Clinical Results of the Implementation of a Breast Milk Bank in Premature Infants (under 37 Weeks) at the Hospital Universitario del Valle 2018–2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
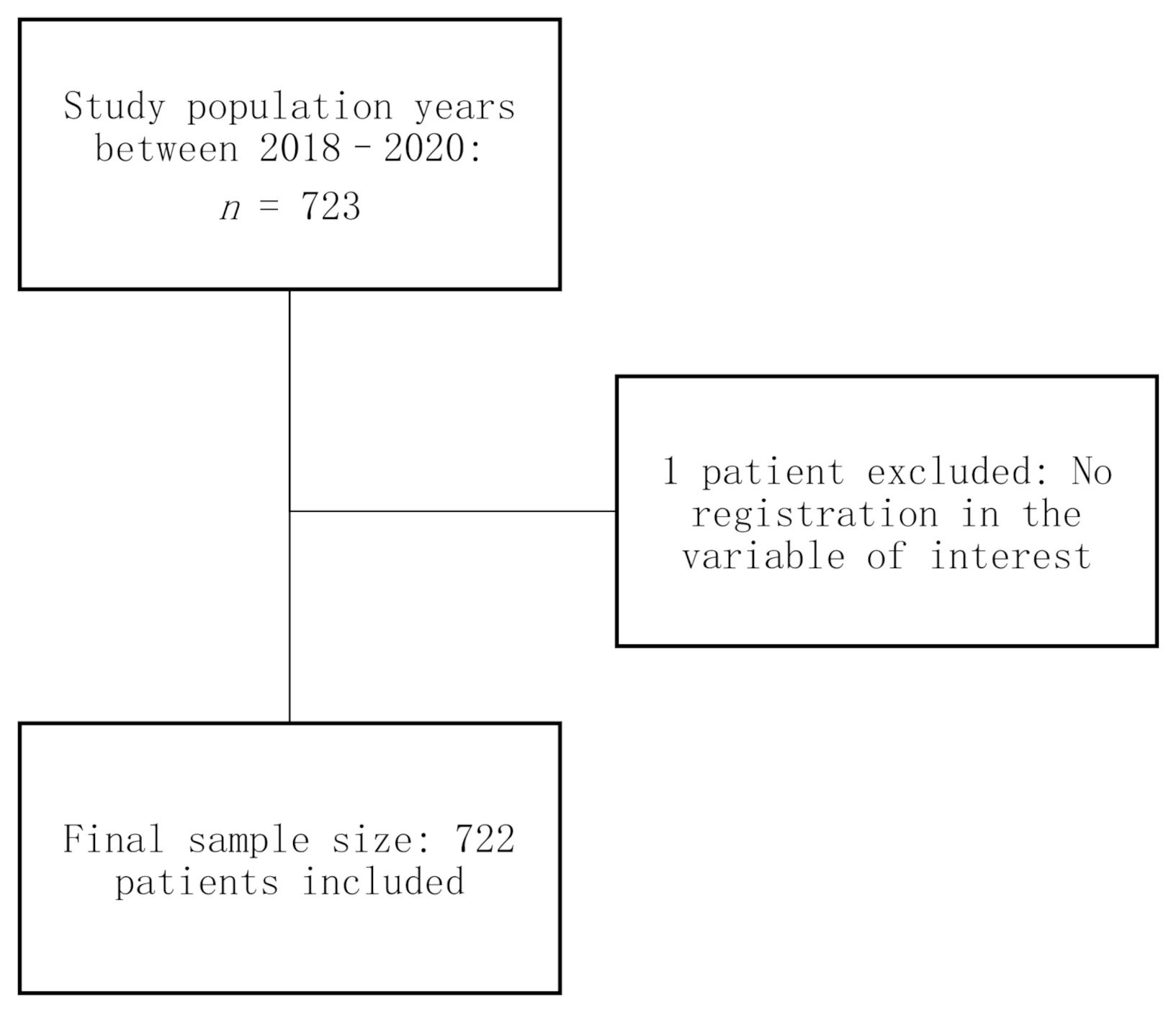
2.2. Object Population of Study
2.3. Sample Size
2.4. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Comparison by Type of Milk that the Newborn Receives at 7 Days of Age and the Number of Days It Takes to Reach Full Breastfeeding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spatz, D.; Edwards, T. The Use of Human Milk and Breastfeeding in the Neonatal Intensive Care Unit. Adv. Neonatal Care 2016, 16, 254. [Google Scholar] [CrossRef] [PubMed]
- Weaver, G.; Bertino, E.; Gebauer, C.; Grovslien, A.; Mileusnic-Milenovic, R.; Arslanoglu, S.; Picaud, J.C. Recommendations for the establishment and operation of human milk banks in Europe: A consensus statement from the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- WHO; UNICEF. Premature Births. 2018. Available online: https://www.who.int/es/news-room/fact-sheets/detail/preterm-birth#:~:text=El%20problema,complicaciones%20en%20el%20parto%201 (accessed on 15 December 2019).
