Possible Neuroprotective Effects of l-Carnitine on White-Matter Microstructural Damage and Cognitive Decline in Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Risk Factors
2.3. Drug Administration and Classification
2.4. Profile of Carnitine Kinetics after l-Carnitine Treatment and Other Laboratory Blood Examinations
2.5. Neuropsychological Tests
2.6. MRI Acquisition
2.7. MRI Preprocessing
2.8. Tract-Based Spatial Statistics Analysis
2.9. Tract-of-Interest (TOI) Analysis
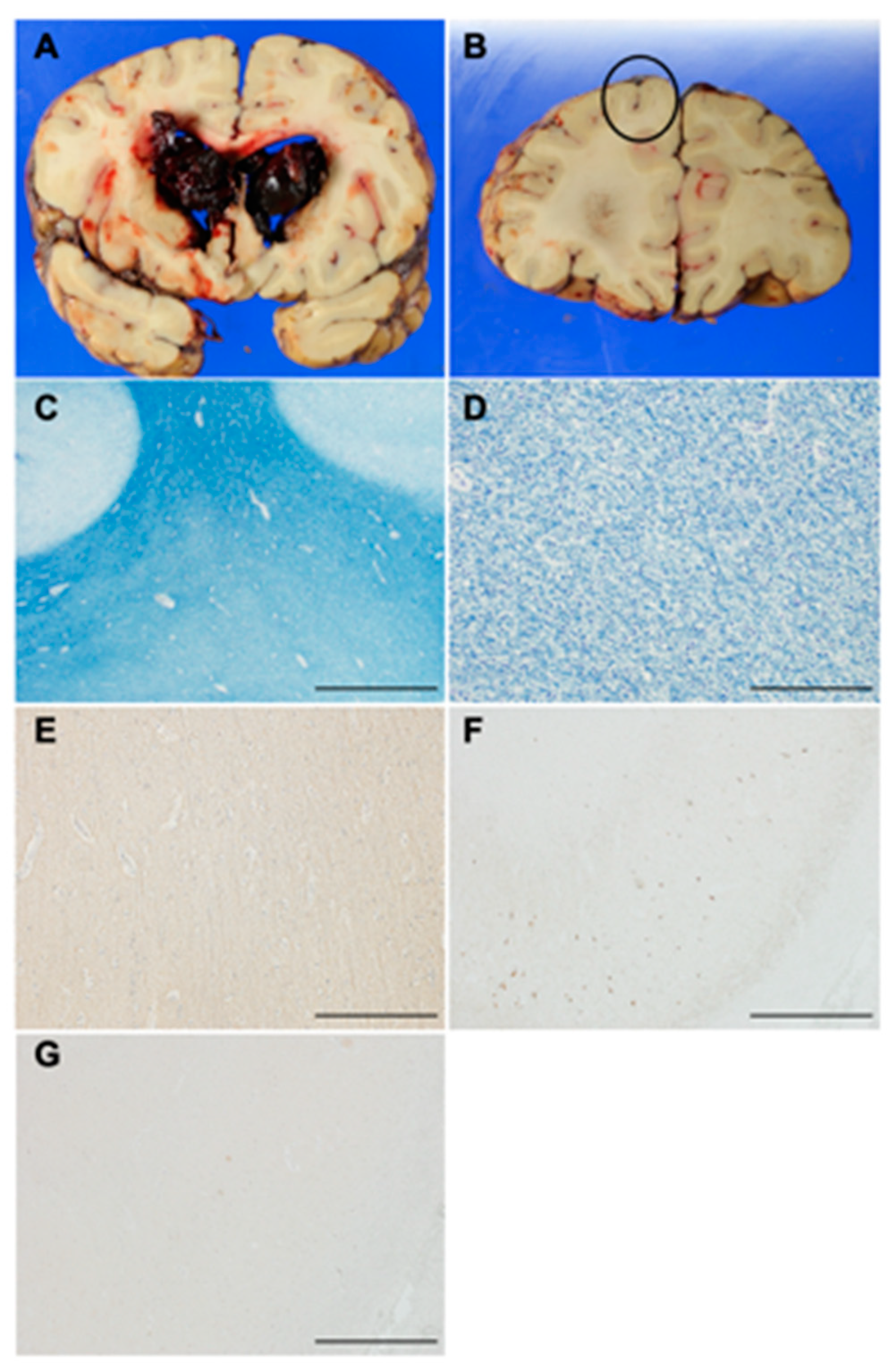
2.10. Histopathological Analysis of an Autopsy Brain
2.11. Statistical Analysis
3. Results
3.1. Study Population
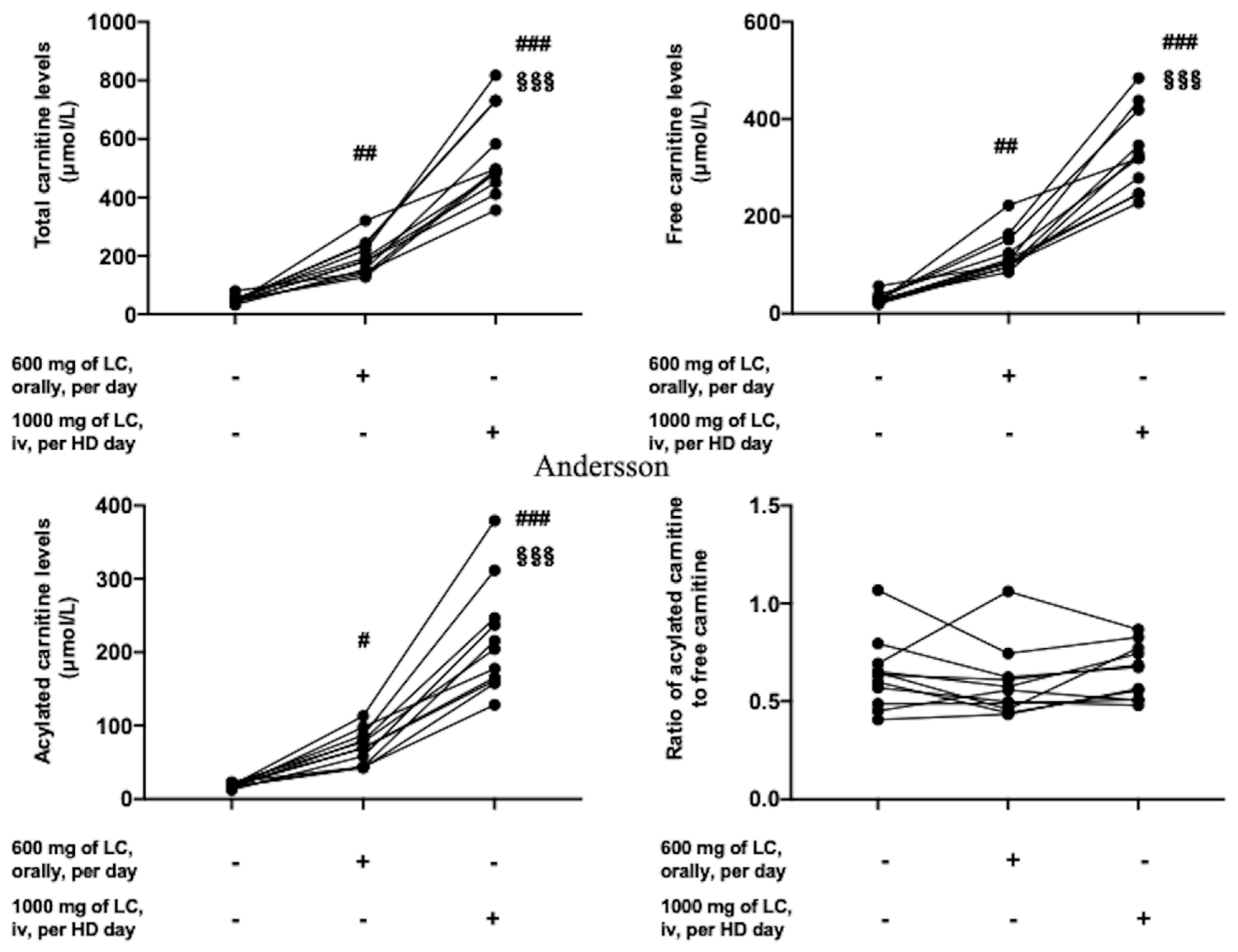
3.2. Profile of Serum Carnitine Kinetics before and after l-Carnitine Treatment and Classification
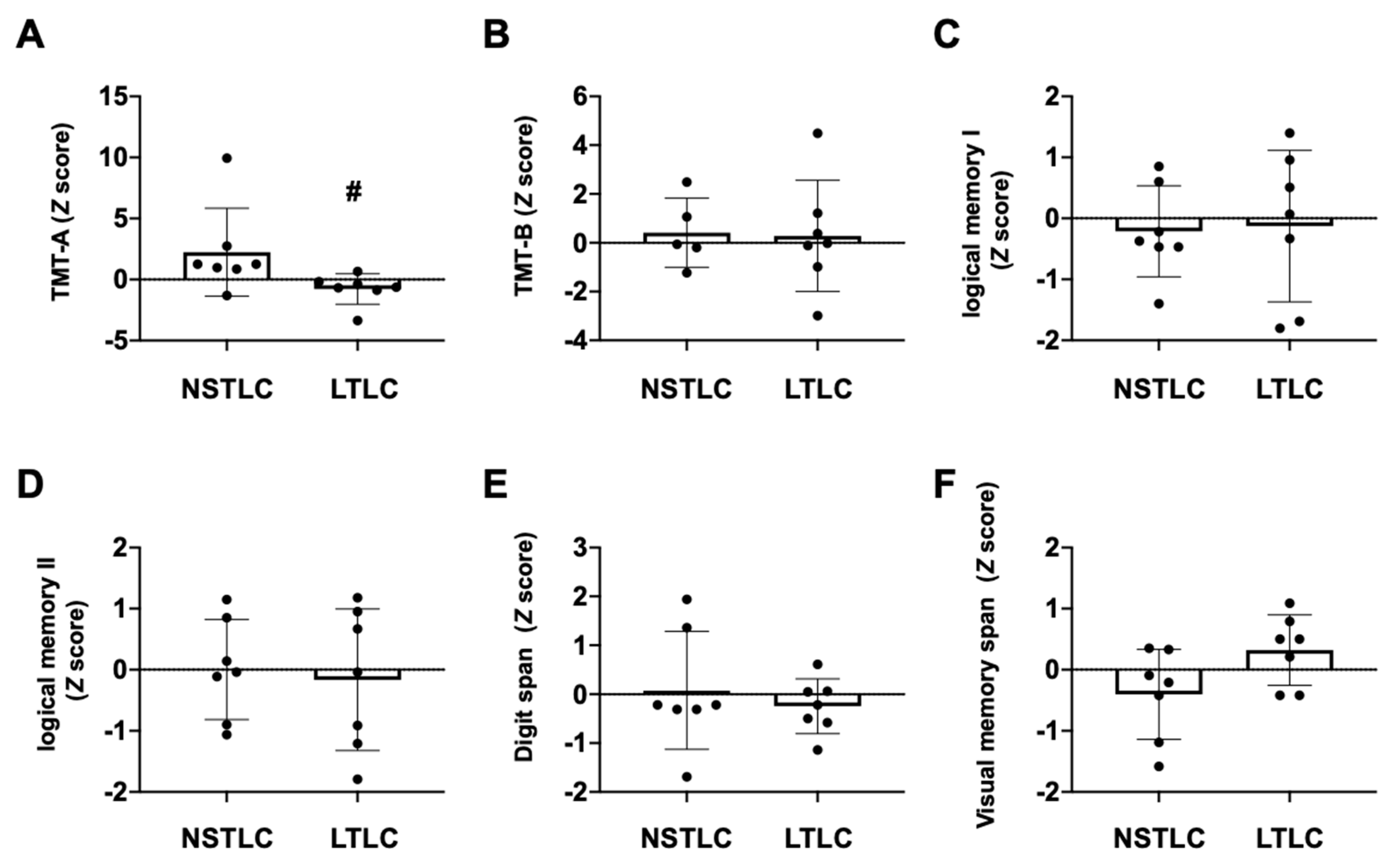
3.3. Baseline Patients’ Profile, Degree of White-Matter Lesions, Psychological Tests, and Laboratory Data in the NSTLC and LTLC Groups
