Association of Plasma Phospholipids with Age-Related Cognitive Impairment: Results from a Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Design
2.2. Demographic Variables
2.3. Cognitive Assessment
2.4. Plasma Collection and Storage
2.5. Analysis of Biochemical Parameters
2.6. Analysis of Plasma Phospholipid Profile
2.6.1. Extraction of Total Phospholipid
2.6.2. Qualitative Analysis of Phospholipid Molecular Species in Plasma
2.6.3. Quantitative Analysis of Phospholipid Molecular Species in Plasma
2.7. Statistical Analysis
3. Results
3.1. General Characteristics of Participants
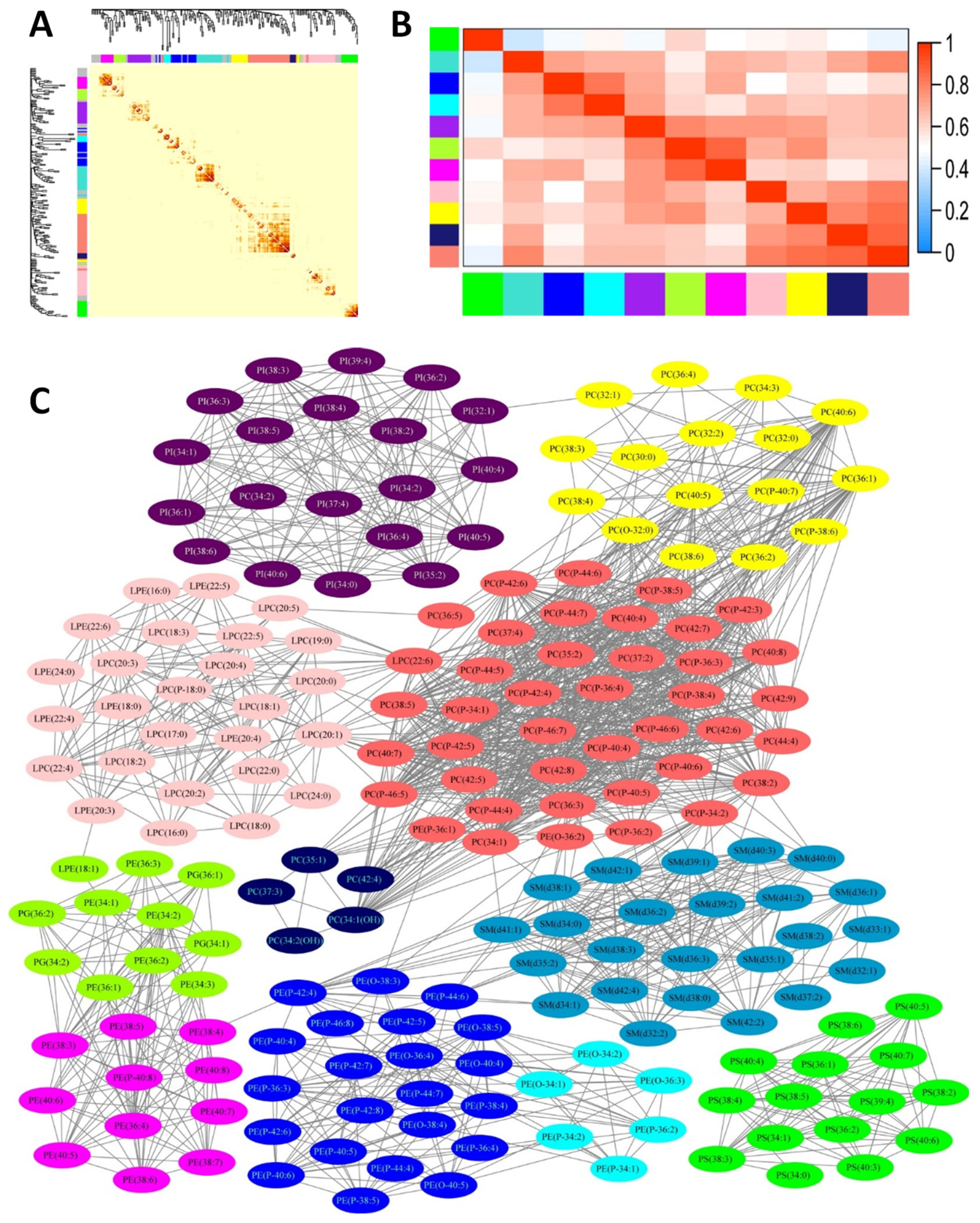
3.2. Characteristics of Plasma Phospholipids
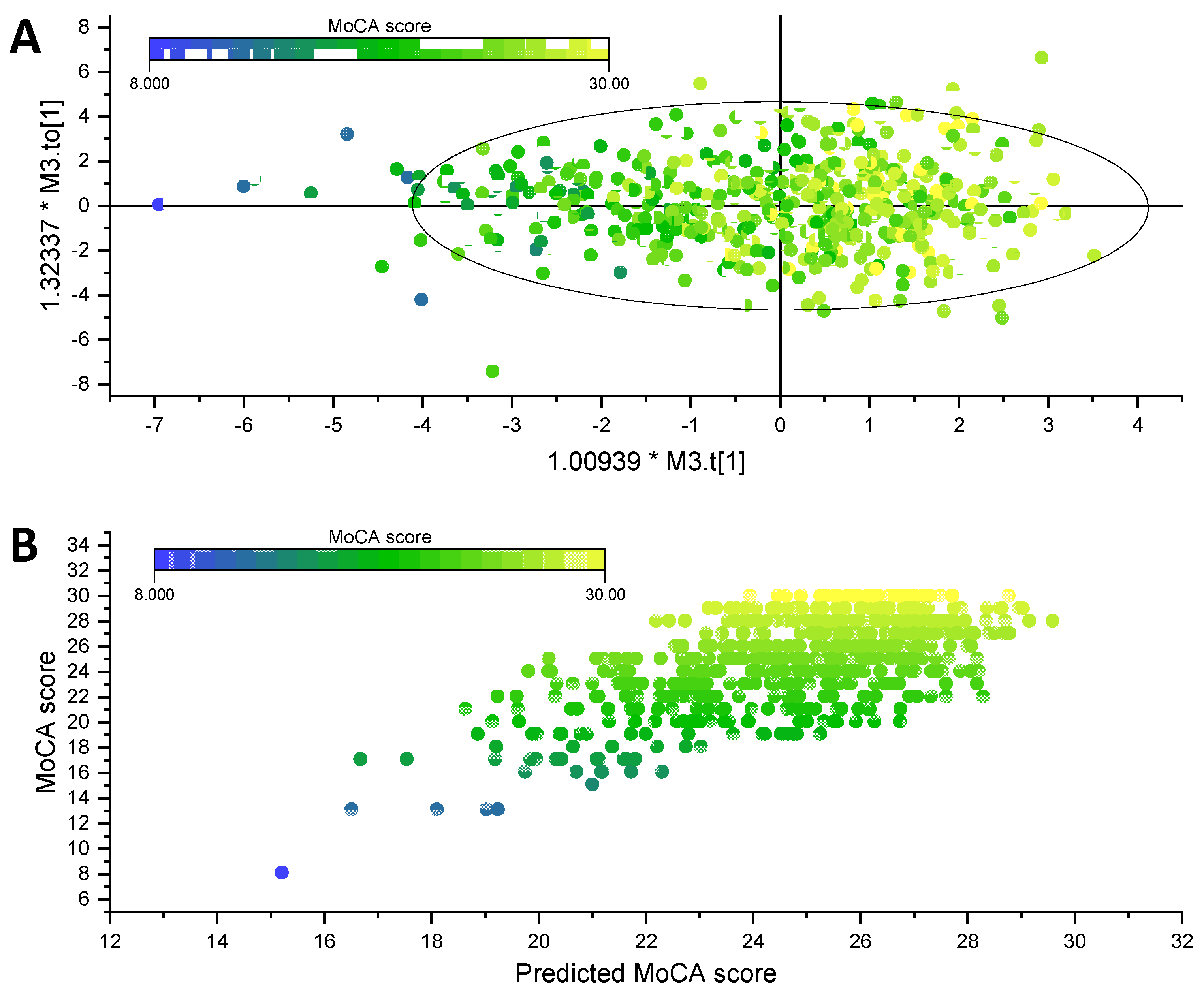
3.3. Association of Phospholipid Matrix with MoCA Score and Related Biochemical Parameters
3.4. Association of Phospholipid Clusters with MoCA Score
3.5. Association of Individual Phospholipid Molecular Species and MoCA Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.J.; Wu, F.; Guo, Y.; Robledo, L.M.G.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Sacuiu, S.F. Chapter 8—Dementias. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 138, pp. 123–151. [Google Scholar]
- Schmidt, D.; Jiang, Q.-X.; MacKinnon, R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature 2006, 444, 775–779. [Google Scholar] [CrossRef]
- Aureli, M.; Grassi, S.; Prioni, S.; Sonnino, S.; Prinetti, A. Lipid membrane domains in the brain. Biochim. Biophys. Acta 2015, 1851, 1006–1016. [Google Scholar] [CrossRef]
- García-Morales, V.; Montero, F.; González-Forero, D.; Rodríguez-Bey, G.; Gómez-Pérez, L.; Medialdea-Wandossell, M.J.; Domínguez-Vías, G.; García-Verdugo, J.M.; Moreno-López, B. Membrane-Derived Phospholipids Control Synaptic Neurotransmission and Plasticity. PLoS Biol. 2015, 13, e1002153. [Google Scholar] [CrossRef] [Green Version]
- Kosicek, M.; Hecimovic, S. Phospholipids and Alzheimer’s disease: Alterations, mechanisms and potential biomarkers. Int. J. Mol. Sci. 2013, 14, 1310–1322. [Google Scholar] [CrossRef]
- He, X.; Huang, Y.; Li, B.; Gong, C.-X.; Schuchman, E.H. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol. Aging 2010, 31, 398–408. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Holtzman, D.M.; McKeel, D.W., Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: Molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J. Alzheimers Dis. 2011, 24, 507–517. [Google Scholar] [CrossRef]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef]