- Colaizy, T.T. Donor human milk for VLBWs: Patterns of usage, outcomes, and unanswered questions. Curr. Opin. Pediatr. 2015, 27, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verd, S.; Porta, R.; Botet, F.; Gutiérrez, A.; Ginovart, G.; Barbero, A.H.; Ciurana, A.; Plata, I.I. Hospital outcomes of extremely low birth weight infants after introduction of donor milk to supplement mother’s milk. Breastfeed. Med. 2015, 10, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Kleigman, R.M. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr. Clin N. Am. 1986, 33, 179. [Google Scholar] [CrossRef]
- Huston, R.K.; Markell, A.M.; McCulley, E.A.; Pathak, M.; Rogers, S.P.; Sweeney, S.L.; Dolphin, N.G.; Gardiner, S.K. Decreasing necrotizing enterocolitis and gastrointestinal bleeding in the neonatal intensive care unit: The role of donor human milk and exclusive human milk diets in infants _1500 g birth weight. Infant. Child Adolesc. Nutr. 2014, 6, 86–93. [Google Scholar] [CrossRef]
- Torres, J.; Espinosa, L.L.; García, Á.M.; Mideros, A.M.; Usubillaga, E. Características de recién nacidos con enterocolitis necrotizante en un hospital universitario de tercer nivel en Colombia. Colomb. Med. 2011, 42, 468–475. [Google Scholar] [CrossRef]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized Trial of Exclusive Human Milk versus Preterm Formula Diets in Extremely Premature Infants. J. Pediatr. 2013, 163, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Tonkin, E.; Damarell, R.A.; McPhee, A.J.; Suganuma, M.; Suganuma, H.; Middleton, P.F.; Makrides, M.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altobelli, E.; Angeletti, P.M.; Verrotti, A.; Petrocelli, R. The Impact of Human Milk on Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1322. [Google Scholar] [CrossRef] [PubMed]
- Van Gysel, M.; Cossey, V.; Fieuws, S.; Schuermans, A. Impact of pasteurization on the antibacterial properties of human milk. Eur. J. Pediatr. 2012, 171, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Dan, L.; Liu, S.; Shang, S.; Zhang, H.; Zhang, R.; Li, N. Expression of recombinant human lysozyme in bacterial artificial chromosome transgenic mice promotes the growth of Bifidobacterium and inhibits the growth of Salmonella in the intestine. J. Biotechnol. 2018, 272–273, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.S.; Martin, S.C.; Gómez-De-Orgaz, C.S. Human milk bank and personalized nutrition in the NICU: A narrative review. Eur. J. Pediatr. 2021, 180, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, N.M.; Chung, S.H. A retrospective study on the effects of exclusive donor human milk feeding in a short period after birth on morbidity and growth of preterm infants during hospitalization. Medicine 2017, 96, e7970. [Google Scholar] [CrossRef] [PubMed]
