Dietary Patterns and Risk Factors of Frailty in Lebanese Older Adults
Abstract
:1. Introduction
2. Materials and Methods
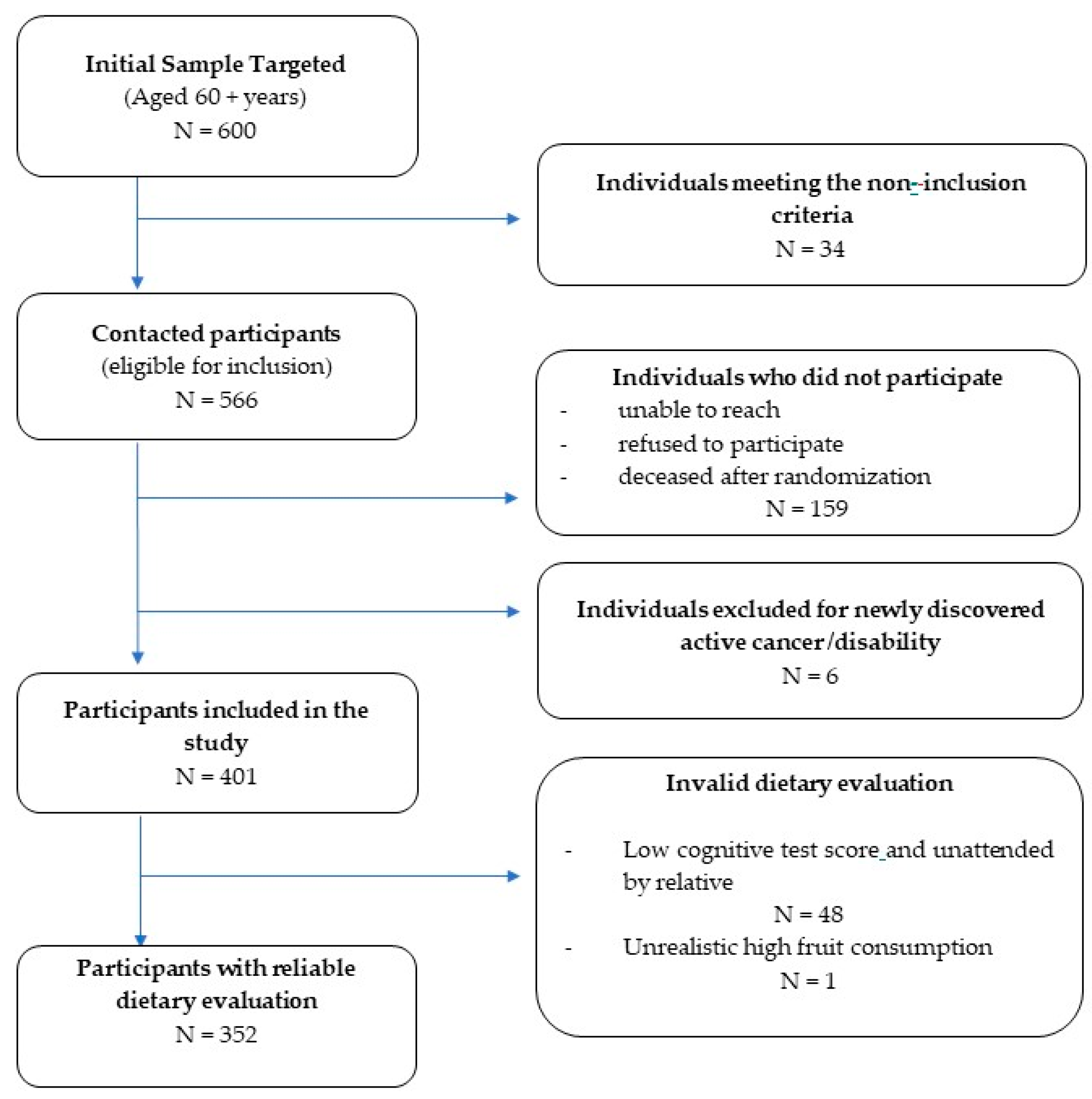
2.1. Study Design and Participants
2.2. Data Collected
2.2.1. Frailty and FRAIL Scale
2.2.2. Sociodemographic and Health-Related Data
2.2.3. Anthropometric and Functional Measurements
2.2.4. Nutritional and Dietary Data
2.3. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Dietary Patterns
3.3. Frailty
3.3.1. Frailty Association with Sociodemographic and Health-Related Factors
3.3.2. Frailty and Dietary Patterns
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Verlaan, S.; Ligthart-Melis, G.C.; Wijers, S.L.J.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. High Prevalence of Physical Frailty Among Community-Dwelling Malnourished Older Adults—A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2017, 18, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Crow, R.S.; Lohman, M.C.; Pidgeon, D.; Bruce, M.L.; Bartels, S.J.; Batsis, J.A. Frailty Versus Stopping Elderly Accidents, Deaths and Injuries Initiative Fall Risk Score: Ability to Predict Future Falls. J. Am. Geriatr. Soc. 2018, 66, 577–583. [Google Scholar] [CrossRef]
- Marques, A.; Queirós, C. Frailty, Sarcopenia and Falls. In Fragility Fracture Nursing; Hertz, K., Santy-Tomlinson, J., Eds.; Perspectives in Nursing Management and Care for Older Adults; Springer International Publishing: Cham, Switzerland, 2018; pp. 15–26. ISBN 978-3-319-76680-5. [Google Scholar]
- Morley, J.E. The New Geriatric Giants. Clin. Geriatr. Med. 2017, 33, xi–xii. [Google Scholar] [CrossRef] [Green Version]
- Kojima, G. Frailty as a Predictor of Disabilities among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Disabil. Rehabil. 2017, 39, 1897–1908. [Google Scholar] [CrossRef]
- Nwagwu, V.C.; Cigolle, C.; Suh, T. Reducing Frailty to Promote Healthy Aging. Clin. Geriatr. Med. 2020, 36, 613–630. [Google Scholar] [CrossRef]
- Lorenzo-López, L.; López-López, R.; Maseda, A.; Buján, A.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Changes in Frailty Status in a Community-Dwelling Cohort of Older Adults: The VERISAÚDE Study. Maturitas 2019, 119, 54–60. [Google Scholar] [CrossRef]
- Zamudio-Rodríguez, A.; Letenneur, L.; Féart, C.; Avila-Funes, J.A.; Amieva, H.; Pérès, K. The Disability Process: Is There a Place for Frailty? Age Ageing 2020, 49, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Cohen, A.A.; Xue, Q.-L.; Walston, J.; Bandeen-Roche, K.; Varadhan, R. The Physical Frailty Syndrome as a Transition from Homeostatic Symphony to Cacophony. Nat. Aging 2021, 1, 36–46. [Google Scholar] [CrossRef]
- Kojima, G.; Walters, K.; Iliffe, S.; Taniguchi, Y.; Tamiya, N. Kojima Gotaro Marital Status and Risk of Physical Frailty: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2020, 21, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Kingston, A.; Davies, K.; Collerton, J.; Robinson, L.; Duncan, R.; Kirkwood, T.B.L.; Jagger, C. The Enduring Effect of Education-Socioeconomic Differences in Disability Trajectories from Age 85 Years in the Newcastle 85+ Study. Arch. Gerontol. Geriatr. 