- Toledo, J.B.; Arnold, M.; Kastenmüller, G.; Chang, R.; Baillie, R.A.; Han, X.; Thambisetty, M.; Tenenbaum, J.D.; Suhre, K.; Thompson, J.W.; et al. Metabolic network failures in Alzheimer’s disease: A biochemical road map. Alzheimers Dement. 2017, 13, 965–984. [Google Scholar] [CrossRef]
- Wood, P.L.; Mankidy, R.; Ritchie, S.; Heath, D.; Wood, J.A.; Flax, J.; Goodenowe, D.B. Circulating plasmalogen levels and Alzheimer Disease Assessment Scale-Cognitive scores in Alzheimer patients. J. Psychiatry Neurosci. 2010, 35, 59–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St John-Williams, L.; Blach, C.; Toledo, J.B.; Rotroff, D.M.; Kim, S.; Klavins, K.; Baillie, R.; Han, X.; Mahmoudiandehkordi, S.; Jack, J.; et al. Targeted metabolomics and medication classification data from participants in the ADNI1 cohort. Sci. Data 2017, 4, 170140. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Rozen, S.; Boyle, S.H.; Hellegers, C.; Cheng, H.; Burke, J.R.; Welsh-Bohmer, K.A.; Doraiswamy, P.M.; Kaddurah-Daouk, R. Metabolomics in early Alzheimer’s disease: Identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS ONE 2011, 6, e21643. [Google Scholar] [CrossRef]
- Chatterjee, P.; Lim, W.L.F.; Shui, G.; Gupta, V.B.; James, I.; Fagan, A.M.; Xiong, C.; Sohrabi, H.R.; Taddei, K.; Brown, B.M.; et al. Plasma Phospholipid and Sphingolipid Alterations in Presenilin1 Mutation Carriers: A Pilot Study. J. Alzheimers Dis. 2016, 50, 887–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Cheong, L.-Z.; Man, Q.-Q.; Pang, S.-J.; Li, Y.-Q.; Ren, B.; Zhang, J. Characterization of potential plasma biomarkers related to cognitive impairment by untargeted profiling of phospholipids using the HILIC-ESI-IT-TOF-MS system. Anal. Bioanal. Chem. 2018, 410, 2937–2948. [Google Scholar] [CrossRef]
- Song, S.; Liu, T.-T.; Liang, X.; Liu, Z.-Y.; Yishake, D.; Lu, X.-T.; Yang, M.-T.; Man, Q.-Q.; Zhang, J.; Zhu, H.-L. Profiling of phospholipid molecular species in human breast milk of Chinese mothers and comprehensive analysis of phospholipidomic characteristics at different lactation stages. Food Chem. 2021, 348, 129091. [Google Scholar] [CrossRef]
