pajecki, D.; Kawamoto, F.; de Cleva, R.; Antonangelo, L.; Marçal, L.; Cecconello, I. Weight Regain After Gastric Bypass: Influence of Gut Hormones. Obes. Surg. 2016, 5, 919–925. [Google Scholar] [CrossRef]
- Torrego-Ellacuría, M.; Barabash, A.; Larrad-Sainz, A.; Hernández-Nuñez, G.M.; Matía-Martín, P.; Pérez-Ferre, N.; Marcuello, C.; Sánchez-Pernaute, A.; Torres, A.J.; Calle-Pascual, A.L.; et al. Weight Regain Outcomes After Bariatric Surgery in the Long-term Follow-up: Role of Preoperative Factors. Obes. Surg. 2021. [Google Scholar] [CrossRef]
- Karmali, S.; Johnson Stoklossa, C.; Sharma, A.; Stadnyk, J.; Christiansen, S.; Cottreau, D.; Birch, D.W. Bariatric surgery: A primer. Can. Fam. Physician 2010, 56, 873–879. [Google Scholar]
- Schroeder, R.; Harrison, T.D.; McGraw, S.L. Treatment of adult obesity with bariatric surgery. Am. Fam. Physician 2016, 93, 31–37. [Google Scholar] [PubMed]
- Buchwald, H.; Oien, D.M. Metabolic/bariatric surgery worldwide 2008. Obes. Surg. 2009, 19, 1605–1611. [Google Scholar] [CrossRef]
- Mason, E.E. Vertical banded gastroplasty for obesity. Arch. Surgery 1982, 117, 701–706. [Google Scholar] [CrossRef]
- Cobourn, C.; Mumford, D.; Chapman, M.A.; Wells, L. Laparoscopic gastric banding is safe in outpatient surgical centers. Obes. Surg. 2010, 20, 415–422. [Google Scholar] [CrossRef]
- Steffen, R. The history and role of gastric banding. Surg. Obes. Relat. Dis. 2008, 4, S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Burton, P.R.; Brown, W.A.; Laurie, C.; Richards, M.; Hebbard, G.; O’Brien, P.E. Effects of gastric band adjustments on intraluminal pressure. Obes. Surg. 2009, 19, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.F.; Dixon, J.B.; O’Brien, P.E. Laparoscopic adjustable gastric banding induces prolonged satiety: A randomised blind crossover study. J. Clin. Endocrinol. Metab. 2004, 90, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Aylwin, S.J.; Batterham, R.L.; Borg, C.M.; Coyle, F.; Prasad, V.; Shurey, S.; Ghatei, M.A.; Patel, A.G.; Bloom, S.R. Gut Hormone Profiles Following Bariatric Surgery Favor An Anorectic State, Facilitate Weight Loss, And Improve Metabolic Parameters. Ann. Surg. 2006, 243, 108–114. [Google Scholar] [CrossRef]
- Dogan, U.; Bulbuller, N.; Cakir, T.; Habibi, M.; Mayir, B.; Koc, U.; Aslaner, A.; Ellidag, H.Y.; Gomceli, I. Nesfatin-1 Hormone Levels in Morbidly Obese Patients After Laparoscopic Sleeve Gastrectomy. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1023–1031. [Google Scholar]
- Karamanakos, S.N.; Vagenas, K.; Kalfarentzos, F.; Alexan-Drides, T.K. Weight Loss, Appetite Suppression, And Changes in Fasting And Postprandial Ghrelin And Peptide-Yy Levels After Roux-En-Y Gastric Bypass And Sleeve Gastrectomy: A Prospective, Double Blind Study. Ann. Surg. 2008, 247, 401–407. [Google Scholar] [CrossRef]
- Corrodi, P. Jejunoileal bypass: Change in the flora of the small intestine and its clinical impact. Rev. Infect. Dis. 1984, 6 (Suppl. 1), S80–S84. [Google Scholar] [CrossRef]
- Hocking, M.P.; Davis, G.L.; Franzini, D.A.; Woodward, E.R. Long-term consequences after jejunoileal bypass for morbid obesity. Dig. Dis. Sci. 1998, 43, 2493–2499. [Google Scholar] [CrossRef]
- O’Leary, J.P. Hepatic complications of jejunoileal bypass. Semin. Liver Dis. 1983, 3, 203–215. [Google Scholar] [CrossRef]
- Vyberg, M.; Ravn, V.; Andersen, B. Pattern of progression in liver injury following jejunoileal bypass for morbid obesity. Liver 1987, 7, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Scopinaro, N.; Gianetta, E.; Civalleri, D.; Bonalumi, U.; Bachi, V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br. J. Surg. 1979, 66, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Scopinaro, N.; Gianetta, E.; Adami, G.F.; Friedman, D.; Traverso, E.; Marinari, G.M.; Cuneo, S.; Vitale, B.; Ballari, F.; Colombini, M.; et al. Biliopancreatic diversion for obesity at eighteen years. Surgery 1996, 119, 261–268. [Google Scholar] [CrossRef]
- Marceau, P.S.; Bourque, R.A.; Potvin, M.; Hould, F.S.; Simard, S. Biliopancreatic diversion with a new type of gastrectomy. Obes. Surg. 1993, 3, 29–35. [Google Scholar] [CrossRef]
- Marceau, P.; Hould, F.S.; Simard, S.; Lebel, S.; Bourque, R.A.; Potvin, M.; Biron, S. Biliopancreatic diversion with duodenal switch. World J. Surg. 1998, 22, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.F.; Cordera, R.; Camerini, G.; Marinari, G.M.; Scopinaro, N. Long-term normalization of insulin sensitivity following biliopancreatic diversion for obesity. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 671–673. [Google Scholar] [CrossRef]
- Buchwald, H.; Williams, S.E. Bariatric surgery worldwide 2003. Obes. Surg. 2004, 14, 1157–1164. [Google Scholar] [CrossRef]
- Mason, E.E.; Ito, C. Gastric bypass in obesity. Surg. Clin. N. Am. 1967, 47, 1345–1351. [Google Scholar] [CrossRef]
- Chevallier, J.M.; Arman, G.A.; Guenzi, M.; Rau, C.; Bruzzi, M.; Beaupel, N.; Zinzindohoue, F.; Berger, A. One thousand single anastomosis (omega loop) gastric bypasses to treat morbid obesity in a 7-year period: Outcomes show few complications and good efficacy. Obes. Surg. 2015, 25, 951–958. [Google Scholar] [CrossRef]