2015, 60, 405–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soysal, P.; Veronese, N.; Thompson, T.; Kahl, K.G.; Fernandes, B.S.; Prina, A.M.; Solmi, M.; Schofield, P.; Koyanagi, A.; Tseng, P.T.; et al. Pinar Soysal Relationship between Depression and Frailty in Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2017, 36, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Valencia, M.; Izquierdo, M.; Cesari, M.; Casas-Herrero, Á.; Inzitari, M.; Martínez-Velilla, N. The Relationship between Frailty and Polypharmacy in Older People: A Systematic Review: Frailty and Polypharmacy: A Systematic Review. Br. J. Clin. Pharmacol. 2018, 84, 1432–1444. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-López, L.; Maseda, A.; de Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional Determinants of Frailty in Older Adults: A Systematic Review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, R.E. Sex Differences in Frailty. Frailty Aging 2015, 41, 41–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, H.; Gu, H.; Zhao, X. Gender-Associated Factors for Frailty and Their Impact on Hospitalization and Mortality among Community-Dwelling Older Adults: A Cross-Sectional Population-Based Study. PeerJ 2018, 6, e4326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults. JAMA Netw. Open 2019, 2. [Google Scholar] [CrossRef] [Green Version]
- Boulos, C.; Salameh, P.; Barberger-Gateau, P. Malnutrition and Frailty in Community Dwelling Older Adults Living in a Rural Setting. Clin. Nutr. 2016, 35, 138–143. [Google Scholar] [CrossRef]
- Kizilarslanoglu, M.C.; Sumer, F.; Kuyumcu, M.E. Malnutrition Increases Frailty among Older Adults: How? Clin. Nutr. 2016, 35, 979. [Google Scholar] [CrossRef]
- Amiri, S.; Behnezhad, S.; Hasani, J. Amiri Sohrab Body Mass Index and Risk of Frailty in Older Adults: A Systematic Review and Meta-Analysis. Obes. Med. 2020, 18, 100196. [Google Scholar] [CrossRef]
- Watanabe, D.; Yoshida, T.; Watanabe, Y.; Yamada, Y.; Kimura, M. A U-Shaped Relationship between the Prevalence of Frailty and Body Mass Index in Community-Dwelling Japanese Older Adults: The Kyoto–Kameoka Study. J. Clin. Med. 2020, 9, 1367. [Google Scholar] [CrossRef]
- Crow, R.S.; Petersen, C.L.; Cook, S.B.; Stevens, C.J.; Titus, A.J.; Mackenzie, T.A.; Batsis, J.A. Weight Change in Older Adults and Risk of Frailty. J. Frailty Aging. 2020, 9, 74–81. [Google Scholar]
- Tabue-Teguo, M.; Perès, K.; Simo, N.; Le Goff, M.; Perez Zepeda, M.U.; Féart, C.; Dartigues, J.-F.; Amieva, H.; Cesari, M. Gait Speed and Body Mass Index: Results from the AMI Study. PLoS ONE 2020, 15, e0229979. [Google Scholar] [CrossRef]
- Rietman, M.L.; van der A, D.L.; van Oostrom, S.H.; Picavet, H.S.J.; Dollé, M.E.T.; van Steeg, H.; Verschuren, W.M.M.; Spijkerman, A.M.W. The Association Between BMI and Different Frailty Domains: A U-Shaped Curve? J. Nutr. Health Aging 2018, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.F.; Stephens, C.R.; Román-Sicilia, H.; Cesari, M.; Pérez-Zepeda, M.U. Anthropometric Measurements and Mortality in Frail Older Adults. Exp. Gerontol. 2018, 110, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, N.Y.; Krakauer, J.C. Association of Body Shape Index (ABSI) with Hand Grip Strength. Int. J. Environ. Res. Public Health 2020, 17, 6797. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, Y.; Kim, E.-Y.; Park, Y. Mediating Effect of Waist: Height Ratio on the Association between BMI and Frailty: The Korean Frailty and Aging Cohort Study. Available online: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/mediating-effect-of-waistheight-ratio-on-the-association-between-bmi-and-frailty-the-korean-frailty-and-aging-cohort-study/CCF95088B753D1ED366C8C436D963F3F (accessed on 17 October 2020).
