The Effect of Broccoli Sprout Extract on Seasonal Grass Pollen-Induced Allergic Rhinitis
Abstract
:1. Introduction
2. Methods
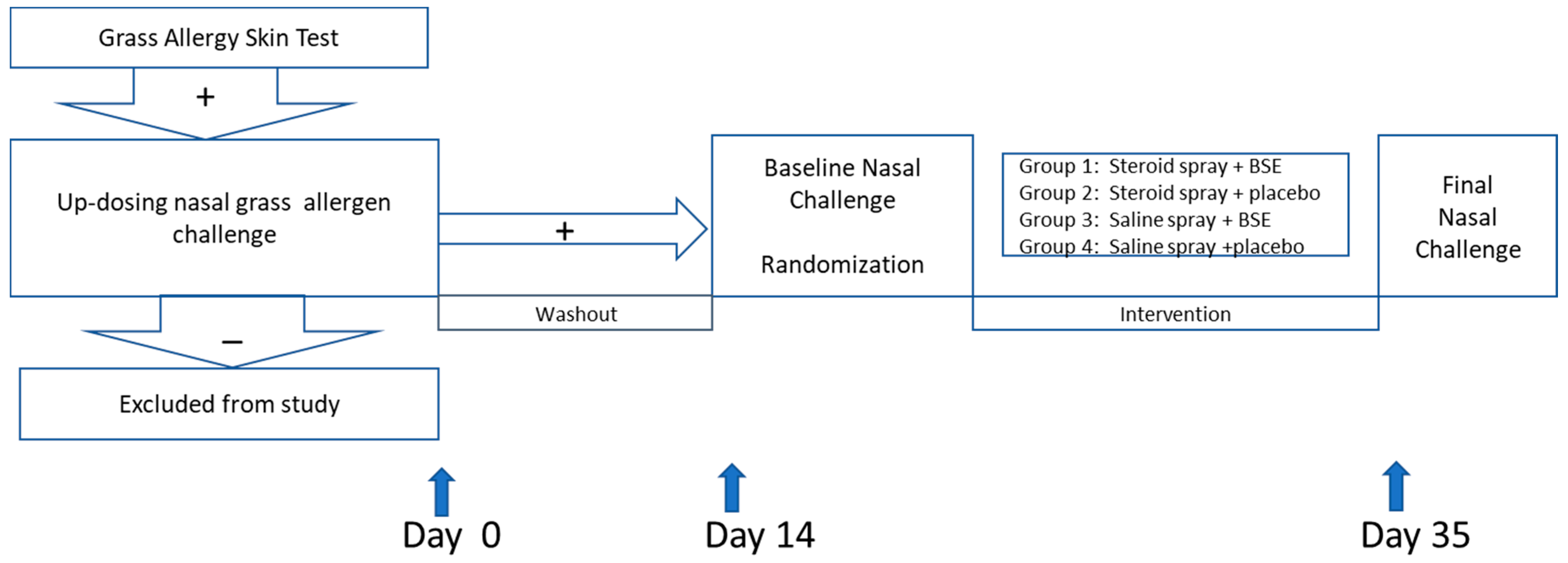
2.1. Study Design
2.2. Participants
2.3. Study Objectives
2.4. Study Products Used during the Study Included
- nasal corticosteroid + placebo tablet
- saline nasal spray + placebo tablet
- nasal corticosteroid + BSE
- saline nasal spray + BSE
2.5. Study Procedures
2.6. Nasal Allergen Challeng
2.7. Cytokine Analysis
2.8. Genotyping GSTP1, GSTM1, GSTP1
2.9. Statistical Analysis
3. Results
3.1. Baseline Characteristics
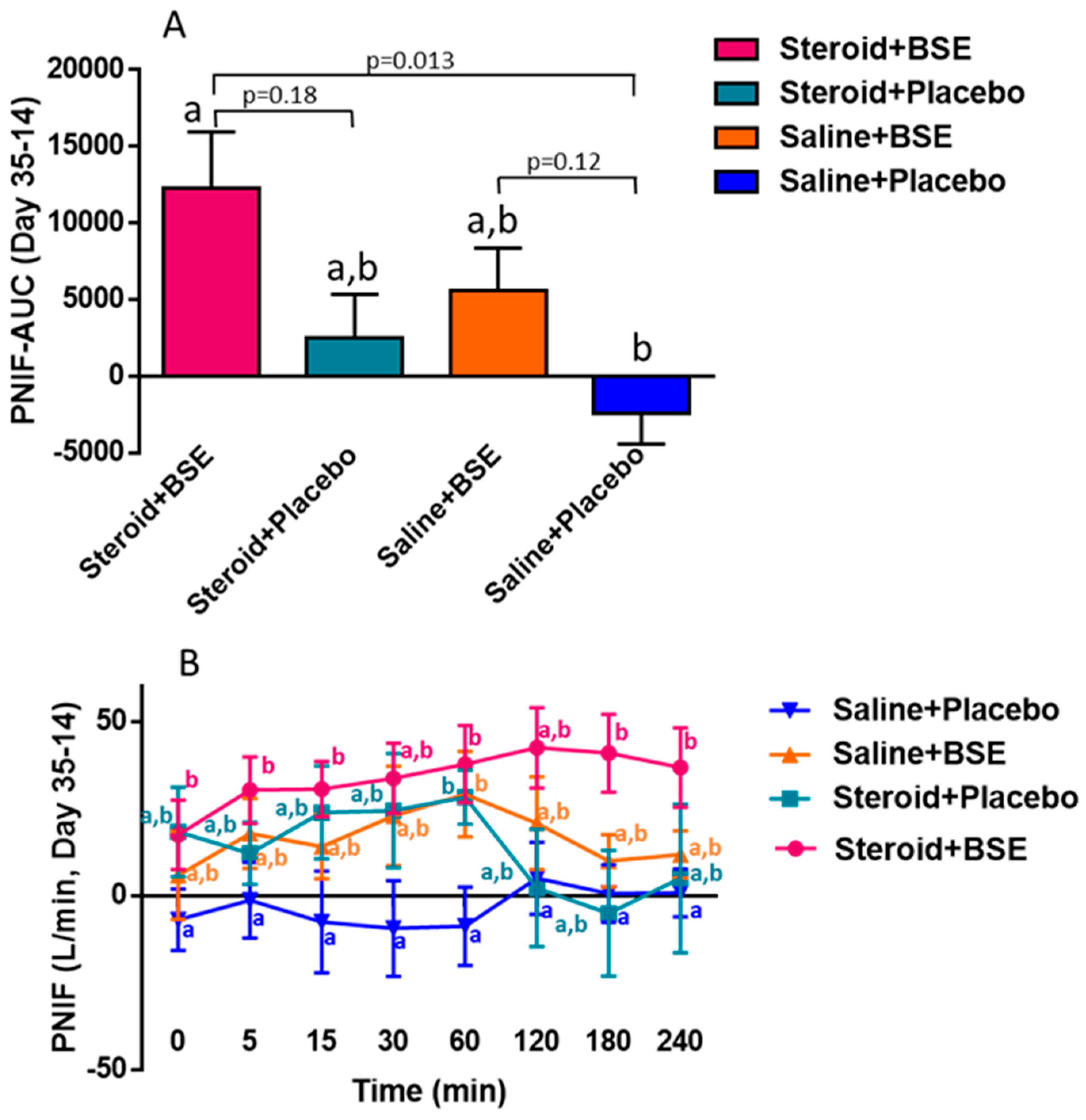
3.2. Peak Nasal Inspiratory Flow (PNIF) and Total Nasal Symptom Score (TNSS)
3.3. Inflammatory Markers
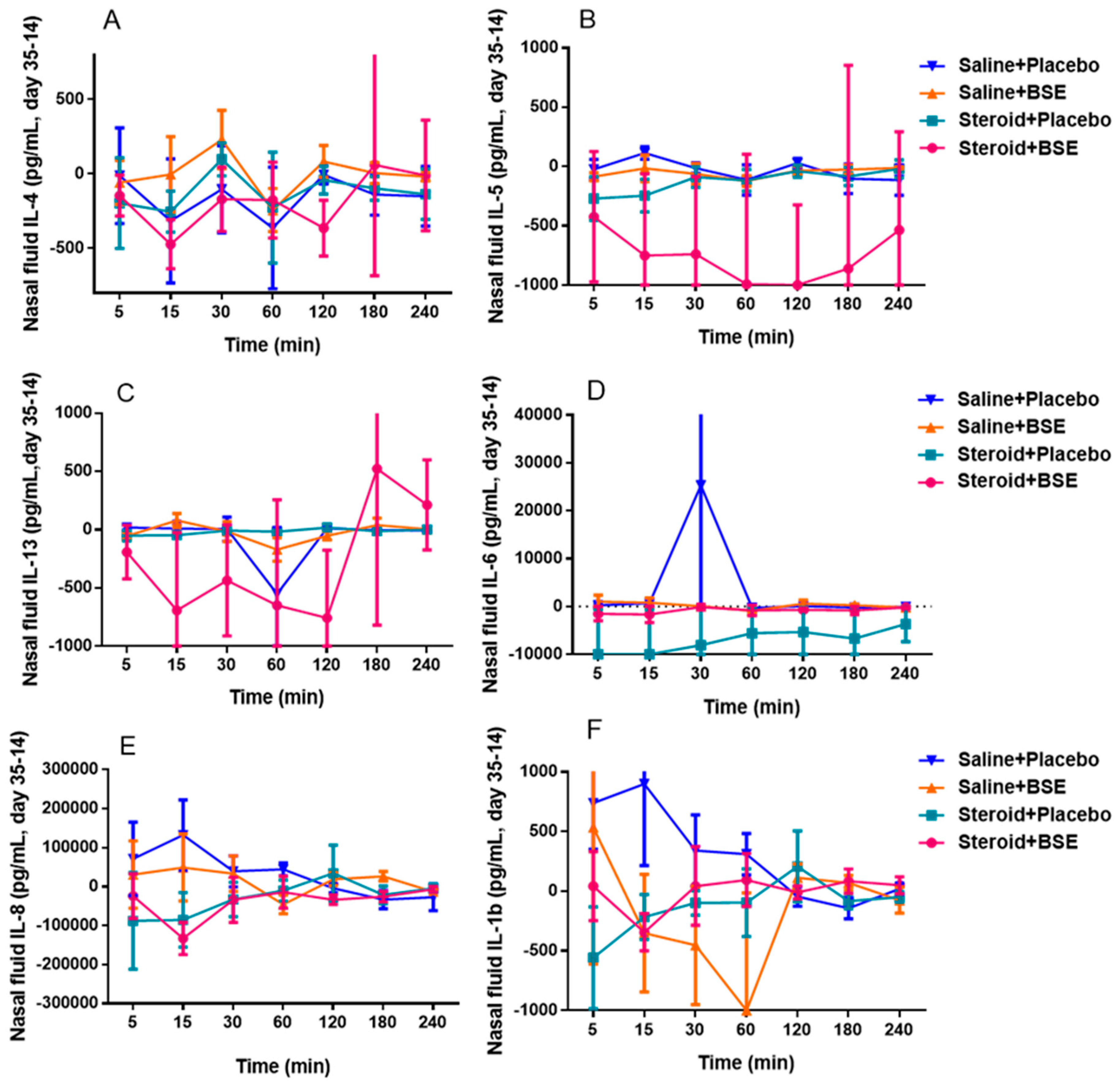
3.3.1. Nasal Mucus Cytokine Levels
3.3.2. GST Polymorphism
4. Discussion
4.1. Clinical Benefit
4.2. Cytokine Levels
4.3. Genetic Analysis
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEP | Diesel Exhaust Particles |
| ROS | Reactive oxygen species |
| GST | Glutathione transferase |
| SFN | Sulforaphane |
| Nrf2 | Nuclear factor E2-related factor 2 |
| BSE | Broccoli sprout extract |
| FEV1 | Forced expiratory volume in 1 s |
| TNSS | Total nasal symptoms score |
| PNIF | Peak nasal inspiratory flow |
| AUC | Area under the curve |
References
- Lee, S.-Y.; Chang, Y.-S.; Cho, S.-H. Allergic diseases and air pollution. Asia Pac. Allergy 2013, 3, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Takafuji, S.; Suzuki, S.; Koizumi, K.; Tadokoro, K.; Miyamoto, T.; Ikemori, R.; Muranaka, M. Diesel-exhaust particulates inoculated by the intranasal route have an adjuvant activity for IgE production in mice. J. Allergy Clin. Immunol. 1987, 79, 639–645. [Google Scholar] [CrossRef]
- Fujimaki, H.; Nohara, O.; Ichinose, T.; Watanabe, N.; Saito, S. IL-4 production in mediastinal lymph node cells in mice intratracheally instilled with diesel exhaust particulates and antigen. Toxicology 1994, 92, 261–368. [Google Scholar] [CrossRef]