- Lee, W.J.; Lin, Y.H. Single-anastomosis gastric bypass (sagb): Appraisal of clinical evidence. Obes. Surg. 2014, 24, 1749–1756. [Google Scholar] [CrossRef]
- Lee, W.J.; Ser, K.H.; Lee, Y.C.; Tsou, J.J.; Chen, S.C.; Chen, J.C. Laparoscopic roux-en-y vs. Mini-gastric bypass for the treatment of morbid obesity: A 10-year experience. Obes. Surg. 2012, 22, 1827–1834. [Google Scholar] [CrossRef]
- Rutledge, R.; Walsh, T.R. Continued excellent results with the mini-gastric bypass: Six-year study in 2,410 patients. Obes. Surg. 2005, 15, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Yu, P.J.; Wang, W.; Chen, T.C.; Wei, P.L.; Huang, M.T. Laparoscopic roux-en-y versus mini-gastric bypass for the treatment of morbid obesity: A prospective randomized controlled clinical trial. Ann. Surg. 2005, 242, 20–28. [Google Scholar] [CrossRef]
- Mahawar, K.K.; Kumar, P.; Carr, W.R.; Jennings, N.; Schroeder, N.; Balupuri, S.; Small, P.K. Current status of mini-gastric bypass. J. Minim. Access. Surg 2016, 12, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Csikesz, N.G.; Nguyen, L.N.; Tseng, J.F.; Shah, S.A. Nationwide volume and mortality after elective surgery in cirrhotic patients. J. Am. Coll. Surg. 2009, 208, 96–103. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef]
- European Association for The Study of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Lam, C.M.; Yuen, W.K.; Yeung, C.; Wong, J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: Analysis of 1222 consecutive patients from a prospective database. Ann. Surg. 2004, 240, 698–708, discussion 708–710. [Google Scholar] [CrossRef]
- Northup, P.G.; Wanamaker, R.C.; Lee, V.D.; Adams, R.B.; Berg, C.L. Model for End-Stage Liver Disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann. Surg. 2005, 242, 244–251. [Google Scholar] [CrossRef]
- Teh, S.H.; Nagorney, D.M.; Stevens, S.R.; Offord, K.P.; Therneau, T.M.; Plevak, D.J.; Talwalkar, J.A.; Kim, W.R.; Kamath, P.S. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology 2007, 132, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, A. Surgical risk in patients with cirrhosis. J. Gastroenterol. Hepatol. 2012, 27, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Seijo, S.; Reverter, E.; Bosch, J. Assessing portal hypertension in liver diseases. Expert Rev. Gastroenterol. Hepatol. 2013, 7, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Boyer, T.D.; Haskal, Z.J. American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005, 41, 386–400. [Google Scholar] [CrossRef]
- Schlenker, C.; Johnson, S.; Trotter, J.F. Preoperative transjugular intrahepatic portosystemic shunt (TIPS) for cirrhotic patients undergoing abdominal and pelvic surgeries. Surg. Endosc. 2009, 23, 1594–1598. [Google Scholar] [CrossRef]
- Azoulay, D.; Buabse, F.; Damiano, I.; Smail, A.; Ichai, P.; Dannaoui, M.; Castaing, D.; Bismuth, H. Neoadjuvant transjugular intrahepatic portosystemic shunt: A solution for extrahepatic abdominal operation in cirrhotic patients with severe portal hypertension. J. Am. Coll. Surg. 2001, 193, 46–51. [Google Scholar] [CrossRef]
- Gil, A.; Martínez-Regueira, F.; Hernández-Lizoain, J.L.; Pardo, F.; Olea, J.M.; Bastarrika, G.; Cienfuegos, J.A.; Bilbao, J.I. The role of transjugular intrahepatic portosystemic shunt prior to abdominal tumoral surgery in cirrhotic patients with portal hypertension. Eur. J. Surg. Oncol. 2004, 30, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Boon-Bee Goh, G.; Schauer, P.R.; McCullough, A.J. Considerations for bariatric surgery in patients with cirrhosis. World J. Gastroenterol. 2018, 24, 3112–3119. [Google Scholar] [CrossRef]
- Dallal, R.M.; Mattar, S.G.; Lord, J.L.; Watson, A.R.; Cottam, D.R.; Eid, G.M.; Hamad, G.; Rabinovitz, M.; Schauer, P.R. Results of laparoscopic gastric bypass in patients with cirrhosis. Obes. Surg. 2004, 14, 47–53. [Google Scholar] [CrossRef]
- Shimizu, H.; Phuong, V.; Maia, M.; Kroh, M.; Chand, B.; Schauer, P.R.; Brethauer, S.A. Bariatric surgery in patients with liver cirrhosis. Surg. Obes. Relat. Dis. 2013, 9, 1–6. [Google Scholar] [CrossRef]
- Minambres, I.; Rubio, M.A.; de Hollanda, A.; Breton, I.; Vilarrasa, N.; Pellitero, S.; Bueno, M.; Lecube, A.; Marcuello, C.; Goday, A.; et al. Outcomes of bariatric surgery in patients with cirrhosis. Obes. Surg. 2019, 29, 585–592. [Google Scholar] [CrossRef]
- Rebibo, L.; Gerin, O.; Verhaeghe, P.; Dhahri, A.; Cosse, C.; Regimbeau, J.M. Laparoscopic sleeve gastrectomy in patients with NASH- related cirrhosis: A case-matched study. Surg. Obes. Relat. Dis. 2014, 10, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Wolter, S.; Duprée, A.; Coelius, C.; El Gammal, A.; Kluwe, J.; Sauer, N.; Mann, O. Influence of Liver Disease on Perioperative Outcome After Bariatric Surgery in a Northern German Cohort. Obes. Surg. 2017, 27, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ortiz, J.; Dallal, R. Is bariatric surgery safe in cirrhotics? Hepat. Mon. 2013, 13, e8536. [Google Scholar] [CrossRef]
