Effect of Dietary Strategies on Respiratory Quotient and Its Association with Clinical Parameters and Organ Fat Loss: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Intervention
2.3. Indirect Calorimetry
2.4. Magnetic Resonance Imaging (MRI)
2.5. Clinical and Anthropometric Outcomes
2.6. Statistical Analysis
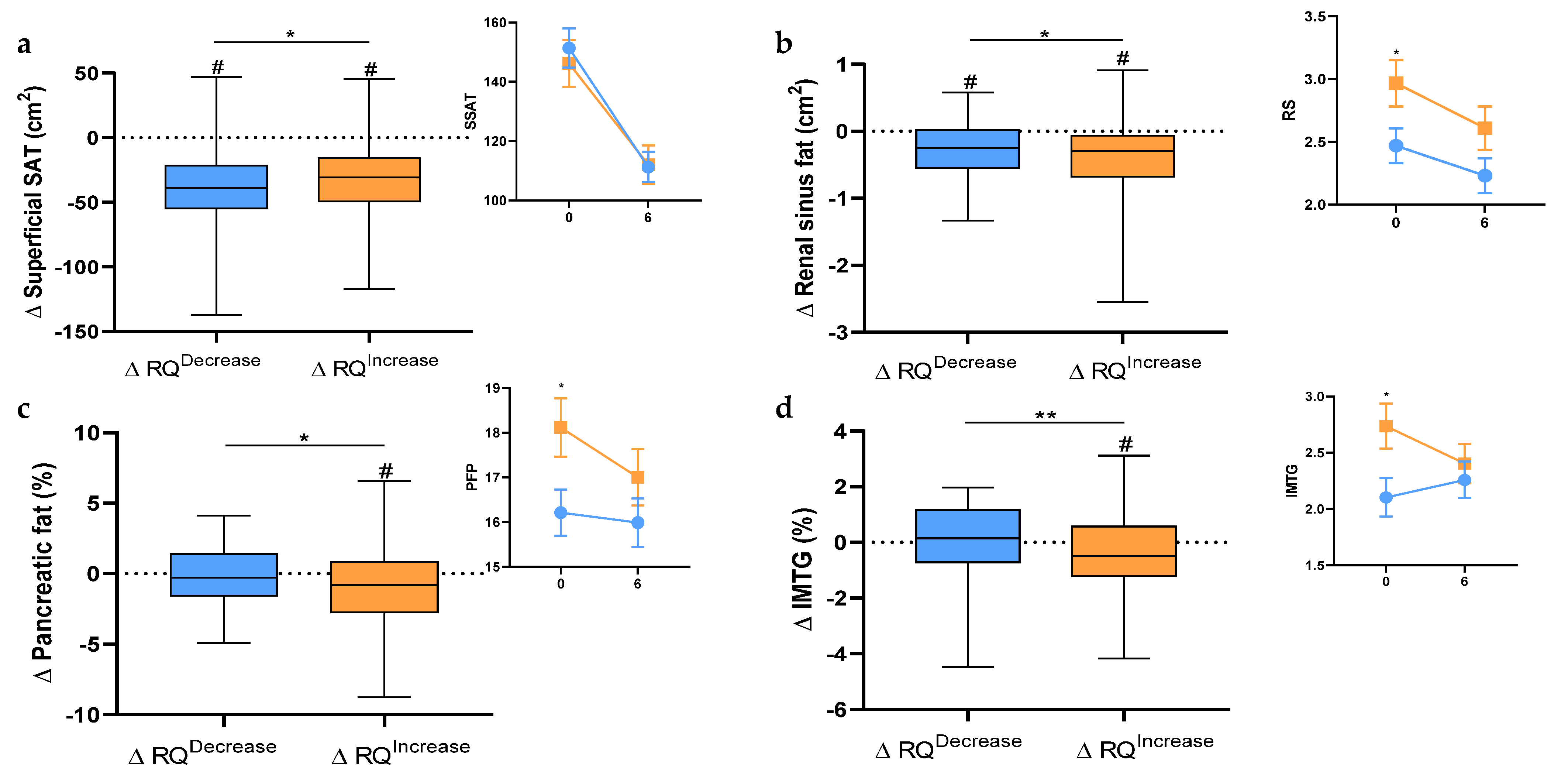
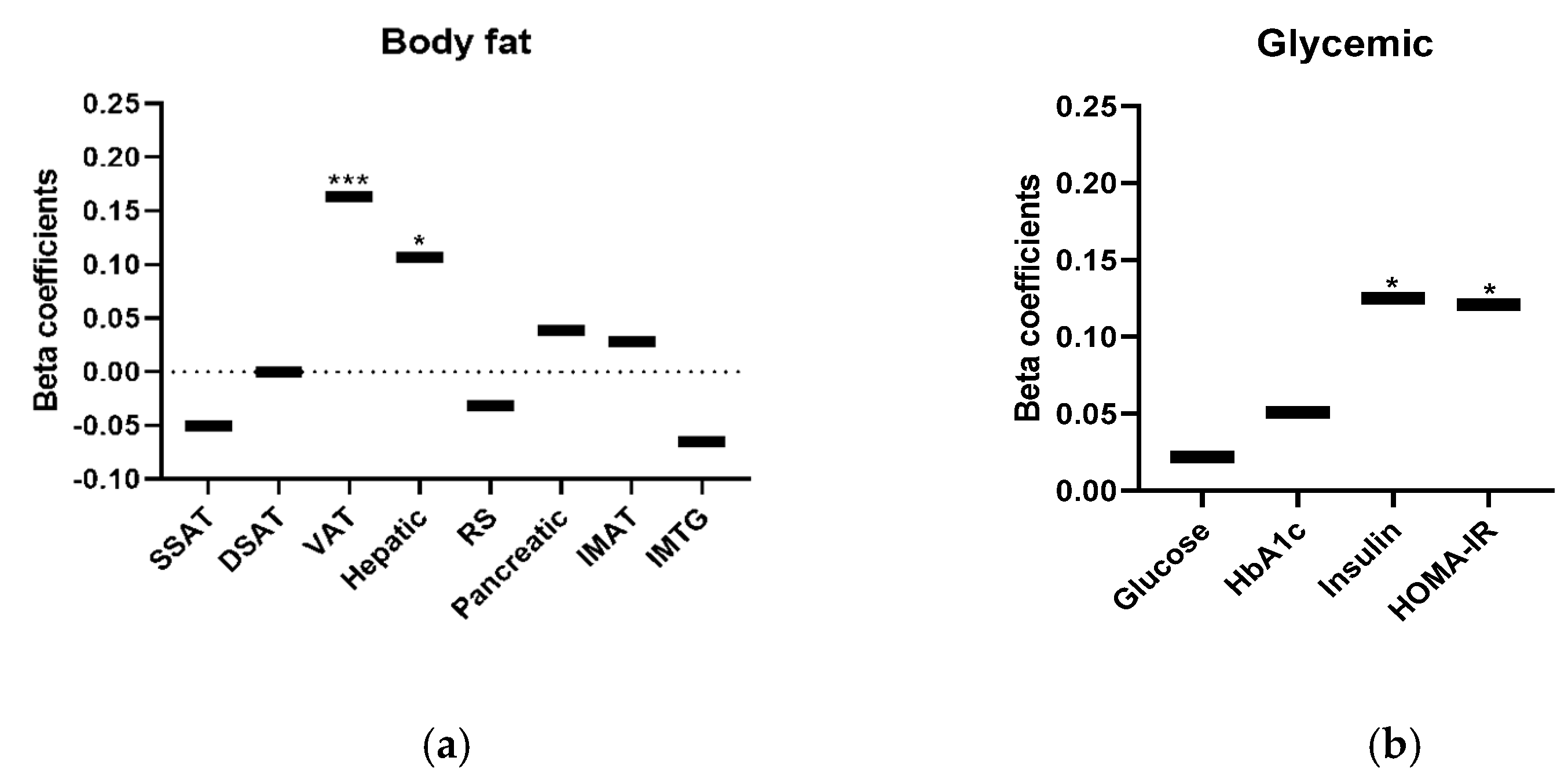
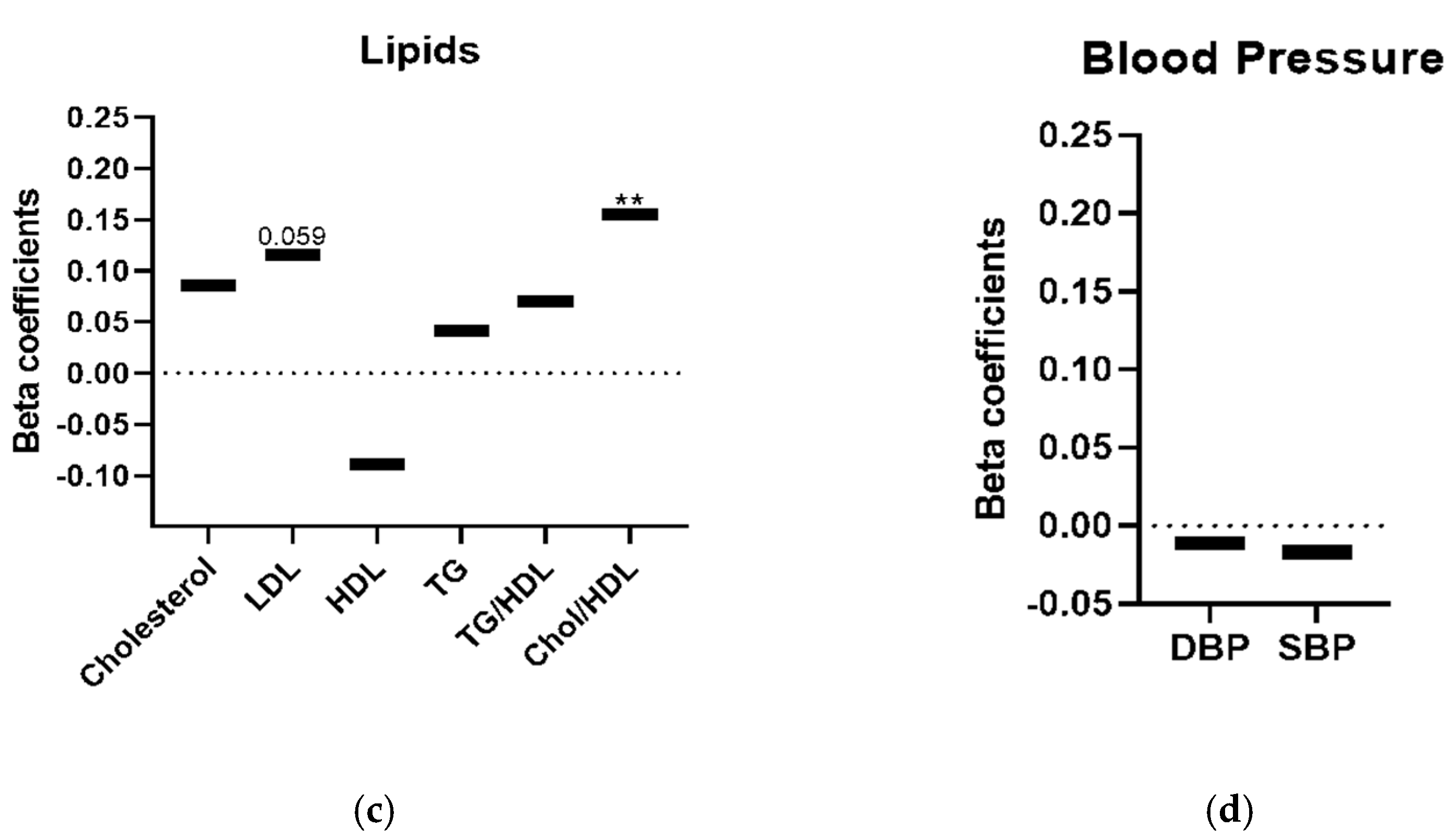
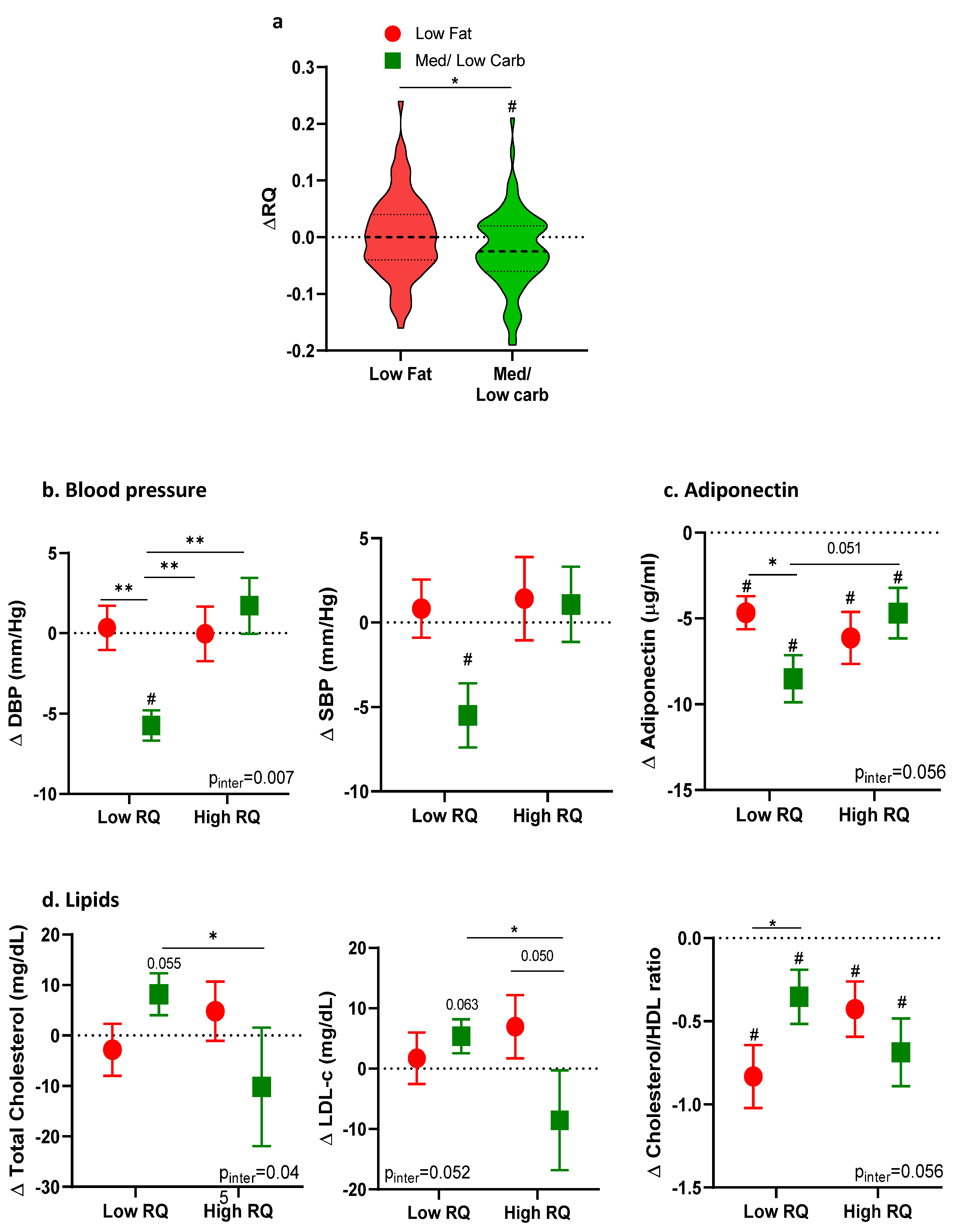
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics | Follow Up n = 159 | Non-Follow Up n = 119 | p |
|---|---|---|---|
| RQ | 0.80 ± 0.04 | 0.80 ± 0.05 | 0.299 |
| Age (years) | 47.7 ± 8.9 | 48.1 ± 9.7 | 0.666 |
| Male n, (%) | 136 (85.5) | 111 (93.2) | 0.042 |
| Weight (Kg) | 91.5 ± 14.4 | 91.3 ± 11.9 | 0.892 |
| Waist circumference (cm) | 106.9 ± 10.3 | 106.3 ± 8.6 | 0.596 |
| BMI (Kg/m2) | 31.1 ± 4.1 | 30.6 ± 3.6 | 0.325 |
| RMR (kcal) | 1927.2 ± 318.5 | 1902.9 ± 266.3 | 0.504 |
| Body fat (cm2) | |||
| Visceral adipose tissue | 177.4 ± 69.7 | 172.6 ± 61.9 | 0.552 |
| Deep Subcutaneous tissue | 221.2 ± 76.1 | 209.9 ± 71.7 | 0.213 |
| Superficial subcutaneous tissue | 148.9 ± 63.9 | 133.9 ± 60.9 | 0.049 |
| Intramuscular triglycerides (%) | 2.4 ± 1.7 | 2.37 ± 1.4 | 0.894 |
| Intermuscular fat | 9.4 ± 4.5 | 9.8 ± 4.7 | 0.513 |
| Renal sinus fat | 2.7 ± 1.4 | 2.6 ± 1.2 | 0.747 |
| Pancreatic fat | 17.1 ± 5.2 | 17.8 ± 4.9 | 0.279 |
| Hepatic fat (%) | 10.8 ± 11.1 | 9.2 ± 9.3 | 0.206 |
| Blood cardio-metabolic markers | |||
| Glucose (mg/dL) | 107.5 ± 19.6 | 107.0 ± 18.9 | 0.821 |
| Insulin (mIU/L) | 17.6 ± 11.2 | 16.1 ± 8.6 | 0.227 |
| HbA1c (%) | 5.5 ± 0.5 | 5.5 ± 0.5 | 0.604 |
| HOMA-IR | 4.7 ± 3.6 | 4.3 ± 2.5 | 0.195 |
| Triglycerides (mg/dL) | 73.1 ± 41.1 | 71.8 ± 41.3 | 0.801 |
| LDL cholesterol (mg/dL) | 123.1 ± 30.9 | 121.2 ± 32.1 | 0.623 |
| HDL cholesterol (mg/dL) | 42.9 ± 11.3 | 43.4 ± 12.1 | 0.729 |
| Blood pressure (mm Hg) | |||
| Systolic | 123.2 ± 15.1 | 125.6 ± 17.1 | 0.231 |
| Diastolic | 79.3 ± 10.5 | 80.8 ± 11.0 | 0.252 |
| Adipokines (ng/mL) | |||
| Leptin | 16.7 ± 14.2 | 11.5 ± 8.9 | <0.001 |
| Adiponectin | 11.4 ± 10.9 | 9.5 ± 8.1 | 0.102 |
| RQ Groups | ||||
|---|---|---|---|---|
| Characteristics | All n = 277 | Lower RQ n = 137 | Higher RQ n = 140 | p |
| RQ | 0.81 ± 0.05 | 0.77 ± 0.02 | 0.85 ± 0.04 | <0.001 |
| Carbohydrate ratio (%) | 36.7% | 23.3% | 50.2% | |
| Age (years) | 47.9 ± 9.3 | 48.7 ± 9.2 | 47.0 ± 9.3 | 0.135 |
| Male n, (%) | 246 (88.8) | 118 (86.1) | 128 (91.4) | 0.162 |
| Weight (Kg) | 91.4 ± 13.4 | 91.0 ± 12.6 | 91.9 ± 14.1 | 0.572 |
| Waist circumference (cm) | 106.7 ± 9.6 | 106.5 ± 9.9 | 106.9 ± 9.4 | 0.727 |