- Suzuki, T.; Kanoh, T.; Ishimori, M.; Ikeda, S.; Ohkuni, H. Adjuvant activity of diesel exhaust particulates (DEP) in production of anti-IgE and anti-IgG1 antibodies to mite allergen in mice. J. Clin. Lab. Immunol. 1996, 48, 187–199. [Google Scholar]
- Takano, H.; Yoshikawa, T.; Ichinose, T.; Miyabara, Y.; Imaoka, K.; Sagai, M. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am. J. Respir. Crit. Care Med. 1997, 156, 36–42. [Google Scholar] [CrossRef]
- Lijima, M.K. Exposure to ozone aggravates nasal allergy like symptoms in guinea pigs. Toxicol. Lett. 2001, 123, 77–85. [Google Scholar]
- Diaz-Sanchez, D.; Dotson, A.R.; Takenaka, H.; Saxon, A. Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms. J. Clin. Invest. 1994, 94, 1417–1425. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Sanchez, D.; Tsien, A.; Fleming, J.; Saxon, A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J. Immunol. Baltim. Md. 1997, 158, 2406–2413. [Google Scholar]
- Bastain, T.M.; Gilliland, F.D.; Li, Y.F.; Saxon, A.; Diaz-Sanchez, D. Intraindividual reproducibility of nasal allergic responses to diesel exhaust particles indicates a susceptible phenotype. Clin. Immunol. 2003, 109, 130–136. [Google Scholar] [CrossRef]
- Wang, X.L.; Takai, T.; Kamijo, S.; Gunawan, H.; Ogawa, H.; Okumura, K. NADPH oxidase activity in allergenic pollen grains of different plant species. Biochem. Biophys. Res. Commun. 2009, 387, 430–434. [Google Scholar] [CrossRef]
- Boldogh, I.; Bacsi, A.; Choudhury, B.K.; Dharajiya, N.; Alam, R.; Hazra, T.K.; Mitra, S.; Goldblum, R.M.; Sur, S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen induced allergic airway inflammation. J. Clin. Invest. 2005, 115, 2169–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takizawa, H.; Abe, S.; Okazaki, H.; Kohyama, T.; Sugawara, I.; Saito, Y.; Ohtoshi, T.; Kawasaki, S.; Desaki, M.; Nakahara, K.; et al. Diesel exhaust particles upregulate eotaxin gene expression in human bronchial epithelial cells via nuclear factor kb dependent pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L1055–L1062. [Google Scholar] [CrossRef] [Green Version]
