Blinded Oral Challenges with Lactose and Placebo Accurately Diagnose Lactose Intolerance: A Real-Life Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Protocol
2.3. Hydrogen Breath Test
2.4. Symptom’s Questionnaire
2.5. Statistical Analysis
3. Results
- (1)
- Overall, female sex was prevalent (100 out of 148 patients, 68%).
- (2)
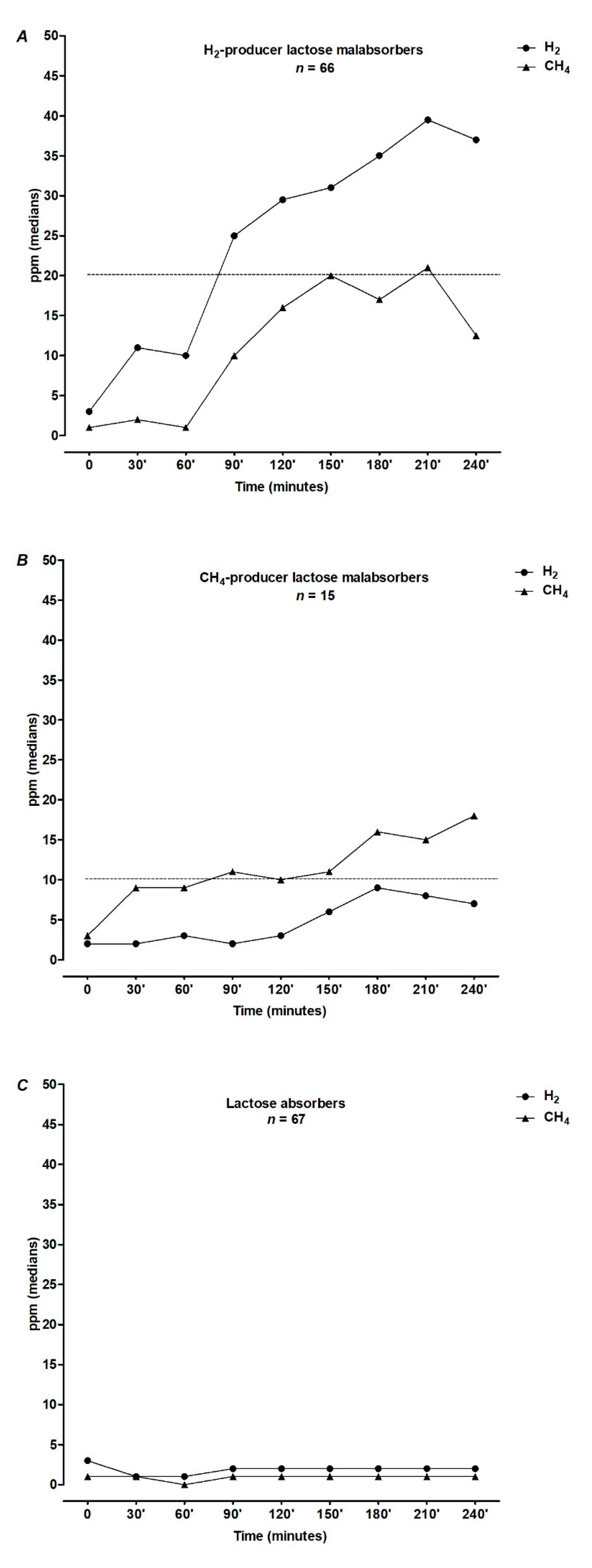
- HBT was positive (Lactose Malabsorbers, LM) in 55% (n = 81, of whom 15 methane producers) and negative (Lactose absorbers, LA) in 45% (n = 67) of subjects after lactose load. Figure 1 shows the results of 25 g lactose HBT expressed as medians of H2 and CH4 excretion in ppm at each time point.
- (3)
- All patients reported clinically relevant symptoms (GSS >7) at the HQ. GSS, diarrhea, and abdominal pain mean scores were significantly higher in LA than in the LM group (p = 0.005, p = 0.001, and p = 0.006, respectively) (Figure 2A–C).
- (4)
- At the LQ, a significantly higher percentage of LM patients reported a GSS >7 in comparison to LA (74% vs. 42%, p = 0.001), although the mean symptom score did not significantly differ between the groups (13.9 ± 9.6 in LM versus 12 ± 9.6 in LA, p = 0.244) (Figure 2A). When we analyzed single symptoms, LM complained of vomiting and bloating more frequently than LA (22% vs. 9%, p = 0.042 and 81% vs. 64%, p = 0.024, respectively). Moreover, bloating scored significantly higher in LM than in LA (5, IQR: 1–8 in LM versus 3, IQR: 0–6 in LA; p = 0.021) (Figure 2B–F).