| BMI (Kg/m2) | 30.9 ± 3.8 | 31.0 ± 3.8 | 30.8 ± 3.9 | 0.609 |
| RMR (kcal) | 1916 ± 297 | 1881 ± 287 | 1951 ± 303 | 0.052 |
| Body fat (cm2) | ||||
| Visceral adipose tissue | 175.4 ± 66.4 | 168.7 ± 58.1 | 182.1 ± 73.5 | 0.095 |
| Deep Subcutaneous tissue | 216.4 ± 74.3 | 216.4 ± 77.1 | 217.1 ± 71.5 | 0.935 |
| Superficial subcutaneous tissue | 142.5 ± 63.0 | 150.0 ± 74.9 | 135.7 ± 47.8 | 0.059 |
| Intra-muscular triglycerides (%) | 2.39 ± 1.56 | 2.58 ± 1.6 | 2.22 ± 1.5 | 0.051 |
| Intermuscular fat | 9.63 ± 4.6 | 9.59 ± 4.4 | 9.69 ± 4.8 | 0.852 |
| Renal sinus fat | 2.68 ± 1.4 | 2.63 ± 1.4 | 2.74 ± 1.4 | 0.493 |
| Pancreatic fat (%) | 17.4 ± 5.09 | 17.31 ± 4.8 | 17.44 ± 5.3 | 0.829 |
| Hepatic fat (%) | 10.17 ± 10.4 | 9.98 ± 10.4 | 10.3 ± 10.4 | 0.765 |
| Blood markers | ||||
| Glucose (mg/dL) | 107.3 ± 19.3 | 107.5 ± 20.5 | 107.1 ± 18.2 | 0.863 |
| Insulin (mIU/L) | 16.9 ± 10.2 | 16.59 ± 10.4 | 17.3 ± 10.0 | 0.558 |
| HbA1c (%) | 5.5 ± 0.5 | 5.6 ± 0.5 | 5.5 ± 0.5 | 0.209 |
| HOMA-IR | 4.6 ± 3.2 | 4.5 ± 3.4 | 4.6 ± 2.9 | 0.687 |
| Triglycerides (mg/dL) | 72.6 ± 41.1 | 73.8 ± 45.8 | 71.4 ± 36.1 | 0.620 |
| LDL cholesterol (mg/dL) | 122.3 ± 31.4 | 119.2 ± 33.1 | 125.5 ± 29.4 | 0.094 |
| HDL cholesterol (mg/dL) | 43.1 ± 11.7 | 43.7 ± 12.2 | 42.7 ± 11.1 | 0.489 |
| Blood pressure (mm Hg) | ||||
| Systolic | 124 ± 16 | 125 ± 16 | 123 ± 16 | 0.207 |
| Diastolic | 80 ± 11 | 80 ± 11 | 79 ± 10 | 0.395 |
| Adipokines (ng/mL) | ||||
| Leptin | 14.5 ± 12.5 | 15.7 ± 15.1 | 13.4 ± 9.2 | 0.134 |
| Adiponectin | 10.6 ± 9.9 | 11.8 ± 10.7 | 9.5 ± 8.9 | 0.060 |
References
- Sam, S.; Mazzone, T. Adipose tissue changes in obesity and the impact on metabolic function. Transl. Res. 2014, 164, 284–292. [Google Scholar] [CrossRef]
- Ross, R.; Bradshaw, A.J. The future of obesity reduction: Beyond weight loss. Nat. Rev. Endocrinol. 2009, 5, 319–326. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Zelicha, H.; Tene, L.; Meir, A.Y.; Tsaban, G.; Cohen, N.; Bril, N.; Rein, M.; et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools CENTRAL magnetic resonance imaging randomized controlled trial. Circulation 2018, 137, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Snel, M.; Jonker, J.T.; Schoones, J.; Lamb, H.; De Roos, A.; Pijl, H.; Smit, J.W.A.; Meinders, A.E.; Jazet, I.M. Ectopic fat and insulin resistance: Pathophysiology and effect of diet and lifestyle interventions. Int. J. Endocrinol. 2012, 2012, 983814. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Huang, T.; Wang, M.; Zheng, Y.; Wang, T.; Heianza, Y.; Sun, D.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; et al. Two-year changes in circulating adiponectin, ectopic fat distribution and body composition in response to weight-loss diets: The POUNDS Lost Trial. Int. J. Obes. 2016, 40, 1723–1729. [Google Scholar] [CrossRef]
