Intravenous Vitamin K1 for the Correction of Prolonged Prothrombin Times in Non-Bleeding Critically Ill Patients: A Prospective Observational Study
Abstract
:1. Introduction
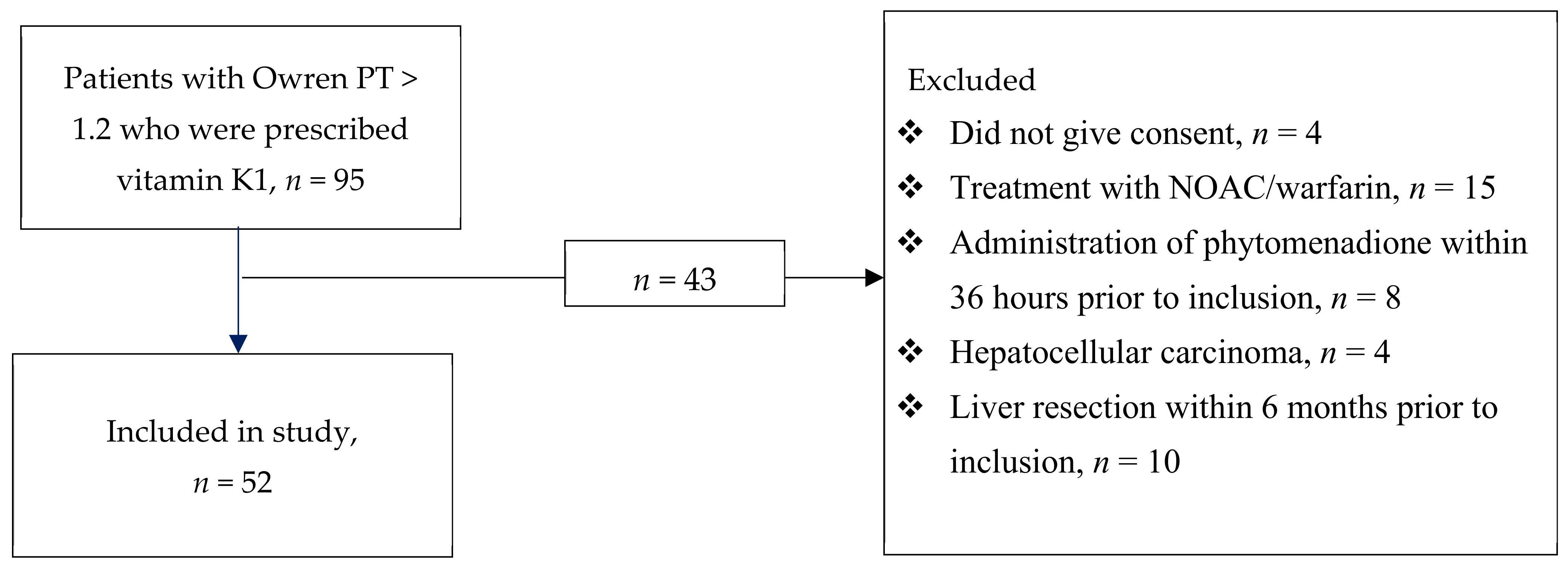
2. Materials and Methods
2.1. Study Design
2.2. Blood Sampling and Laboratory Analysis
2.3. Standard Coagulation Assays
2.4. Vitamin K-Dependent Coagulation Factor Activities
2.5. Thrombin Generation Assay
2.6. ROTEM
2.7. dp-ucMGP and PIVKA-II
2.8. Clinical Data
2.9. Statistical Analysis
3. Results
3.1. Baseline
3.2. Standard Coagulation Assays
3.3. Vitamin K-Dependent Coagulation Factor Activity
3.4. Thrombin Generation Assay
3.5. ROTEM
3.6. dp-ucMGP and PIVKA-II
3.7. Additional Analyses
3.8. Sensitivity Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benediktsson, S.; Frigyesi, A.; Kander, T. Routine coagulation tests on ICU admission are associated with mortality in sepsis: An observational study. Acta Anaesthesiol. Scand. 2017, 61, 790–796. [Google Scholar] [CrossRef]
- Hunt, B.J. Bleeding and coagulopathies in critical care. N. Engl. J. Med. 2014, 370, 847–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Y.; Hsu, C.L.; Chang, C.H.; Chen, K.Y.; Yu, C.J.; Yang, P.C. Hemothorax in a medical intensive care unit: Incidence, comorbidity and prognostic factors. J. Med. Assoc. 2010, 109, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Durila, M.; Lukáš, P.; Astraverkhava, M.; Beroušek, J.; Zábrodský, M.; Vymazal, T. Tracheostomy in intensive care unit patients can be performed without bleeding complications in case of normal thromboelastometry results (EXTEM CT) despite increased PT-INR: A prospective pilot study. BMC Anesth. 2015, 15, 89. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Lin, X.; Zhang, X.; Samir, A.E.; Arellano, R.S. Imaging-Related Risk Factors for Bleeding Complications of US-Guided Native Renal Biopsy: A Propensity Score Matching Analysis. J. Vasc Interv. Radiol. 2019, 30, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef]
- Dahlberg, S.; Schött, U.; Kander, T. The effect of vitamin K on prothrombin time in critically ill patients: An observational registry study. J. Intensive Care 2021, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [Green Version]
- Lees, J.S.; Chapman, F.A.; Witham, M.D.; Jardine, A.G.; Mark, P.B. Vitamin K status, supplementation and vascular disease: A systematic review and meta-analysis. Heart 2019, 105, 938–945. [Google Scholar] [CrossRef]