- (5)
- At the PQ, 15 out of 81 (18%) LM and 24 out of 67 (36%) LA (p = 0.024) had a GSS > 7. The frequency distribution of patients with diarrhea, abdominal pain, vomiting, bowel sounds, and bloating did not significantly differ between the groups. However, abdominal pain severity scored significantly higher in LA than in LM (0, IQR: 0–2 in LM vs 2, IQR: 0–5 in LA; p = 0.007) (Figure 2C).
- (6)
- The distribution of GSS at LQ, according to lactose HBT results expressed as delta increase of H2 or CH4 levels in the breath, is shown in Figure 3. Interestingly, there was no significant correlation between GSS and H2 or CH4 delta increase in the breath (r = 0.204; p = 0.06).
- (7)
- After the placebo challenge, 45 out of 60 (75%) LM patients with GSS > 7 at LQ, did not complain of clinically relevant symptoms, thus fulfilling the criteria for the diagnosis of lactose intolerance, according to NIH Consensus on Lactose Intolerance and Health (Figure 4).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassio, F.; Facioni, M.; Guagnini, F. Lactose Maldigestion, Malabsorption, and Intolerance: A Comprehensive Review with a Fo-cus on Current Management and Future Perspectives. Nutrients 2018, 10, 1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown-Esters, O.; Mc Namara, P.; Savaiano, D. Dietary and biological factors influencing lactose intolerance. Int. Dairy J. 2012, 22, 98–103. [Google Scholar] [CrossRef]
- Casellas, F.; Aparici, A.; Casaus, M.; Rodríguez, P.; Malagelada, J.R. Subjective Perception of Lactose Intolerance Does Not Always Indicate Lactose Malabsorption. Clin. Gastroenterol. Hepatol. 2010, 8, 581–586. [Google Scholar] [CrossRef]
- Zheng, X.; Chu, H.; Cong, Y.; Deng, Y.; Long, Y.; Zhu, Y.; Pohl, D.; Fried, M.; Dai, N.; Fox, M. Self-reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: Prevalence, risk factors, and impact on food choices. Neurogastroenterol. Motil. 2015, 27, 1138–1146. [Google Scholar] [CrossRef]
- Law, D.; Conklin, J.; Pimentel, M. Lactose Intolerance and the Role of the Lactose Breath Test. Am. J. Gastroenterol. 2010, 105, 1726–1728. [Google Scholar] [CrossRef]
- Casellas, F.; Varela, E.; Aparici, A.; Casaus, M.; Rodríguez, P. Development, validation, and applicability of a symptoms question-naire for lactose malabsorption screening. Dig. Dis. Sci. 2009, 54, 1059–1065. [Google Scholar] [CrossRef]
- Vernia, P.; Di Camillo, M.; Foglietta, T.; Avallone, V.E.; De Carolis, A. Diagnosis of lactose intolerance and the “nocebo” effect: The role of negative expectations. Dig. Liver Dis. 2010, 42, 616–619. [Google Scholar] [CrossRef]
- Suchy, F.J.; Brannon, P.M.; Carpenter, T.O.; Fernandez, J.R.; Gilsanz, V.; Gould, J.B.; Hall, K.; Hui, S.L.; Lupton, J.; Mennella, J.; et al. National Institutes of Health Consensus Development Conference: Lactose Intolerance and Health. Ann. Intern. Med. 2010, 152, 792–796. [Google Scholar] [CrossRef] [Green Version]
- Latorre, G.; Besa, P.; Parodi, C.G.; Ferrer, V.; Azocar, L.; Quirola, M.; Villarroel, L.; Miquel, J.F.; Agosin, E.; Chianale, J. Prevalence of lac-tose intolerance in Chile: A double-blind placebo study. Digestion 2014, 90, 18–26. [Google Scholar] [CrossRef]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomer, M.C.E. Review article: The aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment. Pharmacol. Ther. 2015, 41, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, T.A.; Qu, H.; Hughes, S.O.; He, M.; Wagner, S.E.; Foushee, H.R.; Shewchuk, R.M. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am. J. Clin. Nutr. 2011, 94, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, X.; Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. Nutr. Metab. 2021, 18, 1–18. [Google Scholar] [CrossRef]
- Suárez, F.; Levitt, M.D. Abdominal symptoms and lactose: The discrepancy between patients’ claims and the results of blinded trials. Am. J. Clin. Nutr. 1996, 64, 251–252. [Google Scholar] [CrossRef] [Green Version]