- Borel, A.L.; Nazare, J.A.; Smith, J.; Alméras, N.; Tremblay, A.; Bergeron, J.; Poirier, P.; Després, J.P. Visceral and not subcutaneous abdominal adiposity reduction drives the benefits of a 1-year lifestyle modification program. Obesity 2012, 20, 1223–1233. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Bloomfield, H.E.; Koeller, E.; Greer, N.; MacDonald, R.; Kane, R.; Wilt, T.J. Effects on health outcomes of a mediterranean diet with no restriction on fat intake: A systematic review and meta-analysis. Ann. Intern. Med. 2016, 165, 491–500. [Google Scholar] [CrossRef]
- Ahmad, S.; Moorthy, M.V.; Demler, O.V.; Hu, F.B.; Ridker, P.M.; Chasman, D.I.; Mora, S. Assessment of Risk Factors and Biomarkers Associated with Risk of Cardiovascular Disease Among Women Consuming a Mediterranean Diet. JAMA Netw. Open 2018, 1, e185708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2020, 60, 1561–1586. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Iacoviello, L.; et al. Association of a traditional Mediterranean diet and non-Mediterranean dietary scores with all-cause and cause-specific mortality: Prospective findings from the Moli-sani Study. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Kopel, E.; Sidi, Y.; Kivity, S. Prevention of diabetes with Mediterranean diets. Ann. Intern. Med. 2014, 161, 157. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsaban, G.; Wolak, A.; Avni-Hassid, H.; Gepner, Y.; Shelef, I.; Henkin, Y.; Schwarzfuchs, D.; Cohen, N.; Bril, N.; Rein, M.; et al. Dynamics of intrapericardial and extrapericardial fat tissues during long-term, dietary-induced, moderate weight loss. Am. J. Clin. Nutr. 2017, 106, 984–995. [Google Scholar] [CrossRef] [Green Version]
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [Green Version]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [Green Version]
- McClave, S.A.; Lowen, C.C.; Kleber, M.J.; McConnell, J.W.; Jung, L.Y.; Goldsmith, L.J. Clinical use of the respiratory quotient obtained from indirect calorimetry. JPEN J. Parenter Enter. Nutr 2003, 27, 21–26. [Google Scholar] [CrossRef]
- Galgani, J.E.; Moro, C.; Ravussin, E. Metabolic flexibility and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1009–E1017. [Google Scholar] [CrossRef] [Green Version]