- Coelho-Júnior, H.; Rodrigues, B.; Uchida, M.; Marzetti, E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval-Insausti, H.; Pérez-Tasigchana, R.F.; López-García, E.; García-Esquinas, E.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Macronutrients Intake and Incident Frailty in Older Adults: A Prospective Cohort Study. J. Gerontol. Ser. A 2016, 71, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Nanri, H.; Yamada, Y.; Yoshida, T.; Okabe, Y.; Nozawa, Y.; Itoi, A.; Yoshimura, E.; Watanabe, Y.; Yamaguchi, M.; Yokoyama, K.; et al. Sex Difference in the Association Between Protein Intake and Frailty: Assessed Using the Kihon Checklist Indexes Among Older Adults. J. Am. Med. Dir. Assoc. 2018, 19, 801–805. [Google Scholar] [CrossRef]
- Rahi, B.; Colombet, Z.; Gonzalez-Colaço Harmand, M.; Dartigues, J.-F.; Boirie, Y.; Letenneur, L.; Feart, C. Higher Protein but Not Energy Intake Is Associated With a Lower Prevalence of Frailty Among Community-Dwelling Older Adults in the French Three-City Cohort. J. Am. Med. Dir. Assoc. 2016, 17, 672.e7–672.e11. [Google Scholar] [CrossRef] [PubMed]
- Rashidi Pour Fard, N.; Amirabdollahian, F.; Haghighatdoost, F. Dietary Patterns and Frailty: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Walters, K. Adherence to Mediterranean Diet Reduces Incident Frailty Risk: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2018, 66, 783–788. [Google Scholar] [CrossRef]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Dietary Quality Is Related to Frailty in Community-Dwelling Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntanasi, E.; Yannakoulia, M.; Kosmidis, M.-H.; Anastasiou, C.A.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Scarmeas, N. Adherence to Mediterranean Diet and Frailty. J. Am. Med. Dir. Assoc. 2018, 19, 315–322.e2. [Google Scholar] [CrossRef] [PubMed]
- Machón, M.; Mateo-Abad, M.; Vrotsou, K.; Zupiria, X.; Güell, C.; Rico, L.; Vergara, I. Dietary Patterns and Their Relationship with Frailty in Functionally Independent Older Adults. Nutrients 2018, 10, 406. [Google Scholar] [CrossRef] [Green Version]
- Parsons, T.J.; Papachristou, E.; Atkins, J.L.; Papacosta, O.; Ash, S.; Lennon, L.T.; Whincup, P.H.; Ramsay, S.E.; Wannamethee, S.G. Physical Frailty in Older Men: Prospective Associations with Diet Quality and Patterns. Age Ageing 2019, 48, 355–360. [Google Scholar] [CrossRef]
- de Haas, S.C.M.; de Jonge, E.A.L.; Voortman, T.; Graaff, J.S.; Franco, O.H.; Ikram, M.A.; Rivadeneira, F.; Kiefte-de Jong, J.C.; Schoufour, J.D. Dietary Patterns and Changes in Frailty Status: The Rotterdam Study. Eur. J. Nutr. 2018, 57, 2365–2375. [Google Scholar] [CrossRef] [Green Version]
- Pilleron, S.; Ajana, S.; Jutand, M.-A.; Helmer, C.; Dartigues, J.-F.; Samieri, C.; Féart, C. Dietary Patterns and 12-Year Risk of Frailty: Results From the Three-City Bordeaux Study. J. Am. Med. Dir. Assoc. 2017, 18, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [Green Version]
- Siriwardhana, D.D.; Hardoon, S.; Rait, G.; Weerasinghe, M.C.; Walters, K.R. Prevalence of Frailty and Prefrailty among Community-Dwelling Older Adults in Low-Income and Middle-Income Countries: A Systematic Review and Meta-Analysis. BMJ Open 2018, 8, e018195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Zoghbi, M.; Boulos, C.; Awada, S.; Rachidi, S.; All Hajje, A.; Bawab, W.; Saleh, N.; Salameh, P. Prevalence of Malnutrition and Its Correlates in Older Adults Living in Long Stay Institutions Situated in Beirut, Lebanon. J. Res. Health Sci. 2013, 14, 11–17. [Google Scholar] [CrossRef]
- El-Hayeck, R.; Baddoura, R.; Wehbé, A.; Bassil, N.; Koussa, S.; Khaled, K.; Richa, S.; Khoury, R.; Alameddine, A.; Sellal, F. An Arabic Version of the Mini-Mental State Examination for the Lebanese Population: Reliability, Validity, and Normative Data. J. Alzheimer’s Dis. 2019, 71, 525–540. [Google Scholar] [CrossRef]
- Living Conditions of Households 2007 | UNDP in Lebanon. Available online: https://www.lb.undp.org/content/lebanon/en/home/library/poverty/living-conditions-of-households-2007.html (accessed on 13 October 2020).