- Goodenowe, D.B.; Cook, L.L.; Liu, J.; Lu, Y.; Jayasinghe, D.A.; Ahiahonu, P.W.K.; Heath, D.; Yamazaki, Y.; Flax, J.; Krenitsky, K.F.; et al. Peripheral ethanolamine plasmalogen deficiency: A logical causative factor in Alzheimer’s disease and dementia. J. Lipid Res. 2007, 48, 2485–2498. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.L.; Locke, V.A.; Herling, P.; Passaro, A.; Vigna, G.B.; Volpato, S.; Valacchi, G.; Cervellati, C.; Zuliani, G. Targeted lipidomics distinguishes patient subgroups in mild cognitive impairment (MCI) and late onset Alzheimer’s disease (LOAD). BBA Clin. 2016, 5, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Goodenowe, D.B.; Senanayake, V. Relation of Serum Plasmalogens and APOE Genotype to Cognition and Dementia in Older Persons in a Cross-Sectional Study. Brain Sci. 2019, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.L.; Barnette, B.L.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer’s disease subjects. Acta Neuropsychiatr. 2015, 27, 270–278. [Google Scholar] [CrossRef]
- Klouwer, F.C.C.; Berendse, K.; Ferdinandusse, S.; Wanders, R.J.A.; Engelen, M.; Poll-The, B.T. Zellweger spectrum disorders: Clinical overview and management approach. Orphanet J. Rare Dis. 2015, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Berger, J.; Dorninger, F.; Forss-Petter, S.; Kunze, M. Peroxisomes in brain development and function. Biochim. Biophys. Acta 2016, 1863, 934–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, M.O.; Kuchenbecker, J.; Rothhaar, T.L.; Grösgen, S.; Hundsdörfer, B.; Burg, V.K.; Friess, P.; Müller, U.; Grimm, H.S.; Riemenschneider, M.; et al. Plasmalogen synthesis is regulated via alkyl-dihydroxyacetonephosphate-synthase by amyloid precursor protein processing and is affected in Alzheimer’s disease. J. Neurochem. 2011, 116, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A. Studies on plasmalogen-selective phospholipase A2 in brain. Mol. Neurobiol. 2010, 41, 267–273. [Google Scholar] [CrossRef]
- Rodemer, C.; Thai, T.P.; Brugger, B.; Kaercher, T.; Werner, H.; Nave, K.A.; Wieland, F.; Gorgas, K.; Just, W.W. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum. Mol. Genet. 2003, 12, 1881–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luoma, A.M.; Kuo, F.; Cakici, O.; Crowther, M.N.; Denninger, A.R.; Avila, R.L.; Brites, P.; Kirschner, D.A. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic. Biol. Med. 2015, 84, 296–310. [Google Scholar] [CrossRef]