- Meinzen-Derr, J.; Poindexter, B.; Wrage, L.; Morrow, A.L.; Stoll, B.; Donovan, E.F. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J. Perinatol. 2008, 29, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisk, P.M.; A Lovelady, C.; Dillard, R.G.; Gruber, K.J.; O’Shea, T.M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. 2007, 27, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2019, 7, CD002971. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.A.; Henderson, G.; Anthony, M.Y.; McGuire, W.L. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2007, 17, CD002971. [Google Scholar] [CrossRef]
- Costeloe, K.L.; Hennessy, E.M.; Haider, S.; Stacey, F.; Marlow, N.; Draper, E. Short term outcomes after extreme preterm birth in England: Comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012, 345, e7976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Frequency (n = 722) | % | |
|---|---|---|---|
| Maternal variables | |||
| Age | <19 years | 103 | 14.27 |
| 19–35 | 548 | 75.90 | |
| >35 | 67 | 9.28 | |
| Scholarship | No schooling | twenty | 2.77 |
| Primary | 215 | 29.78 | |
| Secondary | 313 | 43.35 | |
| Further education | 122 | 16.90 | |
| With partner | Yes | 548 | 75.9 |
| Origin | Cali | 434 | 60.11 |
| Donor type | Homologous Donor * | 709 | 98.2 |
| Heterologous Donor ** | 13 | 1.8 | |
| Institutional Collection | HUV **** | 714 | 98.89 |
| Received beastfeeding education | Yes | 241 | 33.38 |
| Pregnancy | First | 304 | 42.11 |
| Prenatal controls | Suitable *** | 536 | 74.24 |
| Maternal pathologies | None | 156 | 21.61 |
| Hypertensive illness | 261 | 36.15 | |
| Infectious disease | 227 | 31.44 | |
| Other | 60 | 8.31 | |
| Newborn variables | |||
| Sex | Female | 358 | 49.58 |
| Male | 363 | 50.28 | |
| Gestational age | Equal to or less than 33 weeks | 366 | 50.69 |
| Between 34–37 | 356 | 49.31 | |
| Discharged | Alive | 685 | 94.88 |
| Dead | 36 | 4.99 | |
| Way of birth | Vaginal | 363 | 50.28 |
| Intubated | Yes | 685 | 94.88 |
| Hospitalized days | Less than 7 | 158 | 21.88 |
| 7 or more | 564 | 78.12 | |
| Necrotizing enterocolitis | Stage II | 9 | 1.25 |
| Stage III | 3 | 0.42 | |
| Intraventricular hemorrhage | Stage I | 28 | 3.88 |
| Stage II | 4 | 0.55 | |
| Stage III | 7 | 0.97 | |
| Bronchopulmonary dysplasia | Mild | 7 | 0.97 |
| Moderate | 13 | 1.8 | |
| Severe | 8 | 1.11 | |
| Retinopathy | Stage I | 11 | 1.52 |
| Stage II | 2 | 0.28 | |
| Late Sepsis | Yes | 275 | 38.09 |
| Breastfeeding variables | |||
| Start day of consumption of Breast milk | Three days | 503 | 69.67 |
| Between 4 to 7 days | 136 | 18.84 | |
| 8 and more days | 70 | 9.7 | |
| 100% Breast Milk Days | Three days | 230 | 31.86 |
| Between 4 to 7 days | 154 | 21.33 | |
| 8 and more days | 304 | 42.11 | |
| Type of milk received at 7 days old | Breastmilk | 412 | 57.06 |
| Artificial milk | 5 | 0.69 | |
| Mixed | 224 | 31.02 | |
| Variable | Type of Milk Received at 7 Days Old | p-Value | OR (95% CI) | ||
|---|---|---|---|---|---|
| Maternal n = 412 (%) | Other * n = 229 (%) | ||||