- Maillet, D.; Matharan, F.; Clésiau, H.; Bailon, O.; Peres, K.; Amieva, H.; Belin, C. TNI-93: A New Memory Test for Dementia Detection in Illiterate and Low-Educated Patients. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2016, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A Simple Frailty Questionnaire (FRAIL) Predicts Outcomes in Middle Aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”: A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Lipschitz, D.A. Screening for Nutritional Status in the Elderly. Prim. Care 1994, 21, 55–67. [Google Scholar] [CrossRef]
- Ambagtsheer, R.; Visvanathan, R.; Cesari, M.; Yu, S.; Archibald, M.; Schultz, T.; Karnon, J.; Kitson, A.; Beilby, J. Feasibility, Acceptability and Diagnostic Test Accuracy of Frailty Screening Instruments in Community-Dwelling Older People within the Australian General Practice Setting: A Study Protocol for a Cross-Sectional Study. BMJ Open 2017, 7, e016663. [Google Scholar] [CrossRef]
- Topolski, T.D.; LoGerfo, J.; Patrick, D.L.; Williams, B.; Patrick, M.M.B. The Rapid Assessment of Physical Activity (RAPA) Among Older Adults. Prev. Chronic Dis. 2006, 3, 8. [Google Scholar]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466. [Google Scholar]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 25 June 2021).
- Gray, W.K.; Richardson, J.; McGuire, J.; Dewhurst, F.; Elder, V.; Weeks, J.; Walker, R.W.; Dotchin, C.L. Frailty Screening in Low- and Middle-Income Countries: A Systematic Review. J. Am. Geriatr. Soc. 2016, 64, 806–823. [Google Scholar] [CrossRef] [PubMed]
- Sibai, A.M.; Sen, K.; Baydoun, M.; Saxena, P. Population Ageing in Lebanon: Current Status, Future Prospects and Implications for Policy. Bull. World Health Organ. 2004, 82, 219–225. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019, Volume II: Demographic Profiles (ST/ESA/SER.A/427); United Nations: San Francisco, CA, USA, 2019. [Google Scholar]
- Hoogendijk, E.O.; van Hout, H.P.J.; Heymans, M.W.; van der Horst, H.E.; Frijters, D.H.M.; Broese van Groenou, M.I.; Deeg, D.J.H.; Huisman, M. Explaining the Association between Educational Level and Frailty in Older Adults: Results from a 13-Year Longitudinal Study in the Netherlands. Ann. Epidemiol. 2014, 24, 538–544.e2. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Pilotto, A.; Vaona, A.; Demurtas, J.; Mueller, C.; Huntley, J.; Crepaldi, G.; et al. Polypharmacy Is Associated With Higher Frailty Risk in Older People: An 8-Year Longitudinal Cohort Study. J. Am. Med. Dir. Assoc. 2018, 18, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, F.F.; Bernabé, E.; Sabbah, W. Association between Oral Health and Frailty: A Systematic Review of Longitudinal Studies. Gerodontology 2019, 36, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Blackwell, T.L.; Redline, S.; Ancoli-Israel, S.; Paudel, M.L.; Cawthon, P.M.; Dam, T.-T.L.; Barrett-Connor, E.; Leung, P.C.; Stone, K.L. Sleep Disturbances and Frailty Status in Older Community-Dwelling Men. J. Am. Geriatr. Soc. 2009, 57, 2085–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamil, R.J.; Li, L.; Lin, F.R. Association of Hearing Impairment and Frailty in Older Adults. J. Am. Geriatr. Soc. 2014, 62, 1186–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swenor, B.K.; Lee, M.J.; Tian, J.; Varadaraj, V.; Bandeen-Roche, K. Visual Impairment and Frailty: Examining an Understudied Relationship. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 596–602. [Google Scholar] [CrossRef] [PubMed]