- Alisi, L.; Cafolla, C.; Gentili, A.; Tartaglione, S.; Curini, R.; Cafolla, A. Vitamin K Concentration and Cognitive Status in Elderly Patients on Anticoagulant Therapy: A Pilot Study. J. Aging Res. 2020, 2020, 9695324. [Google Scholar] [CrossRef] [Green Version]
- Dahlberg, S.; Ede, J.; Schött, U. Vitamin K and cancer. Scand. J. Clin. Lab. Investig. 2017, 77, 555–567. [Google Scholar] [CrossRef]
- Stenflo, J.; Fernlund, P.; Egan, W.; Roepstorff, P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc. Nati. Acad. Sci. USA 1974, 71, 2730–2733. [Google Scholar] [CrossRef] [Green Version]
- Hauschka, P.V.; Lian, J.B.; Gallop, P.M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc. Nati. Acad. Sci. USA 1975, 72, 3925–3929. [Google Scholar] [CrossRef] [Green Version]
- Dahlberg, S.; Ede, J.; Schurgers, L.; Vermeer, C.; Kander, T.; Klarin, B.; Schött, U. Desphospho-Uncarboxylated Matrix-Gla Protein Is Increased Postoperatively in Cardiovascular Risk Patients. Nutrients 2018, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Dauti, F.; Hjaltalin Jonsson, M.; Hillarp, A.; Bentzer, P.; Schött, U. Perioperative changes in PIVKA-II. Scand. J. Clin. Lab. Investig. 2015, 75, 562–567. [Google Scholar] [CrossRef]
- Thomas, O.; Rein, H.; Strandberg, K.; Schött, U. Coagulative safety of epidural catheters after major upper gastrointestinal surgery: Advanced and routine coagulation analysis in 38 patients. Perioper. Med. 2016, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Dahlberg, S.; Schurgers, L.; Schött, U.; Kander, T. Vitamin K deficiency in critical ill patients; a prospective observational study. J. Crit. Care 2019, 49, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.A.; McDonald, E.; Johnston, M.; Cook, D. Vitamin K deficiency and D-dimer levels in the intensive care unit: A prospective cohort study. Blood Coagul. Fibrinolysis 2002, 13, 49–52. [Google Scholar] [CrossRef]
- Nilsson, C.U.; Strandberg, K.; Reinstrup, P. Warfarin monitoring with viscoelastic haemostatic assays, thrombin generation, coagulation factors and correlations to Owren and Quick prothrombin time. Scand. J. Clin. Lab. Investig. 2018, 78, 358–364. [Google Scholar] [CrossRef]
- Delanaye, P.; Krzesinski, J.-M.; Warling, X.; Moonen, M.; Smelten, N.; Médart, L.; Pottel, H.; Cavalier, E. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol. 2014, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cranenburg, E.C.; Koos, R.; Schurgers, L.J.; Magdeleyns, E.J.; Schoonbrood, T.H.; Landewé, R.B.; Brandenburg, V.M.; Bekers, O.; Vermeer, C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [CrossRef]