- Miville-Godbout, E.; Bourque, M.; Morissette, M.; Al-Sweidi, S.; Smith, T.; Mochizuki, A.; Senanayake, V.; Jayasinghe, D.; Wang, L.; Goodenowe, D.; et al. Plasmalogen Augmentation Reverses Striatal Dopamine Loss in MPTP Mice. PLoS ONE 2016, 11, e0151020. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.; Phillipps, A.; Woltjer, R.L.; Kaye, J.; Quinn, J. Increased lysophosphatidylethanolamine and diacylglycerol levels in Alzheimer’s disease plasma. JSM Alzheimer’s Dis. Relat. Dement. 2014, 1, 1001. [Google Scholar]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef]
- Li, D.; Misialek, J.R.; Boerwinkle, E.; Gottesman, R.F.; Sharrett, A.R.; Mosley, T.H.; Coresh, J.; Wruck, L.M.; Knopman, D.S.; Alonso, A. Plasma phospholipids and prevalence of mild cognitive impairment and/or dementia in the ARIC Neurocognitive Study (ARIC-NCS). Alzheimers Dement. 2016, 3, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Li, N.J.; Liu, W.T.; Li, W.; Li, S.Q.; Chen, X.H.; Bi, K.S.; He, P. Plasma metabolic profiling of Alzheimer’s disease by liquid chromatography/mass spectrometry. Clin. Biochem. 2010, 43, 992–997. [Google Scholar] [CrossRef]
- Tsukahara, T.; Matsuda, Y.; Haniu, H. Lysophospholipid-Related Diseases and PPARγ Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 2730. [Google Scholar] [CrossRef] [Green Version]
- Mielke, M.M.; Bandaru, V.V.R.; Haughey, N.J.; Rabins, P.V.; Lyketsos, C.G.; Carlson, M.C. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol. Aging 2010, 31, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Lagace, T.A. Phosphatidylcholine: Greasing the Cholesterol Transport Machinery. Lipid Insights 2016, 8, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, Å.; Duan, R.-D. Absorption and lipoprotein transport of sphingomyelin. J. Lipid Res. 2006, 47, 154–171. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Yin, Z.; Zhu, P.; Luo, J.; Shi, X.; Gao, X. Blood cholesterol in late-life and cognitive decline: A longitudinal study of the Chinese elderly. Mol. Neurodegener. 2017, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Glade, M.J.; Smith, K. Phosphatidylserine and the human brain. Nutrition 2015, 31, 781–786. [Google Scholar] [CrossRef]