- Whitekus, M.J.; Li, N.; Zhang, M.; Wang, M.; Horwitz, M.A.; Nelson, S.K.; Horwitz, L.D.; Brechun, N.; Diaz-Sanchez, D.; Nel, A.E. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in murine model for ovalubumin sensitization. J. Immunol. 2002, 168, 2560–2567. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, A.; Lewis, S.A.; Scrivener, S.L.; Antoniak, M.; Pacey, S.; Pringle, M.; Britton, J. Oral magnesium and vitamin C supplements in asthma: A parallel group randomized placebo controlled trial. Clin. Exp. Allergy 2003, 33, 1355–1359. [Google Scholar] [CrossRef]
- Pearson, P.J.K.; Lewis, S.A.; Britton, J.; Fogarty, A. Vitamin E supplments in asthma: A parallel group randomized placebo controlled trial. Thorax 2004, 59, 652–656. [Google Scholar] [CrossRef] [Green Version]
- Allam, M.F. Selenium supplementation for asthma. Cochrane Database Syst. Rev. 2004, 2, CD003538. [Google Scholar] [CrossRef] [PubMed]
- Tamer, L.; Calikoğlu, M.; Ates, N.A.; Yildirim, H.; Ercan, B.; Saritas, E.; Unlü, A.; Atik, U. Glutathione-S-transferase gene polymorphisms (GSTT1, GSTM1, GSTP1) as increased risk factors for asthma. Respirology 2004, 9, 493–498. [Google Scholar] [CrossRef]
- Gilliland, F.D.; Li, Y.-F.; Saxon, A.; Diaz-Sanchez, D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: Randomised, placebo-controlled crossover study. Lancet 2004, 363, 119–125. [Google Scholar] [CrossRef]
- Romieu, I.; Sienra-Monge, J.J.; Ramírez-Aguilar, M.; Moreno-Macías, H.; Reyes-Ruiz, N.I.; del Río-Navarro, B.E.; Hernandez-Avila, M.; London, S.J. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax 2004, 59, 8–10. [Google Scholar]