- García-Esquinas, E.; José García-García, F.; León-Muñoz, L.M.; Carnicero, J.A.; Guallar-Castillón, P.; Gonzalez-Colaço Harmand, M.; López-García, E.; Alonso-Bouzón, C.; Rodríguez-Mañas, L.; Rodríguez-Artalejo, F. Obesity, Fat Distribution, and Risk of Frailty in Two Population-Based Cohorts of Older Adults in Spain. Obesity 2015, 23, 847–855. [Google Scholar] [CrossRef] [Green Version]
- Bollwein, J.; Volkert, D.; Diekmann, R.; Kaiser, M.J.; Uter, W.; Vidal, K.; Sieber, C.C.; Bauer, J.M. Nutritional Status According to the Mini Nutritional Assessment (MNA®) and Frailty in Community Dwelling Older Persons: A Close Relationship. J. Nutr. Health Aging 2013, 17, 351–356. [Google Scholar] [CrossRef]
- Chang, S.-F. Frailty Is a Major Related Factor for at Risk of Malnutrition in Community-Dwelling Older Adults: Frail Assessment for Nutrition. J. Nurs. Scholarsh. 2017, 49, 63–72. [Google Scholar] [CrossRef]
- Laclaustra, M.; Rodriguez-Artalejo, F.; Guallar-Castillon, P.; Banegas, J.R.; Graciani, A.; Garcia-Esquinas, E.; Ordovas, J.; Lopez-Garcia, E. Prospective Association between Added Sugars and Frailty in Older Adults. Am. J. Clin. Nutr. 2018, 107, 772–779. [Google Scholar] [CrossRef]
- Struijk, E.A.; Rodríguez-Artalejo, F.; Fung, T.T.; Willett, W.C.; Hu, F.B.; Lopez-Garcia, E. Sweetened Beverages and Risk of Frailty among Older Women in the Nurses’ Health Study: A Cohort Study. PLoS Med. 2020, 17, e1003453. [Google Scholar] [CrossRef]
- Feart, C. Nutrition and Frailty: Current Knowledge. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109703. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Tramontano, G.; De Luca, V.; Illario, M.; Colao, A.; Savastano, S. Association between Mediterranean Diet and Hand Grip Strength in Older Adult Women. Clin. Nutr. 2019, 38, 721–729. [Google Scholar] [CrossRef]
- Fung, T.T.; Struijk, E.A.; Rodriguez-Artalejo, F.; Willett, W.C.; Lopez-Garcia, E. Fruit and Vegetable Intake and Risk of Frailty in Women 60 Years Old or Older. Am. J. Clin. Nutr. 2020, 112, 1540–1546. [Google Scholar] [CrossRef]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Jivraj, S.; Sekiguchi, K.; Walters, K. Fruit and Vegetable Consumption and Frailty: A Systematic Review. J. Nutr. Health Aging 2018, 22, 1010–1017. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Esquinas, E.; Rahi, B.; Peres, K.; Colpo, M.; Dartigues, J.-F.; Bandinelli, S.; Feart, C.; Rodriguez-Artalejo, F. Consumption of Fruit and Vegetables and Risk of Frailty: A Dose-Response Analysis of 3 Prospective Cohorts of Community-Dwelling Older Adults. Am. J. Clin. Nutr. 2016, 104, 132–142. [Google Scholar] [CrossRef]
| Total (N = 352) | Men (N = 176) | Women (N = 176) | p Value | ||
|---|---|---|---|---|---|
| Sociodemographic Status | |||||
| Age (years) | 73 (67–79) | 73 (67.3–80) | 73 (67–78) | 0.288 | |
| Marital status | Married | 232 (65.9) | 143 (81.3) | 89 (50.6) | <0.001 |
| Divorced | 10 (2.8) | 4 (2.3) | 6 (3.4) | ||
| Single | 18 (5.1) | 6 (3.4) | 12 (6.8) | ||
| Widowed | 92 (26.1) | 23 (13.1) | 69 (39.2) | ||
| Living conditions | Living Alone | 53 (15.1) | 16 (9.1) | 37 (21) | <0.001 |
| Living with partner | 202 (57.4) | 131 (74.4) | 71 (30.3) | ||
| Living with others | 97 (27.6) | 29 (16.5) | 68 (38.6) | ||
| Education | Low level | 149 (42.3) | 65 (36.9) | 84 (47.7) | 0.04 |
| Economic status | Insufficient income | 198 (56.3) | 98 (55.7) | 100 (56.8) | 0.159 |
| Health and Functional Status | |||||
| Frailty status | Frail | 53 (15.1) | 18 (10.2) | 35 (19.9) | 0.01 |