| N (%) or Mean ± SD 1 or Median (IQR) 2 | |
|---|---|
| Sociodemographic variables | |
| Gender (male; n) | 230 (36.7%) |
| Ethnicity (Han; n) | 617 (98.6%) |
| Age (years) | 64 (61–69) |
| Education level (n) | |
| ≤6 years | 145 (23.2%) |
| 7–9 years | 364 (58.1%) |
| >9 years | 117 (18.7%) |
| MoCA score | 25 (22–28) |
| Glu (mmol/L) | 5.3 (4.9–6.0) |
| Lipidemia variables | |
| TC (mmol/L) | 5.2 ± 0.9 |
| TG (mmol/L) | 1.2 (0.9–1.8) |
| LDL-C (mmol/L) | 3.1 ± 0.9 |
| HDL-C (mmol/L) | 1.2 (1.0–1.4) |
| Lipoproteins | |
| Lp(a) (mg/L) | 111.0 (54.5–265.4) |
| Apo A1 (g/L) | 1.5 ± 0.3 |
| Apo B (g/L) | 0.9 ± 0.2 |
| Apo E (mg/dL) | 4.0 (3.2, 4.8) |
| Inflammatory variables | |
| CRP (mg/L) | 0.9 (0.4, 1.8) |
| TNF-a (pmol/L) | 10.2 (7.7, 14.5) |
| IL-6 (pg/mL) | 102.5 (68.1, 145.7) |
| Hcy (μmol/L) | 16.1 (13.0, 20.7) |
| Molecular Species (n) | Concentration (mg/L) | |||
|---|---|---|---|---|
| Median | Q1 1 | Q3 2 | ||
| PC | 62 | 1416.6 | 1155 | 1649.6 |
| plasmanyl/plasmenyl PC | 24 | 69.9 | 51.8 | 89.8 |
| PE | 55 | 42.9 | 33.5 | 54.7 |
| plasmanyl/plasmenyl PE | 39 | 7.3 | 5.9 | 8.9 |
| SM | 30 | 371.4 | 300.3 | 0.5 |
| PS | 22 | 1.6 | 1.3 | 2391.5 |
| PI | 19 | 35.3 | 27.1 | 46 |
| PG | 5 | 0.6 | 0.4 | 0.8 |
| PA | 3 | 0.3 | 0.2 | 2.3 |
| LPC | 22 | 135.4 | 99.3 | 464.6 |
| LPE | 11 | 4.1 | 3.2 | 175.3 |
| Total | 229 | 2046.7 | 1700.6 | 2391.7 |
| R2 | SStrace | Pseudo-F | p | |
|---|---|---|---|---|
| MoCA | 0.054 | 92.173 | 4.278 | <0.001 |
| Glu | 0.003 | 35.820 | 1.950 | 0.063 |
| TC | 0.066 | 931.140 | 46.375 | <0.001 |
| TG | 0.027 | 374.060 | 19.177 | <0.001 |
| LDL-C | 0.010 | 149.630 | 7.755 | <0.001 |
| HDL-C | 0.004 | 52.088 | 2.707 | 0.019 |
| Lp(a) | 0.002 | 33.893 | 1.764 | 0.087 |
| Apo A1 | 0.019 | 267.110 | 14.198 | <0.001 |
| Apo B | 0.017 | 246.920 | 13.389 | <0.001 |
| Apo E | 0.004 | 45.640 | 2.481 | 0.024 |
| CRP | 0.002 | 29.301 | 1.597 | 0.116 |
| TNF-α | 0.002 | 28.265 | 1.542 | 0.141 |
| IL-6 | 0.001 | 17.332 | 0.945 | 0.422 |
| Hcy | 0.002 | 29.396 | 1.605 | 0.102 |
| Module | Module Eigengenes | Spearman’s Correlation | |||
|---|---|---|---|---|---|
| Median | Q1 | Q3 | rs | p | |
| Blue | −0.001 | −0.029 | 0.027 | 0.140 | 0.001 |
| Cyan | −0.004 | −0.028 | 0.025 | 0.047 | 0.239 |