- Henry, C.J.; Kaur, B.; Quek, R.Y.C.; Camps, S.G. A low glycaemic index diet incorporating isomaltulose is associated with lower glycaemic response and variability, and promotes fat oxidation in asians. Nutrients 2017, 9, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Lam, Y.Y.; Leibel, R.L.; Mayer, L.E.S.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T.; et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 2016, 104, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shook, R.P.; Hand, G.A.; Paluch, A.E.; Wang, X.; Moran, R.; Hébert, J.R.; Jakicic, J.M.; Blair, S.N. High respiratory quotient is associated with increases in body weight and fat mass in young adults. Eur. J. Clin. Nutr. 2016, 70, 1197–1202. [Google Scholar] [CrossRef]
- Fechner, E.; Bilet, L.; Peters, H.P.F.; Hiemstra, H.; Jacobs, D.M.; Op ‘t Eyndt, C.; Kornips, E.; Mensink, R.P.; Schrauwen, P. Effects of a whole diet approach on metabolic flexibility, insulin sensitivity and postprandial glucose responses in overweight and obese adults—A randomized controlled trial. Clin. Nutr. 2020, 39, 2734–2742. [Google Scholar] [CrossRef]
- Zhang, S.; Takano, J.; Murayama, N.; Tominaga, M.; Abe, T.; Park, I.; Seol, J.; Ishihara, A.; Tanaka, Y.; Yajima, K.; et al. Subacute ingestion of caffeine and oolong tea increases fat oxidation without affecting energy expenditure and sleep architecture: A randomized, placebo-controlled, double-blinded cross-over trial. Nutrients 2020, 12, 3671. [Google Scholar] [CrossRef]
- Ellis, A.C.; Hyatt, T.C.; Hunter, G.R.; Gower, B.A. Respiratory quotient predicts fat mass gain in premenopausal women. Obesity 2010, 18, 2255–2259. [Google Scholar] [CrossRef]
- Weinsier, R.L.; Nelson, K.M.; Hensrud, D.D.; Darnell, B.E.; Hunter, G.R.; Schutz, Y. Metabolic Predictors of Obesity: Contribution of Resting Energy Expenditure, Thermic Effect of Food, and Fuel Utilization to Four-year Weight Gain of Post-obese and Never-obese Women. J. Clin. Investig. 1995, 95, 980–985. [Google Scholar] [CrossRef] [Green Version]
- Weyer, C.; Pratley, R.E.; Salbe, A.D.; Bogardus, C.; Ravussin, E.; Tataranni, P.A. Energy expenditure, fat oxidation, and body weight regulation: A study of metabolic adaptation to long-term weight change. J. Clin. Endocrinol. Metab. 2000, 85, 1087–1094. [Google Scholar] [CrossRef]
- Weinsier, R.L.; Hunter, G.R.; Zuckerman, P.A.; Darnell, B.E. Low resting and sleeping energy expenditure and fat use do not contribute to obesity in women. Obes. Res. 2003, 11, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, S.R.; Halset, E.H.; Gåsbakk, S.; Rehfeld, J.F.; Kulseng, B.; Truby, H.; Martins, C. Compensatory mechanisms activated with intermittent energy restriction: A randomized control trial. Clin. Nutr. 2018, 37, 815–823. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American heart association nutrition committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.M.; Smallwood, C.D.; Joosten, K.F.M.; Hulst, J.M.; Tasker, R.C.; Duggan, C.P. Accuracy of a simplified equation for energy expenditure based on bedside volumetric carbon dioxide elimination measurement—A two-center study. Clin. Nutr. 2015, 34, 151–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best Practice Methods to Apply to Measurement of Resting Metabolic Rate in Adults: A Systematic Review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Golan, R.; Shelef, I.; Rudich, A.; Gepner, Y.; Shemesh, E.; Chassidim, Y.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Ben Avraham, S.; et al. Abdominal superficial subcutaneous fat: A putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 2012, 35, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Tene, L.; Shelef, I.; Schwarzfuchs, D.; Gepner, Y.; Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Bilitzky, A.; Komy, O.; Cohen, N.; et al. The effect of long-term weight-loss intervention strategies on the dynamics of pancreatic-fat and morphology: An MRI RCT study. Clin. Nutr. ESPEN 2018, 24, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zelicha, H.; Schwarzfuchs, D.; Shelef, I.; Gepner, Y.; Tsaban, G.; Tene, L.; Yaskolka Meir, A.; Bilitzky, A.; Komy, O.; Cohen, N.; et al. Changes of renal sinus fat and renal parenchymal fat during an 18-month randomized weight loss trial. Clin. Nutr. 2018, 37, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.Y.; Shelef, I.; Schwarzfuchs, D.; Gepner, Y.; Tene, L.; Zelicha, H.; Tsaban, G.; Bilitzky, A.; Komy, O.; Cohen, N.; et al. Intermuscular adipose tissue and thigh muscle area dynamics during an 18-month randomized weight loss trial. J. Appl. Physiol. 2016, 121, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Cohen, N.; Bril, N.; Rein, M.; Tsaban, G.; Zelicha, H.; Meir, A.Y.; Tene, L.; et al. Intramyocellular triacylglycerol accumulation across weight loss strategies; Sub-study of the CENTRAL trial. PLoS ONE 2017, 12, e0188431. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Bemis, T.; Brychta, R.; Chen, K.Y.; Courville, A.; Crayner, E.J.; Goodwin, S.; Guo, J.; Howard, L.; Knuth, N.D.; et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 2015, 22, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, M.E.; Wright, O.R.L.; Hickman, I.J. A Review of the Effect of Dietary Composition on Fasting Substrate Oxidation in Healthy and Overweight Subjects. Crit. Rev. Food Sci. Nutr. 2016, 56, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclerc, I.; Davignon, I.; Lopez, D.; Garrel, D.R. No change in glucose tolerance and substrate oxidation after a high-carbohydrate, low-fat diet. Metabolism 1993, 42, 365–370. [Google Scholar] [CrossRef]