- Kaul, A.; Singla, V.; Baksi, A.; Aggarwal, S.; Bhambri, A.; Shalimar, D.; Yadav, R. Safety and efficacy of bariatric surgery in advanced liver fibrosis. Obes. Surg. 2020, 30, 4359–4365. [Google Scholar] [CrossRef] [PubMed]
- Quezada, N.; Maturana, G.; Irarrázaval, M.J.; Muñoz, R.; Morales, S.; Achurra, P.; Azócar, C.; Crovari, F. Bariatric Surgery in Cirrhotic Patients: A Matched Case-Control Study. Obes. Surg. 2020, 30, 4724–4731. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.A.; Mikhail, H.M.S.; Nafea, M.A.; Sultan, A.A.E.A.; Elshafey, H.E.; Tourky, M.; Awad, A.; Abouelregal, T.E.; Ahmed, R.A.; Ashoush, O.; et al. Impact of laparoscopic sleeve gastrectomy on fibrosis stage in patients with child-A NASH-related cirrhosis. Surg. Endosc. 2020, 3, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Lai, D.D.; Ni, B.; Sun, K.X. Comparison of laparoscopic Roux- en-Y gastric bypass with laparoscopic sleeve gastrectomy for morbid obesity or type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Can. J. Surg. 2013, 56, E158–E164. [Google Scholar] [CrossRef]
- Ahmed, S.; Pouwels, S.; Parmar, C.; Kassir, R.; de Luca, M.; Graham, Y.; Mahawar, K. Outcomes of Bariatric Surgery in Patients with Liver Cirrhosis: A Systematic Review. Obes. Surg. 2021, 31, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Narwaria, M.; Mahawar, K.K. A systematic review of bariatric surgery in patients with liver cirrhosis. Obes. Surg. 2015, 25, 1518–1526. [Google Scholar] [CrossRef]
- Duailibi, D.F.; Ribeiro, M.A., Jr. Biliary complications following deceased and living donor liver transplantation: A review. Transplant. Proc. 2010, 42, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Watt, K.D.; Poterucha, J.J.; Ziller, N.F.; Cecco, S.D.; Charlton, M.R.; Hay, J.E.; Wiesner, R.H.; Sanchez, W.; Rosen, C.B.; et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am. J. Transplant. 2013, 2, 363–368. [Google Scholar] [CrossRef]
- Lin, M.Y.; Tavakol, M.M.; Sarin, A.; Amirkiai, S.M.; Rogers, S.J.; Carter, J.T.; Posselt, A.M. Safety and feasibility of sleeve gastrectomy in morbidly obese patients following liver transplantation. Surg. Endosc. 2013, 27, 81–85. [Google Scholar] [CrossRef]
- Fipps, D.C.; Goetze, R.E.; Clark, M.M.; Mara, K.; Watt, K.D.; Jowsey-Gregoire, S.G.; Heimbach, J.K.; Grothe, K. Liver Transplantation after Bariatric Surgery: A Clinical Cohort Study. Obes. Surg. 2021. [Google Scholar] [CrossRef]
- Butte, J.M.; Devaud, N.; Jarufe, N.P.; Boza, C.; Pérez, G.; Torres, J.; Pérez-Ayuso, R.M.; Arrese, M.; Martínez, J. Sleeve gastrectomy as treatment for severe obesity after orthotopic liver transplantation. Obes. Surg. 2007, 17, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Pajecki, D.; Cesconetto, D.M.; Macacari, R.; Joaquim, H.; Andraus, W.; de Cleva, R.; Santo, M.A.; D’Albuquerque, L.A.; Cecconello, I. Bariatric surgery (sleeve gastrectomy) after liver transplantation: Case report. ABCD Arq. Bras. Cir. Dig. 2014, 1 (Suppl. 27), 81–83. [Google Scholar] [CrossRef][Green Version]
- Lopez-Lopez, V.; Ruiz-Manzanera, J.J.; Eshmuminov, D.; Lehmann, K.; Schneider, M.; von der Groeben, M.; de Angulo, D.R.; Gajownik, U.; Pons, J.A.; Sánchez-Bueno, F.; et al. Are We Ready for Bariatric Surgery in a Liver Transplant Program? A Meta-Analysis. Obes. Surg. 2021, 31, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Mummadi, R.R.; Kasturi, K.S.; Chennareddygari, S.; Sood, G.K. Effect of bariatric surgery on nonalcoholic fatty liver disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2008, 6, 1396–1402. [Google Scholar] [CrossRef]
- Bower, G.; Athanasiou, T.; Isla, A.M.; Harling, L.; Li, J.V.; Holmes, E.; Efthimiou, E.; Darzi, A.; Ashrafian, H. Bariatric surgery and nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 755–768. [Google Scholar] [CrossRef]
- Chavez-Tapia, N.C.; Tellez-Avila, F.I.; Barrientos-Gutierrez, T.; Mendez-Sanchez, N.; Lizardi-Cervera, J.; Uribe, M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst. Rev. 2010, 2010, CD007340. [Google Scholar] [CrossRef]
- Luyckx, F.H.; Desaive, C.; Thiry, A.; Dewé, W.; Scheen, A.J.; Gielen, J.E.; Lefèbvre, P.J. Liver abnormalities in severely obese subjects: Effect of drastic weight loss after gastroplasty. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Boase, S.; Wahlroos, S.; Dugar, M.; Kow, L.; Stahl, J.; Slavotinek, J.P.; Valentine, R.; Toouli, J.; Thompson, C.H. Associates of change in liver fat content in the morbidly obese after laparoscopic gastric banding surgery. Diabetes Obes. Metab. 2008, 10, 661–667. [Google Scholar] [CrossRef]
- Busetto, L.; Tregnaghi, A.; De Marchi, F.; Segato, G.; Foletto, M.; Sergi, G.; Favretti, F.; Lise, M.; Enzi, G. Liver volume and visceral obesity in women with hepatic steatosis undergoing gastric banding. Obes. Res. 2002, 10, 408–411. [Google Scholar] [CrossRef]