| Not frail | 299 (84.9) | 158 (89.8) | 141 (80.1) | ||
| Cognitive Status | Low cognitive test | 116 (33) | 57 (32.4) | 59 (33.5) | 0.821 |
| Polypharmacy | ≥6 drugs | 254 (73.8) | 133(77.8) | 121 (69.9) | 0.098 |
| Multi-morbidity | ≥2 chronic illnesses | 270 (76.9) | 127 (72.6) | 143 (81.3) | 0.054 |
| Age-related conditions | ≥2 conditions | 189 (53.7) | 84 (47.7) | 105 (59.7) | 0.025 |
| Physical Activity | Sedentary | 275 (78.1) | 146 (83) | 129 (73.3) | 0.301 |
| Regular Active | 74 (21) | 30 (17) | 44 (25) | ||
| Optimal active | 3 (0.9) | 0 (0) | 3 (1.7) | ||
| Strength | HGS (kg) | 23.1 (17.8–31.2) | 31 (24.5–35.7) | 18.3 (13.6–22.3) | <0.001 |
| Nutritional Status and Nutrient Intake | |||||
| BMI | BMI(kg/m2) | 28.2 (25–32.8) | 27.5 (24.3–31.2) | 29.7 (25.9–35.1) | <0.001 |
| BMI < 22 | 109 (31) | 64 (36.4) | 45 (25.6) | 0.042 | |
| BMI (22–27) | 30 (8.5) | 17 (9.7) | 13 (7.4) | ||
| BMI > 27 | 213 (60.5) | 95 (54) | 118 (67) | ||
| WTHR | 0.64 (0.59–0.70) | 0.62 (0.57–0.67) | 0.67 (0.61–0.75) | <0.001 | |
| Nutritional status | Normal | 225 (63.9) | 114 (64.8) | 111 (63.1) | 0.74 |
| (MNA-SF score) | Poor | 127 (36.1) | 62 (35.2) | 65 (36.9) | |
| Energy and macronutrients intake | Energy (Kcal/d) | 1824 (1509–2299) | 2031 (1638–2447) | 1726 (1408–2094) | <0.001 |
| Calories (Kcal/kg BW) | 25.3 (20.1–31.8) | 26.1 (20.1–31.8) | 24.5 (19.5–31.9) | 0.214 | |
| Carbohydrates(g/d) | 201 (161.3–258) | 226 (178.5–293) | 185.5 (145.3–218.5) | <0.001 | |
| Proteins (g/d) | 66.7 (50.1–84.1) | 72.3 (58.9–92.3) | 59.6 (46.1–73.2) | <0.001 | |
| Proteins (g/kg BW) | 0.91 (0.68–1.15) | 0.94 (0.73–1.17) | 0.85 (0.65–1.13) | 0.022 | |
| Fats (g/d) | 89.2 (72.5–111) | 90.4 (74.1–112.8) | 86.3 (70.6–109.8) | 0.076 | |
| WDP | HI-MEDDP | MOD-MEDDP | p Value | |
|---|---|---|---|---|
| N = 42 (11.9%) | N = 81 (23%) | N = 229 (65.1%) | ||
| Kilocalories/day | 2261 (1953–2845) | 2028 (1652–2473) | 1743 (1453–2123) | <0.001 |
| Refined flour products | 5.09 (3.38–7.84) | 2.25 (0.7–5.09) | 3.32 (1.15–5.11) | <0.001 |
| Whole breads and cereals (including burghul) | 0.35 (0.11–1.46) | 1.49 (0.36–2.82) | 0.76 (0.18–2.33) | 0.008 |
| Potato | 0.4 (0.14–0.78) | 0.27 (0.1–0.51) | 0.25 (0.1–0.43) | 0.024 |
| Vegetables | 2.9 (2.1–3.84) | 3.74 (2.59–5.23) | 2.93 (2.09–4.52) | 0.002 |
| Fruits | 2.1 (1.09–2.78) | 2.45 (1.52–3.4) | 1.89 (1.25–2.86) | 0.033 |
| Legumes | 0.3 (0.18–0.57) | 0.57 (0.24–0.86) | 0.29 (0.13–0.57) | 0.006 |
| Meat and poultry | 2.26 (1.49–3.6) | 2.02 (1.5–2.94) | 1.93 (1.2–3.03) | 0.137 |
| Eggs | 0.57 (0.25–0.86) | 0.29 (0.18–0.57) | 0.29 (0.1–0.57) | 0.002 |
| Fish and shellfish | 0.42 (0.08–0.78) | 0.43 (0.17–0.83) | 0.4 (0.13–0.73) | 0.568 |
| Milk and dairy products | 1.89 (1.05–2.61) | 1.71 (0.99–2.43) | 1.39 (0.85–2.05) | 0.002 |
| Vegetable oils | 3 (0.94–4) | 3 (1–3.62) | 3 (2–3.08) | 0.704 |
| Olive, seeds and oleaginous fruits | 4.03 (3–6.04) | 9.87 (8.02–12.67) | 4.16 (3.07–6.18) | <0.001 |
| Processed and saturated fats | 0.02 (0–1) | 0 (0–0.17) | 0 (0–0.18) | 0.024 |
| Low fat sweets | 0.49 (0.07–2.11) | 0.43 (0.08–1.33) | 0.14 (0–0.36) | <0.001 |
| High fat sweets | 0.19 (0.07–0.49) | 0.14 (0–0.43) | 0.07 (0–0.26) | <0.001 |
| Sugars and jams | 8.5 (6.05–12.36) | 3 (0.96–5.37) | 1 (0.15–2.13) | <0.001 |
| Total | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 352 | 176 (50) | 176 (50) | ||||||||
| Non-Frail | Frail | p Value | Non-Frail | Frail | p Value | Non-Frail | Frail | p Value | ||
| N (%) | 299 (84.9) | 53 (15.1) | 158 (89.8) | 18 (10.2) | 141 (80.1) | 35 (19.9) | ||||