- Vasilaras, T.H.; Raben, A.; Astrup, A. Twenty-four hour energy expenditure and substrate oxidation before and after 6 months’ ad libitum intake of a diet rich in simple or complex carbohydrates or a habitual diet. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 954–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, K.; Hornemann, S.; Petzke, K.J.; Kemper, M.; Markova, M.; Rudovich, N.; Grune, T.; Kramer, A.; Pfeiffer, A.F.H.; Pivovarova-Ramich, O. Diurnal distribution of carbohydrates and fat affects substrate oxidation and adipokine secretion in humans. Am. J. Clin. Nutr. 2018, 108, 1209–1219. [Google Scholar] [CrossRef]
- Bisschop, P.H.; De Metz, J.; Ackermans, M.T.; Endert, E.; Pijl, H.; Kuipers, F.; Meijer, A.J.; Sauerwein, H.P.; Romijn, J.A. Dietary fat content alters insulin-mediated glucose metabolism in healthy men. Am. J. Clin. Nutr. 2001, 73, 554–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siervo, M.; Lara, J.; Celis-Morales, C.; Vacca, M.; Oggioni, C.; Battezzati, A.; Leone, A.; Tagliabue, A.; Spadafranca, A.; Bertoli, S. Age-related changes in basal substrate oxidation and visceral adiposity and their association with metabolic syndrome. Eur. J. Nutr. 2016, 55, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Montalcini, T.; Lamprinoudi, T.; Morrone, A.; Mazza, E.; Gazzaruso, C.; Romeo, S.; Pujia, A. Nutrients utilization in obese individuals with and without hypertriglyceridemia. Nutrients 2014, 6, 790–798. [Google Scholar] [CrossRef] [Green Version]
- Galgani, J.E.; Mizgier, M.L.; Mari, A.; Ravussin, E. Relationship between whole-body macronutrient oxidative partitioning and pancreatic insulin secretion/β-cell function in non-diabetic humans. Metabolism 2014, 63, 1426–1431. [Google Scholar] [CrossRef] [Green Version]
- Solomon, T.P.J.; Sistrun, S.N.; Krishnan, R.K.; Aguila, L.F.; Del Marchetti, C.M.; Carroll, S.M.O.; Leary, V.B.O.; Kirwan, J.P. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J. Appl. Physiol. 2008, 104, 1313–1319. [Google Scholar] [CrossRef] [Green Version]
- Croci, I.; Byrne, N.M.; Choquette, S.; Hills, A.P.; Chachay, V.S.; Clouston, A.D.; O’Moore-Sullivan, T.M.; Macdonald, G.A.; Prins, J.B.; Hickman, I.J. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut 2013, 62, 1625–1633. [Google Scholar] [CrossRef] [Green Version]
- Ferro, Y.; Gazzaruso, C.; Coppola, A.; Romeo, S.; Migliaccio, V.; Giustina, A.; Pujia, A.; Montalcini, T. Fat utilization and arterial hypertension in overweight/obese subjects. J. Transl. Med. 2013, 11, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalcini, T.; Gazzaruso, C.; Ferro, Y.; Migliaccio, V.; Rotundo, S.; Castagna, A.; Pujia, A. Metabolic fuel utilization and subclinical atherosclerosis in overweight/obese subjects. Endocrine 2013, 44, 380–385. [Google Scholar] [CrossRef]