| Magenta | −0.003 | −0.031 | 0.028 | 0.089 | 0.027 |
| Salmon | 0.000 | −0.032 | 0.028 | −0.010 | 0.796 |
| Yellow | −0.006 | −0.027 | 0.022 | 0.059 | 0.142 |
| Midnightblue | −0.006 | −0.029 | 0.027 | −0.050 | 0.210 |
| Green | −0.008 | −0.024 | 0.010 | 0.047 | 0.240 |
| Purple | −0.002 | −0.028 | 0.028 | 0.024 | 0.548 |
| Turquoise | −0.003 | −0.030 | 0.029 | 0.056 | 0.166 |
| Pink | 0.001 | −0.033 | 0.031 | −0.075 | 0.060 |
| Greenyellow | −0.001 | −0.029 | 0.027 | 0.048 | 0.236 |
| Class | Molecular Species | Spearman’s Rank Correlation Coefficient | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||
| PE | PE(P-46:8) | 0.119 * | 0.104 * | 0.112 * | 0.102 * | 0.100 * |
| PE(P-46:7) | 0.149 * | 0.137 * | 0.130 * | 0.115 * | 0.114 * | |
| PE(P-44:8) | 0.108 * | 0.092 * | 0.094 * | 0.081 * | 0.079 * | |
| PE(P-44:7) | 0.135 * | 0.117 * | 0.122 * | 0.107 * | 0.107 * | |
| PE(P-44:6) | 0.108 * | 0.091 * | 0.101 * | 0.092 * | 0.092 * | |
| PE(P-44:5) | 0.099 * | 0.086 * | 0.086 * | 0.072 * | 0.072 * | |
| PE(P-42:8) | 0.099 * | 0.076 | 0.077 | 0.060 | 0.062 | |
| PE(P-42:7) | 0.108 * | 0.086 * | 0.086 * | 0.071 * | 0.073 * | |
| PE(P-42:6) | 0.134 * | 0.112 * | 0.112 * | 0.097 * | 0.100 * | |
| PE(P-42:5) | 0.125 * | 0.102 * | 0.103 * | 0.093 * | 0.095 * | |
| PE(P-42:4) | 0.104 * | 0.084 * | 0.089 * | 0.08 * | 0.081 * | |
| PE(P-40:8) | 0.082 * | 0.074 | 0.075 | 0.061 | 0.060 | |
| PE(P-40:7) | 0.138 * | 0.120 * | 0.114 * | 0.103 * | 0.105 * | |
| PE(P-40:6) | 0.119 * | 0.095 * | 0.088 * | 0.075 * | 0.079 * | |
| PE(P-40:5) | 0.105 * | 0.083 * | 0.073 | 0.060 | 0.064 | |
| PE(P-40:4) | 0.093 * | 0.066 | 0.064 | 0.044 | 0.048 | |
| PE(P-38:6) | 0.134 * | 0.122 * | 0.115 * | 0.103 * | 0.104 * | |
| PE(P-38:5) | 0.109 * | 0.084 * | 0.075 | 0.060 | 0.064 | |
| PE(O-40:4) | 0.079 * | 0.053 | 0.052 | 0.035 | 0.037 | |
| PE(40:7) | 0.082 * | 0.079 | 0.076 | 0.066 | 0.068 | |
| PE(40:6) | 0.080 * | 0.086 * | 0.088 * | 0.089 * | 0.092 * | |
| PE(38:6) | 0.090 * | 0.089 * | 0.093 * | 0.093 * | 0.095 * | |
| PC | PC(O-32:0) | 0.096 * | 0.072 | 0.079 | 0.081 | 0.083 |
| PC(36:2) | 0.079 * | 0.057 | 0.059 | 0.068 | 0.068 | |
| PC(35:3) | 0.119 * | 0.104 * | 0.102 * | 0.104 * | 0.104 * | |
| PC(32:0) | 0.079 * | 0.047 | 0.055 | 0.056 | 0.058 | |