- Bota, D.P.; Melot, C.; Ferreira, F.L.; Ba, V.N.; Vincent, J.-L. The multiple organ dysfunction score (MODS) versus the sequential organ failure assessment (SOFA) score in outcome prediction. Intensive Care Med. 2002, 28, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.P.; Metnitz, P.G.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.-R. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005, 31, 1345–1355. [Google Scholar] [CrossRef] [Green Version]
- Du Clos, T.W.; Mold, C. C-reactive protein. Immunol. Res. 2004, 30, 261–277. [Google Scholar] [CrossRef]
- Dofferhoff, A.S.M.; Piscaer, I.; Schurgers, L.J.; Visser, M.P.J.; van den Ouweland, J.M.W.; de Jong, P.A.; Gosens, R.; Hackeng, T.M.; van Daal, H.; Lux, P.; et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Ingold, C.J.; Sergent, S.R. Phytonadione (Vitamin K1). In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wyskida, K.; Żak-Gołąb, A.; Wajda, J.; Klein, D.; Witkowicz, J.; Ficek, R.; Rotkegel, S.; Spiechowicz, U.; Kocemba Dyczek, J.; Ciepał, J.; et al. Functional deficiency of vitamin K in hemodialysis patients in Upper Silesia in Poland. Int. Urol. Nephrol. 2016, 48, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Krueger, T.; Schlieper, G.; Schurgers, L.; Cornelis, T.; Cozzolino, M.; Jacobi, J.; Jadoul, M.; Ketteler, M.; Rump, L.C.; Stenvinkel, P.; et al. Vitamin K1 to slow vascular calcification in haemodialysis patients (VitaVasK trial): A rationale and study protocol. Nephrol. Dial. Transpl. 2014, 29, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Holden, R.M.; Booth, S.L.; Day, A.G.; Clase, C.M.; Zimmerman, D.; Moist, L.; Shea, M.K.; McCabe, K.M.; Jamal, S.A.; Tobe, S.; et al. Inhibiting the progression of arterial calcification with vitamin K in HemoDialysis patients (iPACK-HD) trial: Rationale and study design for a randomized trial of vitamin K in patients with end stage kidney disease. Can. J. Kidney Health Dis. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Roumeliotis, S.; Roumeliotis, A.; Stamou, A.; Leivaditis, K.; Kantartzi, K.; Panagoutsos, S.; Liakopoulos, V. The Association of dp-ucMGP with Cardiovascular Morbidity and Decreased Renal Function in Diabetic Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 6035. [Google Scholar] [CrossRef]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.; Ahmed, N.; Vermeer, C.; Beulens, J.W. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef]
- Mustonen, E.; Pohjolainen, V.; Aro, J.; Pikkarainen, S.; Leskinen, H.; Ruskoaho, H.; Rysä, J. Upregulation of cardiac matrix Gla protein expression in response to hypertrophic stimuli. Blood Press 2009, 18, 286–293. [Google Scholar] [CrossRef]