- Zurlo, F.; Lillioja, S.; Esposito-Del Puente, A.; Nyomba, B.L.; Raz, I.; Saad, M.F.; Swinburn, B.A.; Knowler, W.C.; Bogardus, C.; Ravussin, E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: Study of 24-h RQ. Am. J. Physiol. 1990, 259, E650–E657. [Google Scholar] [CrossRef]
- Seidell, J.C.; Muller, D.C.; Sorkin, J.D.; Andres, R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: The Baltimore longitudinal study on aging. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 667–674. [Google Scholar]
- Doucet, E.; St. Pierre, S.; Alméras, N.; Mauriège, P.; Richard, D.; Tremblay, A. Changes in energy expenditure and substrate oxidation resulting from weight loss in obese men and women: Is there an important contribution of leptin. J. Clin. Endocrinol. Metab. 2000, 85, 1550–1556. [Google Scholar] [CrossRef]
- Saiki, A.; Koide, N.; Watanabe, F.; Murano, T.; Miyashita, Y.; Shirai, K. Suppression of lipoprotein lipase expression in 3T3-L1 cells by inhibition of adipogenic differentiation through activation of the renin-angiotensin system. Metabolism 2008, 57, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Even, P.; Bloch-Faure, M.; Guerre-Millo, M.; Moustaid-Moussa, N.; Ferre, P.; Quignard-Boulange, A. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes 2005, 54, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Toubro, S.; Sørensen, T.I.A.; Hindsberger, C.; Christensen, N.J.; Astrup, A. Twenty-four-hour respiratory quotient: The role of diet and familial resemblance. J. Clin. Endocrinol. Metab. 1998, 83, 2758–2764. [Google Scholar] [CrossRef]
- Lightowler, H.; Schweitzer, L.; Theis, S.; Henry, C.J. Changes in weight and substrate oxidation in overweight adults following isomaltulose intake during a 12-week weight loss intervention: A randomized, double-blind, controlled trial. Nutrients 2019, 11, 2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]

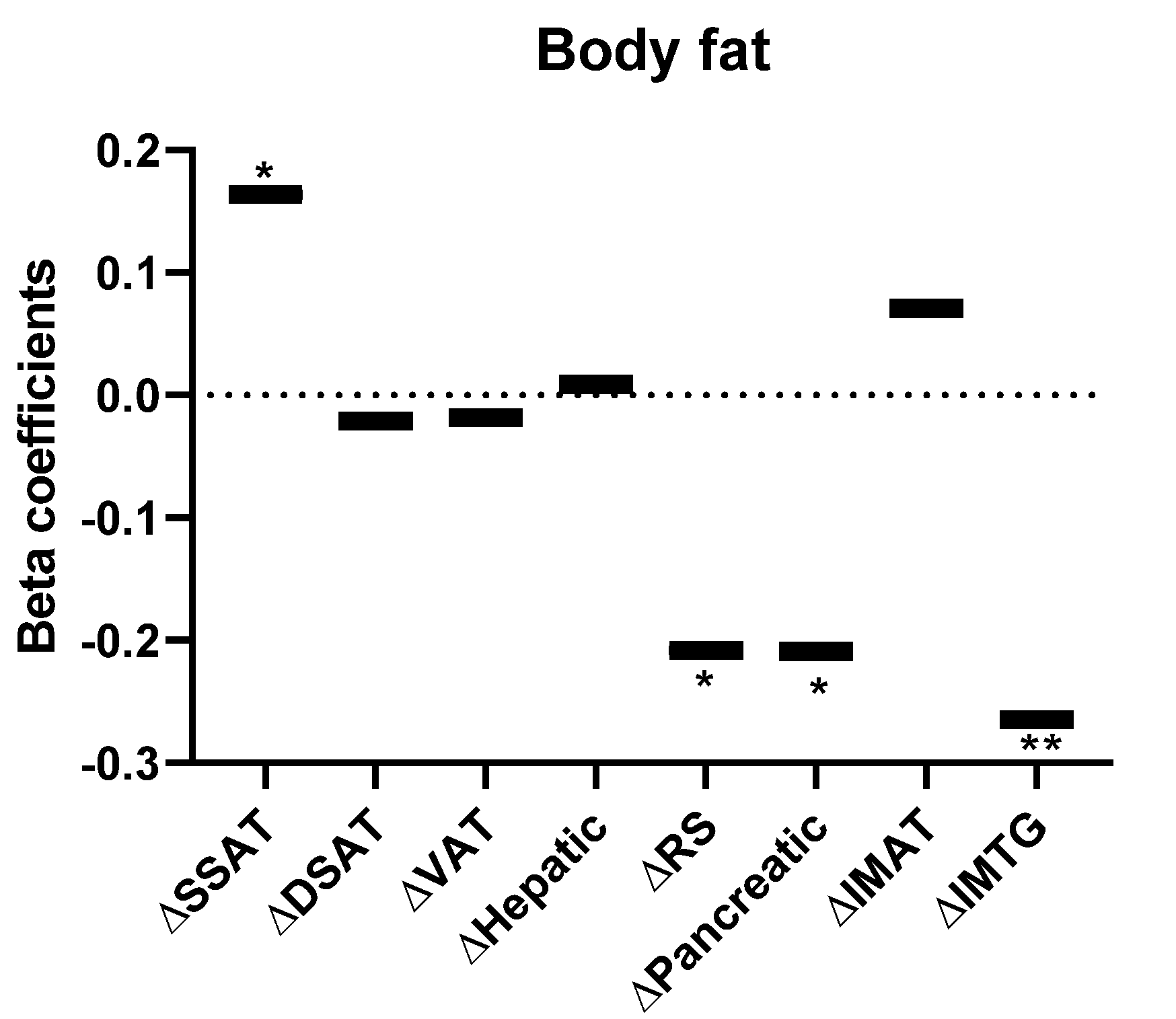
| Intervention Groups | |||||
|---|---|---|---|---|---|
| Characteristics | Participants n = 159 | Low-Fat Diet (n = 79) | Mediterranean/Low Carbohydrate (n = 80) | Mean Difference (95% CI) | p |
| RQ | 0.80 ± 0.04 | 0.80 ± 0.04 | 0.81 ± 0.05 | 0.008 (−0.02–0.006) | 0.26 |
| Age (years) | 47.7 ± 8.9 | 48.9 ± 8.9 | 46.5 ± 8.9 | 2.4 (−0.3–5.2) | 0.09 |
| Male N, (%) | 136 (85.5) | 66 (83.5) | 70 (87.5) | 0.47 | |
| Weight (Kg) | 91.5 ± 14.4 | 90.6 ± 14.6 | 92.4 ± 14.2 | −1.8 (−6.3–2.7) | 0.43 |
| Waist circumference (cm) | 106.9 ± 10.3 | 105.9 ± 9.3 | 107.9 ± 11.2 | −1.9 (−5.1–1.3) | 0.23 |
| BMI (Kg/m2) | 31.1 ± 4.1 | 30.8 ± 3.9 | 31.2 ± 4.2 | −0.4 (−1.6–0.8) | 0.50 |
| RMR (kcal) | 1927 ± 318 | 1898 ± 310 | 1955 ± 325 | −57.5 (−157.1–42.2) | 0.25 |
| Body fat (cm2) | |||||