| Maternal age | <19 years | 43 (10.49) | 42 (18.50) | 0.005 | 0.51 (0.32–0.81) |
| 19 or more | 367 (89.51) | 185 (81.50) | |||
| Origin | Cali | 264 (64.08) | 132 (58.41) | 0.158 | 1.27 (0.91–1.77) |
| Outside of Cali | 148 (35.92) | 94 (41.59) | |||
| Education in breastfeeding | Received | 148 (36.54) | 70 (31.25) | 0.182 | 1.26 (0.89–1.79) |
| Pregnancy | First | 171 (41.50) | 93 (40.61) | 0.826 | 1.03 (0.74–1.44) |
| Gestational age | Equal to or less than 33 weeks | 165 (40.05) | 151 (65.94) | <0.001 | 2.89 (2.06–4.05) |
| Between 34–37 | 247 (59.95) | 78 (34.06) | |||
| Adequate prenatal control | Suitable * | 334 (82.67) | 148 (66.07) | <0.001 | 2.45 (1.67–3.57) |
| Intubation | If required | 403 (97.82) | 210 (91.70) | 0.001 | 4.05 (1.80–9.11) |
| Way of birth | Vaginal | 219 (53.16) | 104 (45.61) | 0.068 | 1.35 (0.97–1.87) |
| Days of hospitalization | Less than 7 | 141 (34.22) | 5 (2.18) | <0.001 | 23.30 (9.38–57.86) |
| Necrotizing enterocolitis | Yes | 1 (0.25) | 4 (1.87) | 0.07 | 0.13 (0.01–1.17) |
| Bronchopulmonary dysplasia | Yes | 6 (1.48) | 10 (4.69) | 0.023 | 0.30 (0.10–0.84) |
| Intraventricular hemorrhage | Yes | 12 (2.96) | 15 (7.04) | 0.022 | 0.40 (0.18–0.87) |
| Retinopathy | Yes | 5 (1.23) | 6 (2.82) | 0.168 | 0.43 (0.12–1.42) |
| Late sepsis | Yes | 121 (29.95) | 108 (50.70) | <0.001 | 0.41 (0.29–0.58) |
| 100% milk | before the 7th day | 331 (80.93) | 40 (18.35) | <0.001 | 18.88 (12.37–28.81) |
| Variable | Starting Days 100% Breast Milk | p-Value | OR (95% CI) | ||
|---|---|---|---|---|---|
| 7 or Less n = 384 (%) | 8 or More n = 304 (%) | ||||
| Maternal age | <19 years | 37 (9.66) | 57 (18.94) | 0.001 | 0.45 (0.29–0.71) |
| 19 or more | 346 (90.34) | 244 (81.06) | |||
| Origin | Cali | 250 (65.10) | 164 (54.49) | 0.005 | 1.55 (1.14–2.12) |
| Outside of Cali | 134 (34.90) | 137 (45.51) | |||
| Education in breastfeeding | Received | 137 (36.34) | 91 (30.23) | 0.095 | 1.31 (0.95–1.82) |
| Pregnancy | First | 162 (42.19) | 125 (41.12) | 0.778 | 1.04 (0.77–1.41) |
| Adequate prenatal control * | Suitable * | 308 (81.05) | 211 (71.53) | 0.004 | 1.70 (1.18–2.44) |
| Intubation | If required | 367 (95.57) | 291 (95.72) | 0.923 | 0.96 (0.46–2.01) |
| Gestational age | Equal to or less than 33 weeks | 129 (33.59) | 215 (70.72) | <0.001 | 4.77 (3.44–6.61) |
| Between 34–37 | 255 (66.41) | 89 (29.28) | |||
| Way of birth | Vaginal | 217 (56.66) | 133 (43.75) | 0.001 | 1.68 (1.24–2.27) |
| Days of hospitalization | Less than 7 | 150 (39.06) | 0 (0.00) | 1 | 1 |
| 7 or more | 234 (60.94) | 304 (100.00) | |||
| Necrotizing enterocolitis | Yes | 2 (0.54) | 9 (3.09) | 0.024 | 0.16 (0.03–0.79) |
| Dysplasia B ** | Yes | 1 (0.27) | 18 (6.16) | 0.002 | 0.04 (0.01–0.31) |
| Intraventricular Hemorrhage *** | Yes | 5 (1.34) | 29 (9.93) | <0.001 | 0.12 (0.04–0.32) |
| Retinopathy **** | Yes | 2 (0.54) | 10 (3.42) | 0.016 | 0.15 (0.03–0.69) |