- Mathurin, P.; Hollebecque, A.; Arnalsteen, L.; Buob, D.; Leteurtre, E.; Caiazzo, R.; Pigeyre, M.; Verkindt, H.; Dharancy, S.; Louvet, A.; et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 2009, 137, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, K.; Raczynska, S.; Rzepko, R.; Sledziński, Z. Nonalcoholic fatty liver disease treated by gastroplasty. Dig. Dis. Sci. 2006, 51, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Taitano, A.A.; Markow, M.; Finan, J.E.; Whheeler, D.E.; Gonzalvo, J.P.; Murr, M.M. Bariatric surgery improves histological features of non-alcholic fatty liver disease and liver fibrosis. J. Gastrointestinal. Surg. 2015, 19, 429–437. [Google Scholar] [CrossRef]
- Moschen, A.R.; Molnar, C.; Wolf, A.M.; Weiss, H.; Graziadei, I.; Kaser, S.; Ebenbichler, C.F.; Stadlmann, S.; Moser, P.L.; Tilg, H. Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J. Hepatol. 2009, 51, 765–777. [Google Scholar] [CrossRef]
- Swierczynski, J.; Sledzinski, T.; Slominska, E.; Smolenski, R.; Sledzinski, Z. Serum phenylalanine concentration as a marker of liver function in obese patients before and after bariatric surgery. Obes. Surg. 2009, 19, 883–889. [Google Scholar] [CrossRef]
- Stratopoulos, C.; Papakonstantinou, A.; Terzis, I.; Spiliadi, C.; Dimitriades, G.; Komesidou, V.; Kitsanta, P.; Argyrakos, T.; Hadjiyannakis, E. Changes in liver histology accompanying massive weight loss after gastroplasty for morbid obesity. Obes. Surg. 2005, 15, 1154–1160. [Google Scholar] [CrossRef]
- Karcz, W.K.; Krawczykowski, D.; Kuesters, S.; Marjanovic, G.; Kulemann, B.; Grobe, H.; Karcz-Socha, I.; Hopt, U.T.; Bukhari, W.; Grueneberger, J.M. Influence of Sleeve Gastrectomy on NASH and Type 2 Diabetes Mellitus. J. Obes. 2011, 2011, 765473. [Google Scholar] [CrossRef]
- Billeter, A.T.; Senft, J.; Gotthardt, D.; Knefeli, P.; Nickel, F.; Schulte, T.; Fischer, L.; Nawroth, P.P.; Büchler, M.W.; Müller-Stich, B.P. Combined Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Sleeve Gastrectomy or Gastric Bypass?—A Controlled Matched Pair Study of 34 Patients. Obes. Surg. 2016, 26, 1867–1874. [Google Scholar] [CrossRef]
- Algooneh, A.; Almazeedi, S.; Al-Sabah, S.; Ahmed, M.; Othman, F. Nonalcoholic fatty liver disease resolution following sleeve gastrectomy. Surg. Endosc. 2016, 30, 1983–1987. [Google Scholar] [CrossRef]
- Praveen Raj, P.; Gomes, R.M.; Kumar, S.; Senthilnathan, P.; Karthikeyan, P.; Shankar, A.; Palanivelu, C. The effect of surgically induced weight loss on nonalcoholic fatty liver disease in morbidly obese Indians: “NASHOST” prospective observational trial. Surg. Obes. Relat. Dis. 2015, 11, 1315–1322. [Google Scholar] [CrossRef]
- Syed-Abdul, M.M.; Moore, M.P.; Wheeler, A.; Ganga, R.R.; Diaz-Arias, A.; Rector, R.S.; Ibdah, J.A.; Parks, E.J. Improvements in nonalcoholic fatty liver disease (NAFLD) after metabolic surgery is linked to an increased hepatic fatty acid oxidation—A case report. AME Surg. J. 2021, 1, 4. [Google Scholar] [CrossRef]
- Clark, J.M.; Alkhuraishi, A.R.; Solga, S.F.; Alli, P.; Diehl, A.M.; Magnuson, T.H. Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes. Res. 2005, 13, 1180–1186. [Google Scholar] [CrossRef]
- Barker, K.B.; Palekar, N.A.; Bowers, S.P.; Goldberg, J.E.; Pulcini, J.P.; Harrison, S.A. Non-alcoholic steatohepatitis: Effect of Roux-en-Y gastric bypass surgery. Am. J. Gastroenterol. 2006, 101, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lazenby, A.J.; Clements, R.H.; Jhala, N.; Abrams, G.A. Resolution of nonalcoholic steatohepatitis after gastric bypass surgery. Obes. Surg. 2007, 17, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Furuya, C.K., Jr.; de Oliveira, C.P.; de Mello, E.S.; Faintuch, J.; Raskovski, A.; Matsuda, M.; Vezozzo, D.C.; Halpern, A.; Garrido, A.B., Jr.; Alves, V.A.; et al. Effects of bariatric surgery on nonalcoholic fatty liver disease: Preliminary findings after 2 years. J. Gastroenterol. Hepatol. 2007, 22, 510–514. [Google Scholar] [CrossRef]
- Tai, C.M.; Huang, C.K.; Hwang, J.C.; Chiang, H.; Chang, C.Y.; Lee, C.T.; Yu, M.L.; Lin, J.T. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes. Surg. 2012, 22, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Cazzo, E.; Jimenez, L.S.; Pareja, J.C.; Chaim, E.A. Effect of Roux-en-Y gastric bypass on nonalcoholic fatty liver disease evaluated through NAFLD fibrosis score: A prospective study. Obes. Surg. 2015, 25, 982–985. [Google Scholar] [CrossRef]
- Abdennour, M.; Reggio, S.; Le Naour, G.; Liu, Y.; Poitou, C.; Aron-Wisnewsky, J.; Charlotte, F.; Bouillot, J.L.; Torcivia, A.; Sasso, M.; et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: Links with diabetes and BMI loss after gastric bypass. J. Clin. Endocrinol. Metab. 2014, 99, 898–907. [Google Scholar] [CrossRef]
- Moretto, M.; Kupski, C.; da Silva, V.D.; Padoin, A.V.; Mottin, C.C. Effect of bariatric surgery on liver fibrosis. Obes. Surg. 2012, 22, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Vargas, V.; Allende, H.; Lecube, A.; Salcedo, M.T.; Baena-Fustegueras, J.A.; Fort, J.M.; Rivero, J.; Ferrer, R.; Catalán, R.; Pardina, E.; et al. Surgically induced weight loss by gastric bypass improves non-alcoholic fatty liver disease in morbid patients with obesity. World J. Hepatol. 2012, 4, 382–388. [Google Scholar] [CrossRef]