- Moreno-Macías, H.; Dockery, D.W.; Schwartz, J.; Gold, D.R.; Laird, N.M.; Sienra-Monge, J.J.; Del Río-Navarro, B.; Ramírez-Aguilar, M.; Barraza-Villarreal, A.; Li, H.; et al. Ozone exposure, vitamin C intake, and genetic susceptibility of asthmatic children in Mexico City: A cohort study. Respir. Res. 2013, 14, 14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef]
- Ritz, S.A.; Wan, J.; Diaz-Sanchez, D. Sulforaphane-stimulated phase II enzyme induction inhibits cytokine production by airway epithelial cells stimulated with diesel extract. Am. J. Physiol. Cell. Mol. Physiol. 2007, 292, L33–L39. [Google Scholar] [CrossRef] [Green Version]
- Riedl, M.A.; Saxon, A.; Diaz-Sanchez, D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin. Immunol. 2009, 130, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Scadding, G.W.; Calderon, M.A.; Bellido, V.; Koed, G.K.; Nielsen, N.C.; Lund, K.; Togias, A.; Phippard, D.; Turka, L.A.; Hansel, T.T.; et al. Optimization of grass pollen nasal challenge for assessment of clinicalo and immunolicial outcomes. J. Immunol. Methods. 2012, 384, 25–32. [Google Scholar] [CrossRef]
- Negovan, A.; Iancu, M.; Moldovan, V.; Mocan, S.; Banescu, C. The Interaction between GSTT1, GSTM1, and GSTP1 Ile105Val Gene Polymorphisms and Environmental Risk Factors in Premalignant Gastric Lesions Risk. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwarith, J.; Kahleova, H.; Crosby, L.; Brooks, A.; Brandon, L.; Levin, S.M.; Barnard, N.D. The role of nutrition in asthma prevention and treatment. Nutr. Rev. 2020, 78, 928–938. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, B.; Gupta, M.; Chauhan, K. Role of antioxidants on the clinical outcome of patients with perennial allergic rhinitis. Allergy Rhinol. 2016, 7, e74–e81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykewicz, M.S.; Wallace, D.V.; Baroody, F.; Bernstein, J.; Craig, T.; Finegold, I.; Huang, F.; Larenas-Linnemann, D.; Meltzer, E.; Steven, G.; et al. Treatment of seasonal allergic rhinitis: An evidence-based focused 2017 guideline update. Ann. Allergy Asthma Immunol. 2017, 119, 489–511.e41. [Google Scholar] [CrossRef]