| Visceral adipose tissue | 177.4 ± 69.7 | 184.6 ± 72.7 | 170.3 ± 66.4 | 14.3 (−7.5–36.1) | 0.19 |
| Deep Subcutaneous tissue | 221.2 ± 76.1 | 213.7 ± 70.3 | 228.5 ± 81.1 | −14.8 (−38.5–9.0) | 0.22 |
| Superficial subcutaneous tissue | 148.9 ± 63.9 | 147.1 ± 65.4 | 150.7 ± 62.7 | −3.6 (−23.6–16.5) | 0.72 |
| Hepatic fat (%) | 10.85 ± 11.1 | 10.2 ± 9.9 | 11.4 ± 12.1 | −1.2 (−4.7–2.2) | 0.49 |
| Blood cardio-metabolic markers | |||||
| Glucose (mg/dL) | 107.5 ± 19.6 | 105.9 ± 14.3 | 109.2 ± 23.7 | −3.3 (−9.4–2.8) | 0.29 |
| Insulin (mIU/L) | 17.6 ± 11.2 | 17.6 ± 11.7 | 17.5 ± 10.7 | 0.1 (−3.4–3.6) | 0.97 |
| HbA1C (%) | 5.5 ± 0.5 | 5.5 ± 0.5 | 5.6 ± 0.5 | 0.0 (−0.2–0.1) | 0.64 |
| Triglycerides (mg/dL) | 73.1 ± 41.1 | 78.4 ± 45.3 | 68.1 ± 36.2 | 10.4 (−2.6–23.3) | 0.11 |
| LDL cholesterol (mg/dL) | 123.1 ± 30.9 | 125.8 ± 31.5 | 120.4 ± 30.2 | 5.4 (−4.3–15.0) | 0.27 |
| HDL cholesterol (mg/dL) | 42.9 ± 11.3 | 42.4 ± 12.2 | 43.4 ± 10.4 | −1.0 (−4.6–2.5) | 0.56 |
| Blood pressure (mm Hg) | |||||
| Systolic | 123.2 ± 15.1 | 123.4 ± 14.7 | 123.1 ± 15.5 | 0.36 (−4.4–5.1) | 0.87 |
| Diastolic | 79.3 ± 10.5 | 78.5 ± 10.9 | 80.1 ± 10.1 | −1.5 (−4.8–1.7) | 0.35 |
| Reported dietary intake | |||||
| Kcal | 2845 ± 1075 | 2841.9 ± 1061.7 | 2848.8 ± 1095.2 | −6.9 (−344.8–331.0) | 0.96 |
| Carbohydrates (%) | 45.4 ± 7.9 | 44.3 ± 8.4 | 46.6 ± 7.3 | −1.8 (−4.3–0.6) | 0.13 |
| Total fat (%) | 34.5 ± 4.8 | 35.5 ± 5.0 | 33.5 ± 4.4 | 1.6 (0.1–3.1) | 0.03 |
| Protein (%) | 20.1 ± 3.8 | 20.2 ± 4.0 | 20.1 ± 3.7 | 0.06 (−1.1–1.2) | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldenshluger, A.; Constantini, K.; Goldstein, N.; Shelef, I.; Schwarzfuchs, D.; Zelicha, H.; Yaskolka Meir, A.; Tsaban, G.; Chassidim, Y.; Gepner, Y. Effect of Dietary Strategies on Respiratory Quotient and Its Association with Clinical Parameters and Organ Fat Loss: A Randomized Controlled Trial. Nutrients 2021, 13, 2230. https://doi.org/10.3390/nu13072230
Goldenshluger A, Constantini K, Goldstein N, Shelef I, Schwarzfuchs D, Zelicha H, Yaskolka Meir A, Tsaban G, Chassidim Y, Gepner Y. Effect of Dietary Strategies on Respiratory Quotient and Its Association with Clinical Parameters and Organ Fat Loss: A Randomized Controlled Trial. Nutrients. 2021; 13(7):2230. https://doi.org/10.3390/nu13072230
Chicago/Turabian StyleGoldenshluger, Ariela, Keren Constantini, Nir Goldstein, Ilan Shelef, Dan Schwarzfuchs, Hila Zelicha, Anat Yaskolka Meir, Gal Tsaban, Yoash Chassidim, and Yftach Gepner. 2021. "Effect of Dietary Strategies on Respiratory Quotient and Its Association with Clinical Parameters and Organ Fat Loss: A Randomized Controlled Trial" Nutrients 13, no. 7: 2230. https://doi.org/10.3390/nu13072230