- Cazzo, E.; Pareja, J.C.; Adami Chaim, E. Nonalcoholic fatty liver disease and bariatric surgery: A comprehensive review. Sao Paulo Med. J. 2017, 135, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; Bhathal, P.S.; Hughes, N.R.; O’Brien, P.E. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology 2004, 39, 1647–1654. [Google Scholar] [CrossRef]
- Nostedt, J.J.; Switzer, N.J.; Gill, R.S.; Dang, J.; Birch, D.W.; de Gara, C.; Bailey, R.J.; Karmali, S. The effect of bariatric surgery on the spectrum of fatty liver disease. Can. J. Gatroenterol. Hepatol. 2016, 2016, 2059245. [Google Scholar] [CrossRef] [PubMed]
- Hassanian, M.; al Muhlim, A.; al Sabhan, A.; al Amro, S.; Bamehriz, F.; Abdo, A.; al Khalidi, H. The effect of bariatric surgeries on non-alcoholic fatty liver disease. Saudi J. Gastroenterol. 2014, 20, 270–278. [Google Scholar] [CrossRef]
- Pournaras, D.J.; Glicksman, C.; Vincent, R.P.; Kuganolipava, S.; Alaghband-Zadeh, J.; Mahon, D.; Bekker, J.H.R.; Ghatei, M.A.; Bloom, S.R.; Walters, J.R.F.; et al. The Role of Bile after Roux-en-Y Gastric Bypass in Promoting Weight Loss and Improving Glycaemic Control. Endocrinology 2012, 153, 3613–3619. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu. Rev. Med. 2011, 62, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.L.; Hagemann, C.A.; Wei, C.; Kazankov, K.; Thomsen, K.L.; Knop, F.K.; Grønbæk, H. Bariatric surgery in patients with non-alcoholic fatty liver disease—From pathophysiology to clinical effects. World J. Hepatol. 2019, 11, 138–149. [Google Scholar] [CrossRef]
- Cordeiro, L.; Campos, J.M.; de Paula, P.S.; Vilar, L.; Lopes, E.; de Arruda, P.C.; Ramos, A.; Ferraz, Á. Nonalcoholic steatohepatitis on preoperative period of gastric bypass: Lack of correlation with degree of obesity. ABCD Arq. Bras Cir. Dig. 2013, 26 (Suppl. 1), 39–42. [Google Scholar] [CrossRef]
- Papadia, F.; Marinari, G.M.; Camerini, G.; Adami, G.F.; Murelli, F.; Carlini, F.; Stabilini, C.; Scopinaro, N. Short-term liver function after biliopancreatic diversion. Obes. Surg. 2003, 13, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Ferrer Márquez, M.; Carvia Pousaillè, C.; Velasco Albendea, J.; del Mar Rico Morales, M.; Casado Martín, M.; Belda Lozano, R.; Ferrer Ayza, M. Influence of bariatric surgery on the non-alcoholic liver steatosis. A histological evaluation. Cir. Esp. 2009, 86, 94–100. [Google Scholar] [CrossRef]
- Kral, J.G.; Thung, S.N.; Biron, S.; Hould, F.S.; Lebel, S.; Marceau, S.; Simard, S.; Marceau, P. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery 2004, 135, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Keshishian, A.; Zahriya, K.; Willes, E.B. Duodenal switch has no detrimental effects on hepatic function and improves hepatic steatohepatitis after 6 months. Obes. Surg. 2005, 15, 1418–1423. [Google Scholar] [CrossRef]
- Aller, R.; Pacheco, D.; Izaola, O.; Primo, D.; deLuis, D.A. Effect on Liver Enzymes of Biliopancreatic Diversion: 4 Years of Follow-Up. Ann. Nutr. Metab. 2015, 66, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Weismann, R.E. Surgical Palliation of Massive and Severe Obesity. Am. J. Surg. 1973, 125, 437–446. [Google Scholar] [CrossRef]
- Griffen, W.O.; Bivins, B.A.; Bell, R.M. The Decline and Fall of the Jejunoileal Bypass. Surg. Gynecol. Obstet. 1983, 157, 301–308. [Google Scholar]
- Iber, F.L.; Copper, M. Jejunoileal Bypass for The Treatment of Massive Obesity. Prevalence, Morbidity, And Short- And Long-Term Consequences. Am. J. Clin. Nutr. 1977, 30, 4–15. [Google Scholar] [CrossRef]
- Requarth, J.A.; Burchard, K.W.; Colacchio, T.A.; Stukel, T.A.; Mott, L.A.; Greenberg, E.R.; Weismann, R.E. Long-term morbidity following jejunoileal bypass. The continuing potential need for surgical reversal. Arch. Surg. 1995, 130, 318–325. [Google Scholar] [CrossRef]
- Vespasiani-Gentilucci, U.; Vorini, F.; Carotti, S.; De Vincentis, A.; Galati, G.; Gallo, P.; Scopinaro, N.; Picardi, A. Hepatic complications of bariatric surgery: The reverse side of the coin. Acta Gastroenterol. Belg. 2017, 80, 505–513. [Google Scholar] [PubMed]
- Moxley, R.T.; Pozefsky, T.; Lockwood, D.H. Protein Nutrition and Liver Disease After Jejunoileal Bypass For Morbid Obesity. N. Engl. J. Med. 1974, 290, 921–926. [Google Scholar] [CrossRef]
- Rosina, M.; Micheletto, G.; Vita, P.M.; Restelli, A.; Caspani, P.; Ferla, G.; Doldi, S.B. Intestinal Microflora Settlement In Patients With Jejunoileal Bypass For Morbid Obesity. Obes. Surg. 1993, 3, 239–245. [Google Scholar] [CrossRef]
- Weiner, R.A. Surgical treatment of non-alcoholic steatohepatitis and non-alcoholic fatty liver disease. Dig. Dis. 2010, 28, 274–279. [Google Scholar] [CrossRef]
- Mottin, C.C.; Moretto, M.; Padoin, A.V.; Kupski, C.; Swarowsky, A.M.; Glock, L.; Duval, V.; da Silva, J.B. Histological behavior of hepatic steatosis in morbidly patients with obesity after weight loss induced by bariatric surgery. Obes. Surg. 2005, 15, 788–793. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, S.R.; Rocha, P.R.; Sanches, M.D.; Leite, V.H.; da Silva, R.A.; Diniz, M.T.; Diniz, M.d.F.; Rocha, A.L. Roux-en-Y gastric bypass improves the nonalcoholic steatohepatitis (NASH) of morbid obesity. Obes. Surg. 2006, 16, 270–278. [Google Scholar] [CrossRef]