3.4. Cognitive Functions in Hemodialysis Patients with NSTLC and LTLC
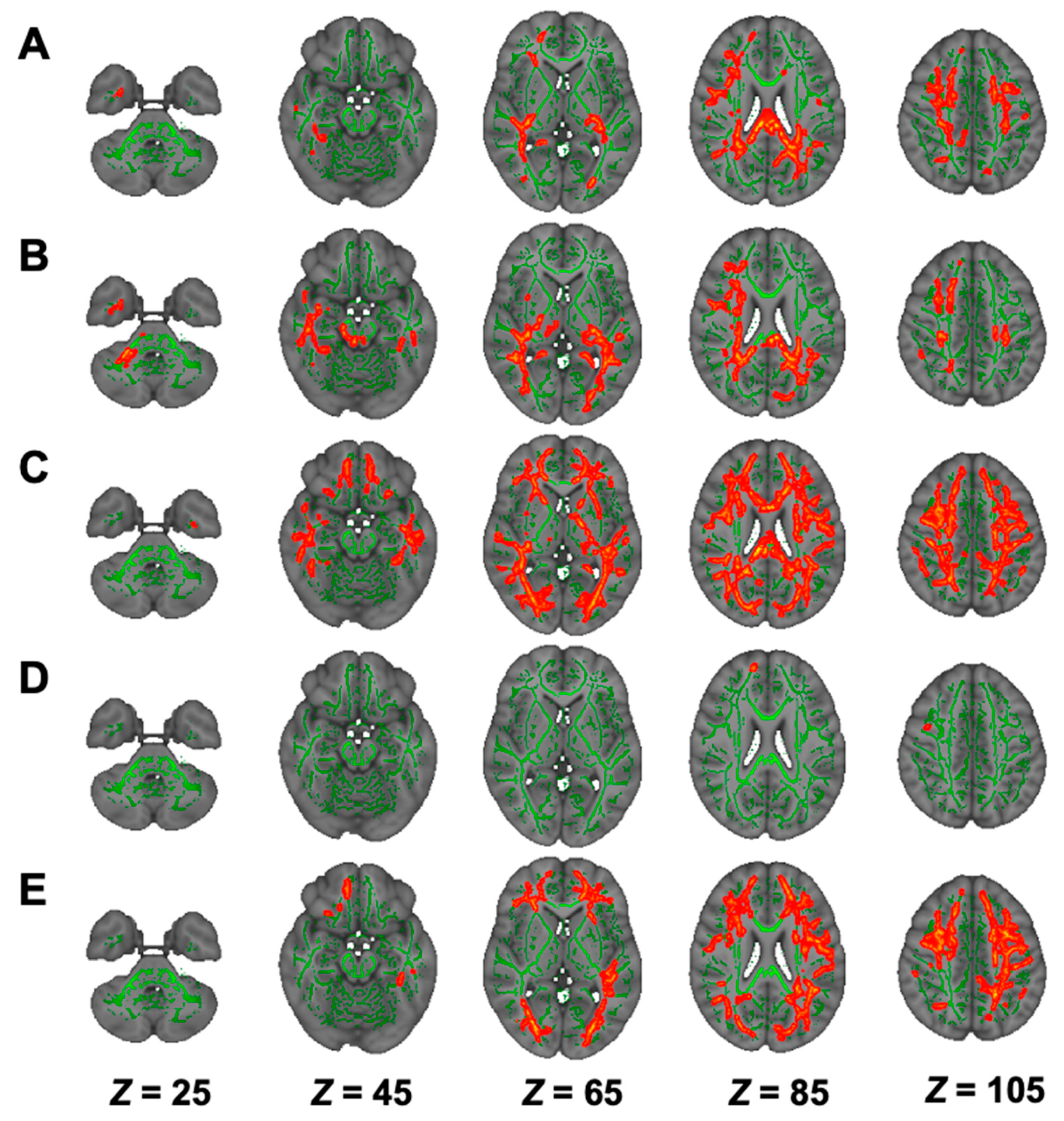
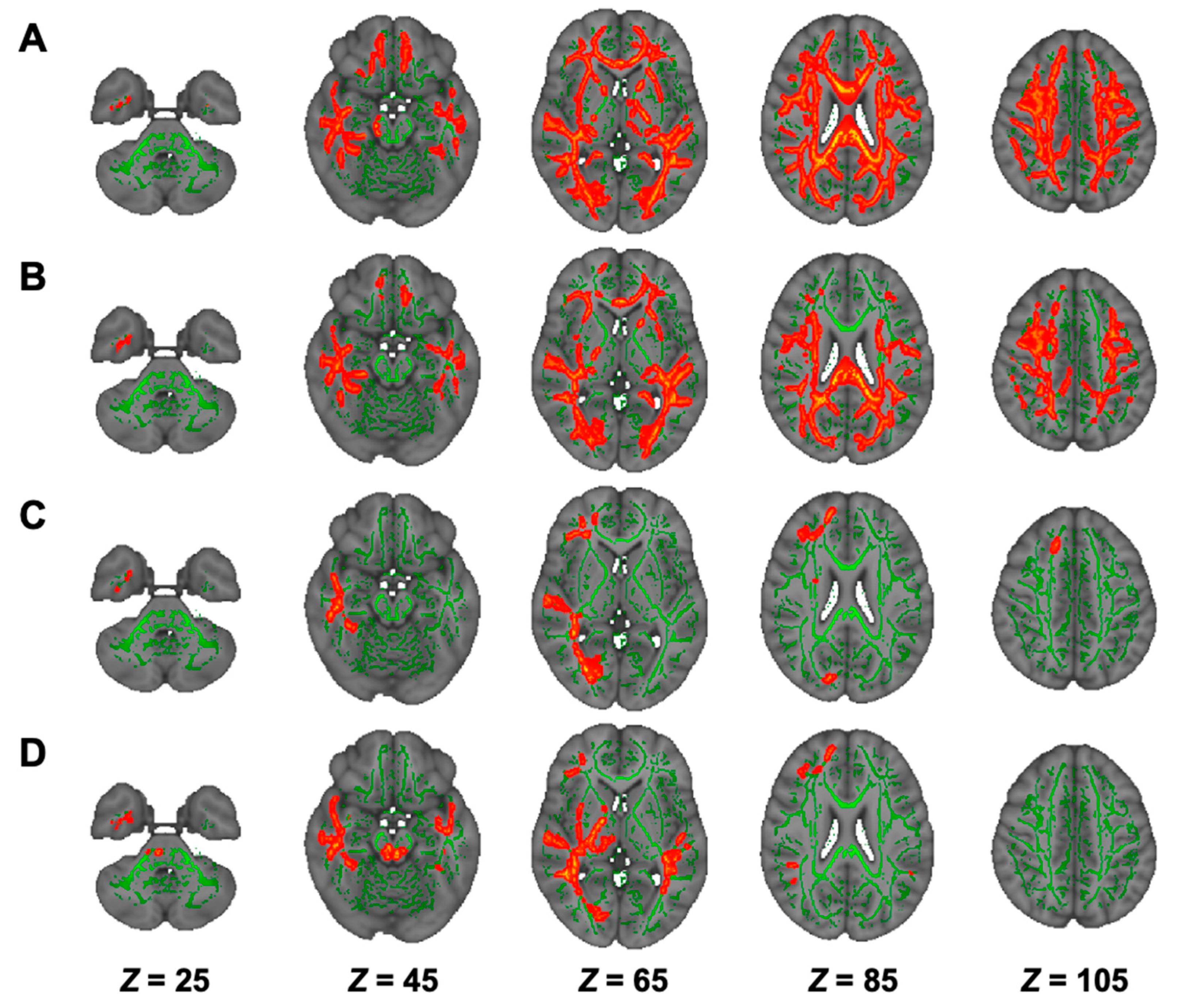
3.5. DTI Analysis in Hemodialysis Patients with NSTLC and LTLC
3.6. DKI Analysis in Hemodialysis Patients with NSTLC and LTLC
3.7. NODDI Analysis in Hemodialysis Patients with NSTLC and LTLC
3.8. Association of Cognitive Function Tests with dMRI in the Comparison between NSTLC and LTLC Groups
3.9. Histopathological Characteristics of an Autopsy Brain
4. Discussion
4.1. Main Findings
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, B.; Wulf, S.; Bikbov, B.; Perico, N.; Cortinovis, M.; de Vaccaro, K.C.; Flaxman, A.; Peterson, H.; Delossantos, A.; Haring, D.; et al. Maintenance Dialysis throughout the World in Years 1990 and 2010. J. Am. Soc. Nephrol. 2015, 26, 2621–2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurella, M.; Mapes, D.L.; Port, F.K.; Chertow, G.M. Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant. 2006, 21, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, J.M.; Godefroy, O.; Chillon, J.M.; Choukroun, G.; Massy, Z.A. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J. Am. Soc. Nephrol. 2013, 24, 353–363. [Google Scholar] [CrossRef]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.E.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Kamagata, K.; Zalesky, A.; Hatano, T.; Ueda, R.; Di Biase, M.A.; Okuzumi, A.; Shimoji, K.; Hori, M.; Caeyenberghs, K.; Pantelis, C.; et al. Gray Matter Abnormalities in Idiopathic Parkinson’s Disease: Evaluation by Diffusional Kurtosis Imaging and Neurite Orientation Dispersion and Density Imaging. Hum. Brain Mapp. 2017. [Google Scholar] [CrossRef] [Green Version]
- Maillard, P.; Fletcher, E.; Singh, B.; Martinez, O.; Johnson, D.K.; Olichney, J.M.; Farias, S.T.; DeCarli, C. Cerebral white matter free water: A sensitive biomarker of cognition and function. Neurology 2019, 92, e2221–e2231. [Google Scholar] [CrossRef]
- Williams, O.A.; Zeestraten, E.A.; Benjamin, P.; Lambert, C.; Lawrence, A.J.; Mackinnon, A.D.; Morris, R.G.; Markus, H.S.; Barrick, T.R.; Charlton, R.A. Predicting Dementia in Cerebral Small Vessel Disease Using an Automatic Diffusion Tensor Image Segmentation Technique. Stroke 2019, 50, 2775–2782. [Google Scholar] [CrossRef]
- Drew, D.A.; Koo, B.B.; Bhadelia, R.; Weiner, D.E.; Duncan, S.; la Garza, M.M.; Gupta, A.; Tighiouart, H.; Scott, T.; Sarnak, M.J. White matter damage in maintenance hemodialysis patients: A diffusion tensor imaging study. BMC Nephrol. 2017, 18, 213. [Google Scholar] [CrossRef] [Green Version]
- Chou, M.C.; Hsieh, T.J.; Lin, Y.L.; Hsieh, Y.T.; Li, W.Z.; Chang, J.M.; Ko, C.H.; Kao, E.F.; Jaw, T.S.; Liu, G.C. Widespread white matter alterations in patients with end-stage renal disease: A voxelwise diffusion tensor imaging study. AJNR Am. J. Neuroradiol. 2013, 34, 1945–1951. [Google Scholar] [CrossRef] [Green Version]