- Linneberg, A.; Kampmann, F.B.; Israelsen, S.B.; Andersen, L.R.; Jørgensen, H.L.; Sandholt, H.; Jørgensen, N.R.; Thysen, S.M.; Benfield, T. The Association of Low Vitamin K Status with Mortality in a Cohort of 138 Hospitalized Patients with COVID-19. Nutrients 2021, 13, 1985. [Google Scholar] [CrossRef] [PubMed]
- Kurland, D.; Hong, C.; Aarabi, B.; Gerzanich, V.; Simard, J.M. Hemorrhagic progression of a contusion after traumatic brain injury: A review. J. Neurotrauma 2012, 29, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Thorn, S.; Güting, H.; Maegele, M.; Gruen, R.L.; Mitra, B. Early Identification of Acute Traumatic Coagulopathy Using Clinical Prediction Tools: A Systematic Review. Medicina 2019, 55, 653. [Google Scholar] [CrossRef] [Green Version]
- Horlocker, T.T.; Vandermeuelen, E.; Kopp, S.L.; Gogarten, W.; Leffert, L.R.; Benzon, H.T. Regional Anesthesia in the Patient Receiving Antithrombotic or Thrombolytic Therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg. Anesth Pain Med. 2018, 43, 263–309. [Google Scholar] [CrossRef] [Green Version]
| Baseline Characteristics 1 | Median (Interquartile Range) or No (%) |
|---|---|
| Age, years | 68 (55–74) |
| Sex, male | 36 (69) |
| SAPS 3 2 | 65 (52–74) |
| SOFA 3 | 7 (5–11) |
| ICU Diagnosis | |
| Cancer | 3 (6) |
| Cardiovascular | 7 (13) |
| Respiratory | 5 (10) |
| Septic shock | 15 (29) |
| Surgical complication | 4 (8) |
| Trauma | 5 (10) |
| Postoperative care | 9 (17) |
| Other | 4 (8) |
| Laboratory values | |
| ALAT 4 (µkat/L) | 0.65 (0.42–1.3) |
| ALP 5 (µkat/L) | 0.92 (0.74–1.3) |
| ASAT 6 (µkat/L) | 0.98 (0.59–1.53) |
| Bilirubin (µmol/L) | 11.5 (9–19.5) |
| Creatinine (µmol/L) | 79 (67–121) |
| Assay | Before | After | Reference Interval | Trend | p-Value |
|---|---|---|---|---|---|
| Standard Coagulation Assays | |||||
| APTT 1 (s) | 31.5 (27–39.5) | 31.5 (28.3–39) | 26–33 | − | 0.81 |
| Owren PT 2 (INR 3) | 1.4 (1.3–1.6) | 1.3 (1.2–1.4) | 0.9–1.2 | ↓ | <0.001 |
| Quick PT (s) | 13.7 (12.5–14.4) | 12.1 (11.4–13.1) | 10–13 | ↓ | <0.001 |
| Fibrinogen (g/L) | 4.2 (3.2–4.7) | 5 (4.3–5.9) | 2–4 | ↑ | <0.001 |
| Vitamin K-dependent proteins | |||||
| dp-ucMGP 4 (pmol/L) | 840 (600–1300) | 580 (450– 660) | < 300 | ↓ | <0.001 |
| PIVKA-II 5 (AU/mL) | 0.36 (0.27–0.56) | 0.23 (0.11–0.37) | < 0.15 | ↓ | 0.016 |
| Coagulation factor activity | |||||
| FII 6 (kIU/L) | 0.61 (0.47–0.76) | 0.70 (0.53–0.86) | 0.7–1.5 | ↑ | <0.001 |
| FVII (kIU/L) | 0.48 (0.34–0.57) | 0.61 (0.51–0.78) | 0.4–1.6 | ↑ | <0.001 |
| FIX (kIU/L) | 0.94 (0.77–1.25) | 1.14 (0.89–1.38) | 0.8–1.5 | ↑ | <0.001 |