- Klein, S.; Mittendorfer, B.; Eagon, J.C.; Patterson, B.; Grant, L.; Feirt, N.; Seki, E.; Brenner, D.; Korenblat, K.; McCrea, J. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology 2006, 130, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Froylich, D.; Corcelles, R.; Daigle, C.; Boules, M.; Brethauer, S.; Schauer, P. Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on nonalcoholic fatty liver disease: A comparative study. Surg. Obes. Relat. Dis. 2016, 12, 127–131. [Google Scholar] [CrossRef]
- Mattar, S.G.; Velcu, L.M.; Rabinovitz, M.; Demetris, A.J.; Krasinskas, A.M.; Barinas-Mitchell, E.; Eid, G.M.; Ramanathan, R.; Taylor, D.S.; Schauer, P.R. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann. Surg. 2005, 242, 610–617, discussion 618–620. [Google Scholar] [CrossRef]
- Kalinowski, P.; Paluszkiewicz, R.; Wróblewski, T.; Remiszewski, P.; Grodzicki, M.; Bartoszewicz, Z.; Krawczyk, M. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux-en-Y gastric bypass—results of a randomized clinical trial. Surg. Obes. Relat. Dis. 2017, 13, 181–188. [Google Scholar] [CrossRef]
- de Brito e Silva, M.B.; Tustumi, F.; de Miranda Neto, A.A.; Dantas, A.C.B.; Santo, M.A.; Cecconello, I. Gastric Bypass Compared with Sleeve Gastrectomy for Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Obes. Surg. 2021, 31, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Wu, W.K.; Chen, Y.H.; Lee, P.C.; Yang, P.J.; Chang, C.C.; Liu, K.L.; Hsu, C.C.; Huang, C.C.; Chuang, H.L.; Sheen, L.Y.; et al. Mining Gut Microbiota from Bariatric Surgery for MAFLD. Front. Endocrinol. 2021, 12, 612946. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Koulas, S.G.; Stefanou, C.K.; Stefanou, S.K.; Tepelenis, K.; Zikos, N.; Tepetes, K.; Kapsoritakis, A. Gut Microbiota in Patients with Morbid Obesity Before and After Bariatric Surgery: A Ten-Year Review Study (2009–2019). Obes. Surg. 2021, 31, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef]
- Cope, K.; Risby, T.; Diehl, A.M. Increased gastrointestinal ethanol production in obese mice: Implications for fatty liver disease pathogenesis. Gastroenterology 2000, 119, 1340–1347. [Google Scholar] [CrossRef]
- Dumas, M.E.; Barton, R.H.; Toye, A.; Cloarec, O.; Blancher, C.; Rothwell, A.; Fearnside, J.; Tatoud, R.; Blanc, V.; Lindon, J.C.; et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. USA 2006, 103, 12511–12516. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4523–4530. [Google Scholar] [CrossRef]
- Spruss, A.; Kanuri, G.; Wagnerberger, S.; Haub, S.; Bischoff, S.C.; Bergheim, I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009, 50, 1094–1104. [Google Scholar] [CrossRef]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef]
- Spencer, M.D.; Hamp, T.J.; Reid, R.W.; Fischer, L.M.; Zeisel, S.H.; Fodor, A.A. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011, 140, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, X.; Cao, M.; Ge, J.; Bao, Q.; Tang, L.; Chen, Y.; Li, L. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci. Rep. 2016, 6, 32002. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Kim, D.K.; Seo, W.; Gao, B.; Yoo, S.H.; Song, B.J. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1-Mediated Oxidative and Nitrative Stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Panasevich, M.R.; Peppler, W.T.; Oerther, D.B.; Wright, D.C.; Rector, R.S. Microbiome and NAFLD: Potential influence of aerobic fitness and lifestyle modification. Physiol. Genom. 2017, 49, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef]
- Sellmann, C.; Priebs, J.; Landmann, M.; Degen, C.; Engstler, A.J.; Jin, C.J.; Gärttner, S.; Spruss, A.; Huber, O.; Bergheim, I. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J. Nutr. Biochem. 2015, 26, 1183–1192. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Kesper, M.S.; Marschner, J.A.; Konrad, L.; Ryu, M.; Kumar, V.S.; Kulkarni, O.P.; Mulay, S.R.; Romoli, S.; Demleitner, J.; et al. Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD-Related Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, A.; Ponziani, F.R.; Biolato, M.; Valenza, V.; Marrone, G.; Sganga, G.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J. Gastroenterol. 2019, 25, 4814–4834. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Zocco, M.A.; Cerrito, L.; Gasbarrini, A.; Pompili, M. Bacterial translocation in patients with liver cirrhosis: Physiology, clinical consequences, and practical implications. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 641–656. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, C.; Cui, J.; Lu, J.; Yan, C.; Wei, X.; Zhao, X.; Li, N.; Li, S.; Xue, G.; et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019, 30, 675–688.e7. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Picca, A.; Marzetti, E.; Calvani, R.; Conta, G.; Del Chierico, F.; Capuani, G.; Faccia, M.; Fianchi, F.; Funaro, B.; et al. Characterization of the gut-liver-muscle axis in cirrhotic patients with sarcopenia. Liver Int. 2021, 41, 1320–1334. [Google Scholar] [CrossRef]