| Sociodemographic Parameters | ||||||||||
| Age (years) | 72 (60–93) | 78 (60–91) | <0.001 | 72 (60–93) | 82 (60–88) | 0.003 | 72 (60–90) | 76 (65–91) | 0.001 | |
| Education | Elementary and lower | 116 (38.8) | 33 (62.3) | 0.001 | 57 (36.1) | 8 (44.4) | 0.486 | 59 (41.8) | 25 (71.4) | 0.002 |
| Health-Related Parameters | ||||||||||
| Polypharmacy | 61 (21) | 29 (54.7) | <0.001 | 32 (20.9) | 6 (33.3) | 0.231 | 29 (21) | 23 (65.7) | <0.001 | |
| Multi-morbidity | 218 (73.2) | 52 (98.1) | <0.001 | 110 (70.1) | 17 (94.4) | 0.028 | 108 (76.6) | 35 (100) | <0.001 | |
| Age-related conditions | 148 (49.5) | 41 (77.4) | <0.01 | 74 (46.8) | 10 (55.6) | 0.483 | 74 (52.5) | 31 (88.6) | <0.001 | |
| Anthropometric Parameters | ||||||||||
| BMI (kg/m2) | 28.1 (16.5–49.4) | 29.8 (18.5–46.2) | 0.096 | 27.5 (16.5–40) | 26.1 (18.5–34) | 0.173 | 28.9 (18.2–49.4) | 32.4 (19.6–46.1) | 0.018 | |
| WHTR | 0.64 (0.42–0.99) | 0.69 (0.49–1) | <0.001 | 0.62 (0.42–0.41) | 0.6 (0.52–0.69) | 0.705 | 0.65 (0.5–0.99) | 0.75 (0.49–1) | <0.001 | |
| Nutritional Status, Strength, and Activity | ||||||||||
| Low HGS | 127 (45) | 44 (86.3) | <0.001 | 64 (43) | 13 (81.2) | 0.004 | 63 (47.4) | 31 (88.6) | <0.001 | |
| Physical activity | Sedentary | 224 (74.9) | 51 (96.2) | <0.001 | 128 (81) | 18 (100) | 0.042 | 96 (68.1) | 33 (94.3) | <0.001 |
| Regular | 72 (24.1) | 2 (3.8) | 30 (19) | 0 (0) | 42 (29.8) | 2 (5.7) | ||||
| Optimal | 3 (1) | 0 (0.0) | 0 (0) | 0 (0) | 3 (2.1) | 0 (0) | ||||
| Poor nutritional status | 96 (32.1) | 31 (58.5) | <0.001 | 51 (32.3) | 10 (55.6) | <0.001 | 44 (31.2) | 21 (60) | <0.001 | |
| Total (N = 352) | Men (N = 176) | Women (N = 176) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Frail 299 (85) | Frail 53 (15) | p | Non-Frail 158 (90) | Frail 18 (10) | p | Non-Frail 141 (80) | Frail 35 (20) | p | |
| Food Patterns | |||||||||
| WDP | 33 (11) | 9 (17) | 0.125 | 26 (16.5) | 3 (16.7) | 0.673 | 7 (5) | 6 (17.1) | 0.01 |
| HI-MEDDP | 65 (21.7) | 16 (30.2) | 20 (12.7) | 1 (5.6) | 45 (31.9) | 15 (42.9) | |||
| MOD-MEDDP | 201 (67.2) | 28 (52.8) | 112 (70.9) | 14 (77.8) | 89 (63.1) | 14 (40) | |||
| Food Intake | |||||||||
| Sugars and jams | 1.8 (0.29–4) | 2 (0.36–5.5) | 0.21 | 1.64 (0.32–4) | 2.14 (0.25–4) | 0.794 | 1.93 (0.3–3.75) | 1.85 (0.43–6.7) | 0.204 |
| Low fat sweets | 0.17 (0.01–0.59) | 0.3 (0.08–1.08) | 0.037 | 0.13 (0–0.47) | 0.15 (0.04–0.5) | 0.53 | 0.24 (0.04–0.8) | 0.37 (0.14–1.3) | 0.097 |
| Fruits and Vegetables | 5.6 (4.1–7.4) | 4.8 (3.1–6.6) | 0.022 | 5.8 (4.08–7.35) | 4.7 (3.8–6.9) | 0.196 | 5.6 (4.2–7.5) | 5.2 (3.1–6.4) | 0.07 |
| Vegetable oils | 3 (1.5–3.1) | 3 (2.5–4) | 0.027 | 3 (1.5–3.16) | 3 (1–3) | 0.96 | 3 (1.5–3.08) | 3 (3–4) | 0.009 |
| Olive, seeds and oleaginous fruits | 5.04 (3.3–7.7) | 6 (3.6–8.3) | 0.513 | 5.2 (3.3–7.5) | 5.1 (3.96–6.7) | 0.786 | 4.8 (3.4–7.82) | 6.4 (3.4–9.1) | 0.305 |
| Energy and Macronutrients | |||||||||
| Caloric intake (Cal/d) | 1851 (1543–2349) | 1763 (1398–2075) | 0.039 | 2077.5 (1667–2487) | 1734 (1422–1900) | 0.025 | 1709 (1416–2082) | 1763 (1388–2120) | 0.859 |
| Kilocalories/kg BW | 25.63 (20.1–32.1) | 23.1 (19.4–30.3) | 0.095 | 26.5 (21.03–32.1) | 22.4 (18.2–29.9) | 0.107 | 24.93 (19.84–31.9) | 23.7 (19.4–31.7) | 0.457 |
| Carbohydrates (g) | 204 (166–263) | 184 (143–217) | 0.009 | 237.5 (181–295) | 198.5 (152–233) | 0.038 | 188 (149–219) | 167 (129–217) | 0.356 |
| Proteins (g) | 67.8 (51.3–84.8) | 57.4 (44.9–70.5) | 0.006 | 74.05 (60.4–94.1) | 62 (51.8–84.6) | 0.033 | 61.1 (46.3–77.4) | 54.4 (44.1–70.5) | 0.295 |