| FX (kIU/L) | 0.62 (0.47–0.79) | 0.72 (0.59–0.93) | 0.7–1.54 | ↑ | <0.001 |
| Protein C (kIU/L) | 0.65 (0.56–0.80) | 0.68 (0.54–0.86) | 0.7–1.3 | − | 0.11 |
| Protein S (kIU/L) | 0.56 (0.46–0.78) | 0.62 (0.50–0.83) | 0.65–1.4 | ↑ | 0.024 |
| Thrombin generation assays | |||||
| TGA 7 RB 8 AUC (nM) | 2400 (2200–2900) | 2600 (2300–3000) | 1500–2700 | ↑ | 0.006 |
| TGA RC 9, AUC (nM) | 2200 (2000–2600) | 2400 (2200–2800) | 1200–2600 | ↑ | 0.005 |
| ROTEM 10 | |||||
| CT 11 (s) | 82 (76–97) | 86 (75–93) | 38–79 | − | 0.29 |
| CFT 12 (s) | 78 (59–99) | 76 (64–94) | 34–159 | − | 0.30 |
| α angle (°) | 74 (70–78) | 75 (71–78) | 63–83 | − | 0.19 |
| MCF 13 (mm) | 67 (60–72) | 67 (62–74) | 50–77 | ↑ | 0.008 |
| ML 14 (%) | 7 (3–10) | 5 (2–10) | 0–18 | − | 0.39 |
| Other analyses and SOFA 15 | |||||
| CRP 16 (mg/L) | 99 (64–235) | 167 (109–239) | <5 | ↑ | 0.01 |
| Haemoglobin (g/L) | 106 (98–113) | 98 (93–103) | 117–170 | ↓ | 0.002 |
| Leukocytes (×109/L) | 11 (8.0–13) | 10.5 (9.0–14) | 3.5–8.8 | − | 0.73 |
| Platelets (×109/L) | 173 (125–229) | 164 (116–235) | 145–387 | − | 0.23 |
| SOFA | 6 (4–9) | 4.5 (3–7) | 0 | ↓ | 0.005 |
| Vitamin K1 in nutrition (µg) 17 | 32 (32–46) | NA | NA | NA | NA |
| Deficiency vitamin K1 in nutrition (µg) 18 | 88 (74–88) | NA | NA | NA | NA |
| Assay | Decreased SOFA 1 Score, n = 27 | Increased or Stagnant SOFA Score, n = 25 | p-Value Delta Changes 3 | ||||
|---|---|---|---|---|---|---|---|
| Before | After | p-Value 2 | Before | After | p-Value | ||
| Standard coagulation assays | |||||||
| APTT 4 (s) | 35 (29–40) | 32 (30– 42) | 0.081 | 30 (28–40) | 32 (28–41) | 0.588 | 0.883 |
| Qwren PT 5 (INR 6) | 1.4 (1.3–1.6) | 1.4 (1.2–1.5) | 0.046 | 1.4 (1.4–1.6) | 1.3 (1.2–1.4) | <0.001 | 0.027 7 |
| Quick PT (s) | 14 (12– 15) | 12 (12–13) | < 0.001 | 14 (12– 14) | 12(11–13) | <0.001 | 0.963 |
| Fibrinogen (g/L) | 4.2 (3.2–4.5) | 5.0 (4.1–5.5) | 0.001 | 4.2 (3.3–4.9) | 5.0 (4.4–6.4) | 0.005 | 0.647 |
| Vitamin K-dependent proteins | |||||||
| dp-ucMGP 8 (pmol/L) | 800 (600–1400) | 560 (450–680) | 0.001 | 970 (610–1270) | 520 (410–660) | <0.001 | 0.589 |
| PIVKA-II 9 (AU/mL) | - | - | - | - | - | - | - |
| Coagulation factor activity | |||||||
| F 10 II (kIU/L) | 0.61 (0.48–0.76) | 0.68 (0.50–0.84) | 0.011 | 0.62 (0.47–0.75) | 0.73 (0.57–0.87) | 0.002 | 0.203 |
| FVII (kIU/L) | 0.48 (0.39–0.56) | 0.60 (0.50–0.79) | < 0.001 | 0.48 (0.31–0.68) | 0.62 (0.51–0.81) | 0.001 | 0.920 |
| FIX (kIU/L) | 0.89 (0.79–1.09) | 1.01 (0.88–1.23) | 0.001 | 1.06 (0.71–1.41) | 1.35 (1.00–1.47) | 0.001 | 0.436 |