- Parséus, A.; Sommer, N.; Sommer, F.; Caesar, R.; Molinaro, A.; Ståhlman, M.; Greiner, T.U.; Perkins, R.; Bäckhed, F. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66, 429–437. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Leite Faria, S.; Santos, A.; Oliveira Magro, D.; Cazzo, E.; Balan Assalin, H.; Guadagnini, D.; Teixeira Vieira, F.; Said Dutra, E.; Abdalla Saad, M.J.; Ito, M.K. Gut Microbiota Modifications and Weight Regain in Morbidly Obese Women After Roux-en-Y Gastric Bypass. Obes. Surg. 2020, 30, 4958–4966. [Google Scholar] [CrossRef]
- Chen, G.; Zhuang, J.; Cui, Q.; Jiang, S.; Tao, W.; Chen, W.; Yu, S.; Wu, L.; Yang, W.; Liu, F.; et al. Two Bariatric Surgical Procedures Differentially Alter the Intestinal Microbiota in Obesity Patients. Obes. Surg. 2020, 30, 2345–2361. [Google Scholar] [CrossRef]
- Ilhan, Z.; DiBaise, J.K.; Dautel, S.E.; Isern, N.G.; Kim, Y.; Hoyt, D.W.; Schepmoes, A.A.; Brewer, H.M.; Weitz, K.K.; Metz, T.O.; et al. Temporospatial shifts in the human gut microbiome and metabolome after gastric bypass surgery. NPJ Biofilms Microbiomes 2020, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, Y.; Li, L.; Kong, Y.; Shi, T.; Xiao, H.; Cao, S.; Zhu, H.; Li, Z.; Zhou, Y. Alterations of Serum Uric Acid Level and Gut Microbiota After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in a Hyperuricemic Rat Model. Obes. Surg. 2020, 30, 1799–1807. [Google Scholar] [CrossRef]
- Sanchez-Carrillo, S.; Ciordia, S.; Rojo, D.; Zubeldia-Varela, E.; Méndez-García, C.; Martínez-Martínez, M.; Barbas, C.; Ruiz-Ruiz, S.; Moya, A.; Garriga, M.; et al. A body weight loss- and health-promoting gut microbiota is established after bariatric surgery in individuals with severe obesity. J. Pharm. Biomed. Anal. 2021, 193, 113747. [Google Scholar] [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota 41 reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, M.; Eybpoosh, S.; Siadat, S.D.; Elyasinia, F.; Soroush, A.; Bouzari, S. Modulation of the Gut Microbiota and Serum Biomarkers After Laparoscopic Sleeve Gastrectomy: A 1-Year Follow-Up Study. Obes. Surg. 2021, 31, 1949–1956. [Google Scholar] [CrossRef]
- Stefura, T.; Zapała, B.; Stój, A.; Gosiewski, T.; Skomarovska, O.; Krzysztofik, M.; Pedziwiatr, M.; Major, P. Does Postoperative Oral and Intestinal Microbiota Correlate with the Weight-Loss Following Bariatric Surgery?—A Cohort Study. J. Clin. Med. 2020, 9, 3863. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, F.; Phetcharaburanin, J.; Glymenaki, M.; Nordbeck, A.; Hankir, M.; Nicholson, J.K.; Holmes, E.; Marchesi, J.R.; Li, J.V. Roux-en-Y gastric bypass surgery in Zucker rats induces bacterial and systemic metabolic changes independent of caloric restriction-induced weight loss. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, A.; Reimann, F.; Habib, A.M.; O’Malley, D.; Williams, L.; Simpson, A.K.; Gribble, F.M. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J. Physiol. 2005, 569, 761–772. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Li, J.V.; Ashrafian, H.; Bueter, M.; Kinross, J.; Sands, C.; le Roux, C.W.; Bloom, S.R.; Darzi, A.; Athanasiou, T.; Marchesi, J.R.; et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 2011, 60, 1214–1223. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation; altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Doré, J.; Clement, K. The importance of the gut microbiota after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Daita, K.; Joyce, A.; Mirshahi, F.; Santhekadur, P.K.; Cazanave, S.; Luketic, V.A.; Siddiqui, M.S.; Boyett, S.; Min, H.K.; et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 2018, 67, 534–548. [Google Scholar] [CrossRef]
- Talavera-Urquijo, E.; Beisani, M.; Balibrea, J.M.; Alverdy, J.C. Is bariatric surgery resolving NAFLD via microbiota-mediated bile acid ratio reversal? A comprehensive review. Surg. Obes. Relat. Dis. 2020, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric Surgery in Obesity: Effects on Gut Microbiota and Micronutrient Status. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef]
- Ishida, R.K.; Faintuch, J.; Paula, A.M.; Risttori, C.A.; Silva, S.N.; Gomes, E.S.; Mattar, R.; Kuga, R.; Ribeiro, A.S.; Sakai, P.; et al. Microbial flora of the stomach after gastric bypass for morbid obesity. Obes. Surg. 2007, 17, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Theisen, J.; Nehra, D.; Citron, D.; Johansson, J.; Hagen, J.A.; Crookes, P.F.; DeMeester, S.R.; Bremner, C.G.; DeMeester, T.R.; Peters, J.H. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J. Gastrointest. Surg. 2000, 4, 50–54. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Mar Rodríguez, M.; Pérez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jové, M.; Pamplona, R.; Ricart, W.; et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Rehman, A.; Souto Lima, E.J.; Agamennone, V.; Schuren, F.H.J.; Gero, D.; Schreiner, P.; Vonlanthen, R.; Ismaeil, A.; Tzafos, S.; et al. Roux-en-Y gastric bypass surgery changes fungal and bacterial microbiota in morbidly obese patients–A pilot study. PLoS ONE 2020, 15, e0236936. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Design | N pts | Child–Pugh Score | MELD Score | Portal Hypertension | Type of Bariatric Surgery | Type of Associated Intervention | Efficacy | Factors Predictive of Efficacy | Safety | Factors Predictive of Safety |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kaul et al. [87] 2020 | Retrospective review of prospective database | 38 (22 cirrhosis; 16 stage 3 fibrosis) | A | - | 1 | 20 cirrhosis 9 controls laparoscopic SG, 2 cirrhosis 4 controls RYGB, 3 controls OAGB | - | Average % EWL 65.8 ± 18.9%; Improvement on fibrosis stage | - | liver decompensation 1 (early) and 2 (late); 1 death | Previous portal hypertension |