| Protein/kg BW | 0.93 (0.69–1.17) | 0.81 (0.66–0.97) | 0.016 | 0.96 (0.74–1.18) | 0.81 (0.73–1.07) | 0.133 | 0.87 (0.65–1.16) | 0.81 (0.63–1) | 0.161 |
| FAT (g) | 89.5 (74.1–111) | 84.9 (66.2–113) | 0.328 | 91.65 (75.8–115) | 81.25 (63.8–107) | 0.058 | 85.8 (70.7–107) | 90.9 (70–119) | 0.638 |
| Associated Factors | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Sociodemographic Parameters | ||||
| Age > 75 years | 3.667 (1.93–6.99) | 3.55 (1.75–7.21) | 2.91(1.42–5.94) | 2.83(1.42–5.63) |
| Female gender | 2.66 (1.33–5.29) | 2.11 (0.99–4.49) | ||
| Low Education level (<7 years of education) | 2.19 (1.17–4.11) | 1.96 (0.98–3.93) | 1.93 (0.94–3.97) | |
| Living conditions (compared with living alone) | ||||
| with partner | 0.64 (0.26–1.59) | |||
| with others | 1.14 (0.35–3.7) | |||
| Marital status (married vs. other status) | 1.95 (0.78–4.9) | |||
| Nutritional and Anthropometric Parameters | ||||
| WHTR>0.718 | 2.94 (1.27–6.76) | 3.27 (1.56–6.83) | 3.78 (1.71–8.33) | |
| Poor nutritional status (malnutrition and at risk vs normal) | 14.26 (4.64–43.81) | 10.79 (3.29–35.35) | 9.67 (3.1–30.18) | |
| BMI (compared with BMI < 22 kg/m2) | ||||
| BMI (22–27) | 0.72 (0.16–3.25) | |||
| BMI (>27) | 1.24 (0.52–2.98) | |||
| Health-Related Parameters | ||||
| Polypharmacy | 2.74 (1.34–5.6) | 4.42 (2.21–8.86) | ||
| Age related conditions | 2.28 (1.03–5.03) | 2.47 (1.17–5.24) | ||
| Multimorbidity | 7.18 (0.92–56.03) | |||
| Dietary Patterns | ||||
| MOD-MEDDP pattern | 1 | |||
| WDP pattern | 2.97 (1.12–7.89) | |||
| HI-MEDDP pattern | 2.27 (0.98–5.25) |
| Dietary Patterns | Frail Individuals N (%) | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| 53 (15) | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Total | 0.176 | 0.074 | 0.083 | |||||
| MOD-MEDDP | 28 (52.8) | 1 | 1 | 1 | ||||
| WDP | 9 (17) | 2.25(0.91–5.53) | 0.078 | 2.44 (0.93–6.43) | 0.07 | 2.68 (0.98–7.29) | 0.054 | |
| HI-MEDDP | 16 (30.2) | 1.45 (0.7–2.98) | 0.316 | 2.08 (0.96–4.54) | 0.065 | 1.96 (0.86–4.45) | 0.109 | |
| Men | 0.482 | 0.735 | 0.011 | |||||
| MOD-MEDDP | 14 (77.8) | 1 | 1 | 1 | ||||
| WDP | 3 (16.7) | 1.14 (0.29–4.53) | 0.85 | 0.89 (0.18–4.39) | 0.885 | 6.63 (1.82–24.21) | 0.004 | |
| HI-MEDDP | 1 (5.6) | 0.28 (0.03–2.35) | 0.243 | 0.43 (0.05–3.62) | 0.434 | 2.23 (0.93–5.32) | 0.071 | |
| Women | 0.027 | 0.013 | 0.013 | |||||
| MOD-MEDDP | 14 (40) | 1 | 1 | 1 | ||||
| WDP | 6 (17.1) | 4.57 (1.25–16.76) | 0.022 | 6.76 (1.56–29.22) | 0.01 | 11.54 (2.02–65.85) | 0.006 | |
| HI-MEDDP | 15 (42.9) | 2.44 (1.02–5.79) | 0.044 | 3.06 (1.15–8.15) | 0.025 | 3.06 (0.97–9.62) | 0.056 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaghi, N.; Yaghi, C.; Abifadel, M.; Boulos, C.; Feart, C. Dietary Patterns and Risk Factors of Frailty in Lebanese Older Adults. Nutrients 2021, 13, 2188. https://doi.org/10.3390/nu13072188
Yaghi N, Yaghi C, Abifadel M, Boulos C, Feart C. Dietary Patterns and Risk Factors of Frailty in Lebanese Older Adults. Nutrients. 2021; 13(7):2188. https://doi.org/10.3390/nu13072188
Chicago/Turabian StyleYaghi, Nathalie, Cesar Yaghi, Marianne Abifadel, Christa Boulos, and Catherine Feart. 2021. "Dietary Patterns and Risk Factors of Frailty in Lebanese Older Adults" Nutrients 13, no. 7: 2188. https://doi.org/10.3390/nu13072188
APA StyleYaghi, N., Yaghi, C., Abifadel, M., Boulos, C., & Feart, C. (2021). Dietary Patterns and Risk Factors of Frailty in Lebanese Older Adults. Nutrients, 13(7), 2188. https://doi.org/10.3390/nu13072188