- Casellas, F.; Aparici, A.; Casaus, M.; Rodríguez, P. Self-perceived lactose intolerance and lactose breath test in elderly. Eur. Geriatr. Med. 2013, 4, 372–375. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Olesen, S.S.; Materna, A.; Drewes, A.M. Fermentable Sugar Ingestion, Gas Production, and Gastrointestinal and Central Nervous System Symptoms in Patients With Functional Disorders. Gastroenterology 2018, 155, 1034–1044.e6. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Fysekidis, M.; Rompteaux, P.; Raynaud, J.; Sabate, J.; Benamouzig, R. Lactose sensitivity and lactose malabsorp-tion. The two faces of lactose intolerance. J. Neurogastroenterol. Motil. 2021, 27, 257–264. [Google Scholar] [CrossRef]
- Vernia, P.; Di Camillo, M.; Marinaro, V.; Caprilli, R. Effect of predominant methanogenic flora on the outcome of lactose breath test in irritable bowel syndrome patients. Eur. J. Clin. Nutr. 2003, 57, 1116–1119. [Google Scholar] [CrossRef] [Green Version]
- Di Stefano, M.; Veneto, G.; Malservisi, S.; Strocchi, A.; Corazza, G.R. Lactose malabsorption and intolerance in the elderly. Scand J. Gastroenterol. 2001, 36, 1274–1278. [Google Scholar] [CrossRef]
- Tomba, C.; Baldassarri, A.; Coletta, M.; Cesana, B.M.; Basilisco, G. Is the subjective perception of lactose intolerance influenced by the psychological profile? Aliment. Pharmacol. Ther. 2012, 36, 660–669. [Google Scholar] [CrossRef]
- Barsky, A.J.; Saintfort, R.; Rogers, M.P.; Borus, J.F. Nonspecific Medication Side Effects and the Nocebo Phenomenon. JAMA 2002, 287, 622–627. [Google Scholar] [CrossRef]
- Benedetti, F.; Lanotte, M.; Lopiano, L.; Colloca, L. When words are painful: Unraveling the mechanisms of the nocebo effect. Neuroscience 2007, 147, 260–271. [Google Scholar] [CrossRef]
- Dekker, P.J.T.; Koenders, D.; Bruins, M.J. Lactose-Free Dairy Products: Market Developments, Production, Nutrition and Health Benefits. Nutrients 2019, 11, 551. [Google Scholar] [CrossRef] [Green Version]
- Lapides, R.A.; Savaiano, D.A. Gender, Age, Race and Lactose Intolerance: Is There Evidence to Support a Differential Symptom Response? A Scoping Review. Nutrients 2018, 10, 1956. [Google Scholar] [CrossRef] [Green Version]
- Mattar, R.; Monteiro, M.D.S.; Villares, C.A.; Dos Santos, A.F.; Carrilho, F.J. Single nucleotide polymorphism C/T-13910, located upstream of the lactase gene, associated with adult-type hypolactasia: Validation for clinical practice. Clin. Biochem. 2008, 41, 628–630. [Google Scholar] [CrossRef]
- Högenauer, C.; Hammer, H.F.; Mellitzer, K.; Renner, W.; Krejs, G.J.; Toplak, H. Evaluation of a new DNA test compared with the lactose hydrogen breath test for the diagnosis of lactase non-persistence. Eur. J. Gastroenterol. Hepatol. 2005, 17, 371–376. [Google Scholar] [CrossRef]
- Pohl, D.; Savarino, E.; Hersberger, M.; Behlis, Z.; Stutz, B.; Goetze, O.; Eckardstein, A.V.; Fried, M.; Tutuian, R. Excellent agreement between genetic and hydrogen breath tests for lactase deficiency and the role of extended symptom assessment. Br. J. Nutr. 2010, 104, 900–907. [Google Scholar] [CrossRef]



| Patients | LM (n = 81) | LA (n = 67) | p * |
|---|---|---|---|
| Age | 41.8 ± 17.3 | 40 ± 18 | 0.7252 |
| Males/Females | 18/63 | 30/37 | 0.0081 |
| BMI, 1 Kg/m2 | 24 ± 3.8 | 24.2 ± 5.2 | 0.8709 |
| § Global Symptom Score >7 | |||
| Home questionnaire | 81 (100%) | 67 (100%) | 1.000 |
| Lactose questionnaire | 60 (74%) | 28 (42%) | 0.001 |
| Placebo questionnaire | 15 (19%) | 24 (36%) | 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, A.; Compare, D.; Sgamato, C.; Martino, A.; De Simone, L.; Coccoli, P.; Melone, M.L.; Nardone, G. Blinded Oral Challenges with Lactose and Placebo Accurately Diagnose Lactose Intolerance: A Real-Life Study. Nutrients 2021, 13, 1653. https://doi.org/10.3390/nu13051653
Rocco A, Compare D, Sgamato C, Martino A, De Simone L, Coccoli P, Melone ML, Nardone G. Blinded Oral Challenges with Lactose and Placebo Accurately Diagnose Lactose Intolerance: A Real-Life Study. Nutrients. 2021; 13(5):1653. https://doi.org/10.3390/nu13051653
Chicago/Turabian StyleRocco, Alba, Debora Compare, Costantino Sgamato, Alberto Martino, Luca De Simone, Pietro Coccoli, Maria Laura Melone, and Gerardo Nardone. 2021. "Blinded Oral Challenges with Lactose and Placebo Accurately Diagnose Lactose Intolerance: A Real-Life Study" Nutrients 13, no. 5: 1653. https://doi.org/10.3390/nu13051653