- Skoner, D.P. Allergic rhinitis: Definition, epidemiology, pathophysiology, detection, and diagnosis. J. Allergy Clin. Immunol. 2001, 108, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Erin, E.M.; Zacharasiewicz, A.S.; Nicholson, G.C.; Tan, A.J.; Higgins, L.A.; Williams, T.J.; Murdoch, R.D.; Durham, S.R.; Barnes, P.J.; Hansel, T.T. Topical corticosteroid inhibits interleukin-4,5 and 13 in nasal secretions following allergen challenge. Clin. Exp. Allergy 2005, 35, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Weido, A.J.; Cook, C.K.; Sim, T.C.; Reece, L.M.; Alam, R. Intranasal fluticasone propionate inhibits recorvery of chemokines and other cytokines in nasal secretions in allergen-induced rhinitis. Ann. Allergy Asthma Immunol. 1996, 77, 407–414. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao-Lin, Z.; Qian, Z.; Xu, Y.; Ya-Qing, D. Association of Glutathione S-Transferase M1 null genotype with inflammatory bowel diseases: A systemic review and meta-analysis. Medicine 2019, 98, 1–8. [Google Scholar] [CrossRef]
- Brasch-Andersen, C.; Christiansen, L.; Tan, Q.; Haagerup, A.; Vestbo, J.; Kruse, T.A. Possible gene dosage effect of glutathione-S-transferases on atopic asthma: Using real-time PCR for quantification of GSTM1 and GSTT1 gene copy numbers. Hum. Mutat. 2004, 24, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.S.P.; Néri, I.A.; Lourenço, G.J.; Faria, I.C.J.; Ribeiro, J.D.; Bertuzzo, C.S. Glutathione S-transferase mu 1 (GATM1) and theta 1 (GSTT1) genetic polymorphisms and atopic asthma in children from Southeastern Brazil. Genet. Mol. 2010, 33, 438–441. [Google Scholar]

| Group 1: Nasal Steroid + BSE | Group 2: Nasal Steroid + Placebo | Group 3: Saline Spray + BSE | Group 4: Saline Spray + Placebo | |
|---|---|---|---|---|
| Number of patients | 16 | 9 | 14 * | 8 |
| Age (years), Mean (SD) | 45.1 (12.2) | 58.6 (13.5) | 48.8 (15.9) | 44.5 (18.5) |