| SM | SM(d42:2) | 0.097 * | 0.073 | 0.076 | 0.074 | 0.073 |
| SM(d40:2) | 0.097 * | 0.068 | 0.067 | 0.063 | 0.064 | |
| SM(d38:2) | 0.105 * | 0.076 | 0.076 | 0.080 | 0.081 | |
| SM(d38:0) | 0.082 * | 0.056 | 0.051 | 0.050 | 0.052 | |
| SM(d37:2) | 0.084 * | 0.059 | 0.051 | 0.049 | 0.051 | |
| SM(d36:1) | 0.143 * | 0.126 * | 0.117 * | 0.113 * | 0.114 * | |
| SM(d35:1) | 0.116 * | 0.099 * | 0.090 * | 0.076 | 0.078 | |
| SM(d34:2) | 0.100 * | 0.077 | 0.072 | 0.054 | 0.054 | |
| SM(d34:1) | 0.104 * | 0.087 * | 0.088 * | 0.076 | 0.076 | |
| SM(d33:1) | 0.113 * | 0.094 * | 0.081 * | 0.057 | 0.060 | |
| PS | PS(36:5) | 0.104 * | 0.092 * | 0.081 * | 0.062 | 0.062 |
| PS(36:1) | 0.096 * | 0.090 * | 0.082 * | 0.079 | 0.081 | |
| PS(34:3) | 0.150 * | 0.147 * | 0.139 * | 0.129 * | 0.132 * | |
| PS(32:0) | 0.096 * | 0.097 * | 0.089 * | 0.068 | 0.068 | |
| PI | PI(35:2) | −0.087 * | −0.121 * | −0.110 * | −0.114 * | −0.114 * |
| LPE | LPE(16:0) | −0.103 * | −0.125 * | −0.116 * | −0.098 * | −0.098 * |
| LPC | LPC(24:0) | −0.098 * | −0.116 * | −0.106 * | −0.096 * | −0.095 * |
| LPC(22:0) | −0.106 * | −0.121 * | −0.109 * | −0.098 * | −0.098 * | |
| LPC(16:1) | −0.094 * | −0.078 * | −0.086 * | −0.088 * | −0.087 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-T.; Pang, S.-J.; Jia, S.-S.; Man, Q.-Q.; Li, Y.-Q.; Song, S.; Zhang, J. Association of Plasma Phospholipids with Age-Related Cognitive Impairment: Results from a Cross-Sectional Study. Nutrients 2021, 13, 2185. https://doi.org/10.3390/nu13072185
Liu T-T, Pang S-J, Jia S-S, Man Q-Q, Li Y-Q, Song S, Zhang J. Association of Plasma Phospholipids with Age-Related Cognitive Impairment: Results from a Cross-Sectional Study. Nutrients. 2021; 13(7):2185. https://doi.org/10.3390/nu13072185
Chicago/Turabian StyleLiu, Ting-Ting, Shao-Jie Pang, Shan-Shan Jia, Qing-Qing Man, Yu-Qian Li, Shuang Song, and Jian Zhang. 2021. "Association of Plasma Phospholipids with Age-Related Cognitive Impairment: Results from a Cross-Sectional Study" Nutrients 13, no. 7: 2185. https://doi.org/10.3390/nu13072185
APA StyleLiu, T.-T., Pang, S.-J., Jia, S.-S., Man, Q.-Q., Li, Y.-Q., Song, S., & Zhang, J. (2021). Association of Plasma Phospholipids with Age-Related Cognitive Impairment: Results from a Cross-Sectional Study. Nutrients, 13(7), 2185. https://doi.org/10.3390/nu13072185