| Late sepsis | Yes | 89 (23.99) | 168 (57.73) | <0.001 | 0.23 (0.16–0.32) |
| Variable | OR Crude (95% CI) | p-Value | Adjusted OR * (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Maternal age | <19 years | 0.51 (0.32–0.81) | 0.005 | 0.69 (0.41–1.18) | 0.179 |
| 19 or more | |||||
| Gestational age | Equal to or less than 33 weeks | 2.89 (2.06–4.05) | <0.001 | 1.55 (1.05–2.30) | 0.027 |
| Adequate prenatal control | Suitable ** | 2.45 (1.67–3.57) | <0.001 | 1.95 (1.26–3.01) | 0.003 |
| Intubation | If required | 4.05 (1.80–9.11) | 0.001 | 1.72 (0.42–6.97) | 0.441 |
| Dysplasia B | Yes | 0.30 (0.10–0.84) | 0.023 | 0.94 (0.31–2.88) | 0.926 |
| Intraventricular hemorrhage | Yes | 0.40 (0.18–0.87) | 0.022 | 0.84 (0.36–1.93) | 0.686 |
| Late sepsis | Yes | 0.41 (0.29–0.58) | <0.001 | 0.63 (0.43–0.93) | 0.023 |
| Variable | OR* Crude (95% CI) | p-Value | Adjusted OR ** (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Maternal age | <19 years | 0.45 (0.29–0.71) | 0.001 | 0.37 (0.22–0.64) | <0.001 |
| 19 or more | |||||
| Origin | Cali | 1.55 (1.14–2.12) | 0.005 | 1.45 (1.01–2.09) | 0.040 |
| Adequate prenatal control | Suitable *** | 1.70 (1.18–2.44) | 0.004 | 1.46 (0.94–2.27) | 0.086 |
| Way of birth | Vaginal | 1.68 (1.24–2.27) | 0.001 | 2.29 (1.58–3.33) | <0.001 |
| Necrotizing enterocolitis | Yes | 0.16 (0.03–0.79) | 0.024 | 0.18 (0.03–0.97) | 0.047 |
| Dysplasia B | Yes | 0.04 (0.005–0.308) | 0.002 | 0.13 (0.01–1.13) | 0.066 |
| Intraventricular hemorrhage | Yes | 0.12 (0.04–0.32) | <0.001 | 0.15 (0.05–0.45) | 0.001 |
| Retinopathy | Yes | 0.15 (0.03–0.69) | 0.016 | 0.51 (0.09–2.88) | 0.452 |
| Late sepsis | Yes | 0.23 (0.16–0.32) | <0.001 | 0.23 (0.15–0.33) | <0.001 |
| Weight | Human Milk | Artificial/Mixed | p-Value |
|---|---|---|---|
| n = 234 | n = 217 | ||
| Upon admission * | 1705 | 1545 | <0.01 |
| On the 7th day * | 1697 | 1570 | 0.06 |
| Upon discharge * | 1910 | 1930 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Muñoz, J.; Jimenez-Fernandez, C.A.; Murillo-Alvarado, J.; Torres-Figueroa, S.; Castro, J.P. Clinical Results of the Implementation of a Breast Milk Bank in Premature Infants (under 37 Weeks) at the Hospital Universitario del Valle 2018–2020. Nutrients 2021, 13, 2187. https://doi.org/10.3390/nu13072187
Torres-Muñoz J, Jimenez-Fernandez CA, Murillo-Alvarado J, Torres-Figueroa S, Castro JP. Clinical Results of the Implementation of a Breast Milk Bank in Premature Infants (under 37 Weeks) at the Hospital Universitario del Valle 2018–2020. Nutrients. 2021; 13(7):2187. https://doi.org/10.3390/nu13072187
Chicago/Turabian StyleTorres-Muñoz, Javier, Carlos Alberto Jimenez-Fernandez, Jennifer Murillo-Alvarado, Sofia Torres-Figueroa, and Juan Pablo Castro. 2021. "Clinical Results of the Implementation of a Breast Milk Bank in Premature Infants (under 37 Weeks) at the Hospital Universitario del Valle 2018–2020" Nutrients 13, no. 7: 2187. https://doi.org/10.3390/nu13072187