| FX (kIU/L) | 0.53 (0.44–0.70) | 0.66 (0.54–0.83) | 0.001 | 0.66 (0.51–0.88) | 0.88 (0.63–1.10) | <0.001 | 0.047 7 |
| Protein C (kIU/L) | 0.60 (0.54–0.75) | 0.64 (0.53–0.76) | 0.775 | 0.70 (0.59–0.89) | 0.79 (0.61–0.98) | 0.013 | 0.041 7 |
| Protein S (kIU/L) | 0.51 (0.44–0.59) | 0.53 (0.47–0.63) | 0.093 | 0.68 (0.56–0.85) | 0.65 (0.56–1.00) | 0.122 | 0.521 |
| Thrombin generation assays | |||||||
| TGA RB 11, AUC (nM) | 2500 (2200–3000) | 2600 (2500–3000) | 0.121 | 2400 (2100–2900) | 2600 (2300–3000) | 0.020 | 0.405 |
| TGA RC 12, AUC (nM) | 2200 (2000–2500) | 2400 (2300–2700) | 0.052 | 2200 (2000–2600) | 2300 (2100–2800) | 0.049 | 0.706 |
| ROTEM 13 | |||||||
| CT 14 (s) | 82 (75–100) | 87 (74–102) | 0.801 | 82 (78–96) | 84 (75–89) | 0.045 | 0.181 |
| CFT 15 (s) | 79 (64–99) | 76 (66–97) | 0.461 | 76 (57–104 | 71 (59–92) | 0.510 | 0.769 |
| α angle (°) | 74 (70–77) | 74 (70–77) | 0.282 | 74 (69–79) | 75 (72–78) | 0.531 | 0.854 |
| MCF 16 (mm) | 65 (60–71) | 67 (62–73) | 0.010 | 67 (57–74) | 68 (62–74) | 0.229 | 0.373 |
| ML 17 (%) | 7 (3–11) | 6 (3–13) | 0.220 | 5 (2–9) | 5 (2–9) | 0.760 | 0.373 |
| Other analyses and SOFA | |||||||
| CRP 18 (mg/L) | 94 (64–234) | 161 (119–218) | 0.012 | 145 (56–275) | 183 (102–262) | 0.426 | 0.240 |
| Haemoglobin (g/L) | 108 (100–113) | 96 (93–102) | 0.001 | 105 (93–114) | 101 (93–112) | 0.162 | 0.047 19 |
| Leukocytes (×109/L) | 9.8 (8.5–12) | 9.8 (8.5–12) | 0.684 | 11 (8.6–15) | 12 (9.6–14) | 0.795 | 0.984 |
| Platelets (×109/L) | 166 (122–231) | 152 (120–289) | 0.289 | 190 (125–228) | 177 (115– 222) | 0.455 | 0.984 |
| SOFA | 6 (4–10) | 3 (2–5) | <0.001 | 4 (3–8) | 6 (3–11) | 0.005 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahlberg, S.; Schött, U.; Eriksson, E.Ä.; Tahirsylaj, Y.; Schurgers, L.; Kander, T. Intravenous Vitamin K1 for the Correction of Prolonged Prothrombin Times in Non-Bleeding Critically Ill Patients: A Prospective Observational Study. Nutrients 2021, 13, 2580. https://doi.org/10.3390/nu13082580
Dahlberg S, Schött U, Eriksson EÄ, Tahirsylaj Y, Schurgers L, Kander T. Intravenous Vitamin K1 for the Correction of Prolonged Prothrombin Times in Non-Bleeding Critically Ill Patients: A Prospective Observational Study. Nutrients. 2021; 13(8):2580. https://doi.org/10.3390/nu13082580
Chicago/Turabian StyleDahlberg, Sofia, Ulf Schött, Emilia Ängeby Eriksson, Yllnor Tahirsylaj, Leon Schurgers, and Thomas Kander. 2021. "Intravenous Vitamin K1 for the Correction of Prolonged Prothrombin Times in Non-Bleeding Critically Ill Patients: A Prospective Observational Study" Nutrients 13, no. 8: 2580. https://doi.org/10.3390/nu13082580