| Quezada et al. [88] 2020 | Retrospective, matched case-control | 64 (16 cirrhosis; 48 controls) | A | 7.4 | 3 | 11 cirrhosis 33 controls laparoscopic RYGB, 5 cirrhosis 15 controls laparoscopic SG | Liver biopsy | Average % EWL 84% | - | 31% minor complications; 13% severe complications; 1 case of HCC after 6 months | Higher rate of complications in cirrhosis group |
| Salman et al. [89] 2020 | Prospective case series | 71 cirrhosis | A | - | 26 | SG | - | Average % EWL 21.7%; Fibrosis regression 67.7% | Amount of weight loss; sex | 11 complications; 2 liver decompensations | Absence of end-stage liver disease, infections, good nutrition |
| Minambres et al. [83] 2019 | Retrospective case series | 41 cirrhosis | 40 A, 1 B | 7.2 | 11 | 28 SG, 11 RYGB, 2 BPD | - | Average % EWL at 5 years 21.16 ± 15.32% | - | 7 complications; 5 liver decompensations | Age |
| Wolter et al. [85] 2016 | Retrospective review of prospectively collected database | 12 cirrhosis | - | - | - | 150 SG, 146 laparoscopic RYGB, 6 BPD/GB | Liver biopsy | - | - | 0.3% mortality; 16 major complications; 50 minor complications | - |
| Rebibo et al. [84] 2014 | Retrospective, matched case-control | 13 cirrhosis; 26 controls | A | 7 | - | SG | - | Average % EWL at 12 months 34.1% in the SG-cirrhosis group vs 33.1% in the SG group | - | No mortality or post-op complications | - |
| Shimizu et al. [82] 2012 | Retrospective review of prospectively collected database | 23 cirrhosis | 22 A, 1 B | - | 2 | 14 laparoscopic RYGB, l8 laparoscopic SG, 1 LAGB | 2 laparoscopic SG with previous TIPS | Average % EWL at 37 months follow-up 67.7 ± 24.8% | - | No liver decompensation; complications in 8 patients | - |
| Dallal et al. [81] 2004 | Retrospective case series | 30 cirrhosis | A | - | - | Laparoscopic RYGB | Previous SG for 3 super-obese patients | Average % EWL 63 ± 15% | - | No perioperative deaths or liver failure; early complications in 9 patients; 1 unrelated death | - |
| Study | Study Design | LT pts (N) | Bariatric Surgery (N) | MELD Score at LT | Portal Hypertension | Type of Bariatric Surgery | Type of Associated Intervention | Efficacy | Safety | Factors Predictive of Safety |
|---|---|---|---|---|---|---|---|---|---|---|
| Fipps et al. [96] 2021 | Retrospective cohort study | 1416 pts who underwent LT for alcoholic liver disease | 18 | 22 previous bariatric surgery 18 no bariatric surgery | / | 16 RYGB; 2 laparoscopic GB | Following LT | 5 years survival: 61.7% if previous bariatric surgery; 78.4% if no history of bariatric surgery | - | - |
| Pajecki et al. [98] 2014 | Case report | - | 1 | 31 | 1 | Laparoscopic SG | Previous LT | EWL = 30 kg | No complications | - |
| Butte et al. [97] 2013 | Case report | - | 1 | - | 1 | SG with Roux-en-Y biliary reconstruction | Intragastric balloon before LT | EWL = 46 kg. Normal liver function tests | No complications | - |
| Heimbach et al. [94] 2013 | Prospective case series | 44 | 7 | 19 LT 32 LT + SG | - | Previous weight loss + LT = 37 LT + SG = 7 | - | Significant weight reduction | LT alone weight gain, complications, 3 deaths, 3 grafts losses | - |
| Lin et al. [95] 2012 | Prospective case series | - | 9 | - | - | Laparoscopic SG = 8 SG = 1 | Previous LT | Average % EWL = 55.5% No graft rejection | 3 complications (mesh dehiscence, bile leak, dysphagia) | Previous surgical procedures and comorbidities |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerreto, M.; Santopaolo, F.; Gasbarrini, A.; Pompili, M.; Ponziani, F.R. Bariatric Surgery and Liver Disease: General Considerations and Role of the Gut–Liver Axis. Nutrients 2021, 13, 2649. https://doi.org/10.3390/nu13082649
Cerreto M, Santopaolo F, Gasbarrini A, Pompili M, Ponziani FR. Bariatric Surgery and Liver Disease: General Considerations and Role of the Gut–Liver Axis. Nutrients. 2021; 13(8):2649. https://doi.org/10.3390/nu13082649
Chicago/Turabian StyleCerreto, Maria, Francesco Santopaolo, Antonio Gasbarrini, Maurizio Pompili, and Francesca Romana Ponziani. 2021. "Bariatric Surgery and Liver Disease: General Considerations and Role of the Gut–Liver Axis" Nutrients 13, no. 8: 2649. https://doi.org/10.3390/nu13082649
APA StyleCerreto, M., Santopaolo, F., Gasbarrini, A., Pompili, M., & Ponziani, F. R. (2021). Bariatric Surgery and Liver Disease: General Considerations and Role of the Gut–Liver Axis. Nutrients, 13(8), 2649. https://doi.org/10.3390/nu13082649