| Gender (male) | 75% | 78% | 83% | 63% |
| Race, No. (%) | ||||
| Asian | 3 (19%) | 1 (12%) | 0 (0%) | 0 (0%) |
| Pacific Islander | 2 (13%) | 0 (0%) | 0 (0%) | 1 (12%) |
| Black | 4 (25%) | 4 (50%) | 5 (42%) | 4 (50%) |
| White | 7 (44%) | 3 (38%) | 7 (58%) | 3 (38%) |
| Group 1 (n = 16) | Group 2 (n = 9) | Group 3 (n = 11) | Group 4 (n = 8) | |
|---|---|---|---|---|
| GSTM1 null | 9 | 3 | 4 | 2 |
| GSTM1 (2,3,4) | 7 | 6 | 7 | 6 |
| % null | 56 | 33 | 36 | 25 |
| GSTT1 null | 5 | 4 | 1 | 2 |
| GSTT1 (1,2) | 11 | 5 | 10 | 6 |
| % null | 31 | 44 | 9 | 25 |
| GSTP1 G/G * | 8 | 4 | 5 | 3 |
| GSTP1 A/G * | 5 | 4 | 4 | 2 |
| GSTP1 A/A | 3 | 1 | 2 | 3 |
| % low activity | 81 | 89 | 82 | 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusin, J.; Wang, V.; Henning, S.M.; Yang, J.; Tseng, C.-H.; Thames, G.; Arnold, I.; Heber, D.; Lee, R.-P.; Sanavio, L.; et al. The Effect of Broccoli Sprout Extract on Seasonal Grass Pollen-Induced Allergic Rhinitis. Nutrients 2021, 13, 1337. https://doi.org/10.3390/nu13041337
Yusin J, Wang V, Henning SM, Yang J, Tseng C-H, Thames G, Arnold I, Heber D, Lee R-P, Sanavio L, et al. The Effect of Broccoli Sprout Extract on Seasonal Grass Pollen-Induced Allergic Rhinitis. Nutrients. 2021; 13(4):1337. https://doi.org/10.3390/nu13041337
Chicago/Turabian StyleYusin, Joseph, Vivian Wang, Susanne M. Henning, Jieping Yang, Chi-Hong Tseng, Gail Thames, Irina Arnold, David Heber, Ru-Po Lee, Laura Sanavio, and et al. 2021. "The Effect of Broccoli Sprout Extract on Seasonal Grass Pollen-Induced Allergic Rhinitis" Nutrients 13, no. 4: 1337. https://doi.org/10.3390/nu13041337
APA StyleYusin, J., Wang, V., Henning, S. M., Yang, J., Tseng, C.-H., Thames, G., Arnold, I., Heber, D., Lee, R.-P., Sanavio, L., Pan, Y., Qin, T., & Li, Z. (2021). The Effect of Broccoli Sprout Extract on Seasonal Grass Pollen-Induced Allergic Rhinitis. Nutrients, 13(4), 1337. https://doi.org/10.3390/nu13041337