- Ueno, Y.; Zhang, N.; Miyamoto, N.; Tanaka, R.; Hattori, N.; Urabe, T. Edaravone attenuates white matter lesions through endothelial protection in a rat chronic hypoperfusion model. Neuroscience 2009, 162, 317–327. [Google Scholar] [CrossRef]
- Ueno, Y.; Koike, M.; Shimada, Y.; Shimura, H.; Hira, K.; Tanaka, R.; Uchiyama, Y.; Hattori, N.; Urabe, T. L-carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in rat brain. J. Cereb. Blood Flow Metab. 2015, 35, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Ringseis, R.; Keller, J.; Eder, K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013, 52, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Abe, M.; Yamazaki, T.; Okawa, E.; Ando, H.; Hotta, S.; Oikawa, O.; Kikuchi, F.; Okada, K.; Soma, M. Levocarnitine Improves Cardiac Function in Hemodialysis Patients With Left Ventricular Hypertrophy: A Randomized Controlled Trial. Am. J. Kidney Dis. 2016, 67, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Delaney, C.L.; Spark, J.I.; Thomas, J.; Wong, Y.T.; Chan, L.T.; Miller, M.D. A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 2013, 229, 1–9. [Google Scholar] [CrossRef]
- Mastropietro, A.; Rizzo, G.; Fontana, L.; Figini, M.; Bernardini, B.; Straffi, L.; Marcheselli, S.; Ghirmai, S.; Nuzzi, N.P.; Malosio, M.L.; et al. Microstructural characterization of corticospinal tract in subacute and chronic stroke patients with distal lesions by means of advanced diffusion MRI. Neuroradiology 2019, 61, 1033–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemanich, S.T.; Mueller, B.A.; Gillick, B.T. Neurite orientation dispersion and density imaging quantifies corticospinal tract microstructural organization in children with unilateral cerebral palsy. Hum. Brain Mapp. 2019, 40, 4888–4900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, Y.; Ueno, Y.; Tanaka, Y.; Okuzumi, A.; Miyamoto, N.; Yamashiro, K.; Tanaka, R.; Hattori, N.; Urabe, T. Aging, aortic arch calcification, and multiple brain infarcts are associated with aortogenic brain embolism. Cerebrovasc. Dis. 2013, 35, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Suzuki, K.; Okada, K.; Miura, R.; Fujii, T.; Etsurou, M.; Yamadori, A. Normative data on tests for frontal lobe functions: Trail Making Test, Verbal fluency, Wisconsin Card Sorting Test (Keio version). No To Shinkei 2004, 56, 567–574. [Google Scholar]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, J.L.R.; Sotiropoulos, S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, G.; Fazekas, F.; Schmidt, R.; Kapeller, P.; Offenbacher, H.; Krejs, G.J. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J. Neurol. Sci. 1995, 134, 83–88. [Google Scholar] [CrossRef]
- Kurella, M.; Chertow, G.M.; Luan, J.; Yaffe, K. Cognitive impairment in chronic kidney disease. J. Am. Geriatr. Soc. 2004, 52, 1863–1869. [Google Scholar] [CrossRef]
- Sehgal, A.R.; Grey, S.F.; DeOreo, P.B.; Whitehouse, P.J. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am. J. Kidney Dis. 1997, 30, 41–49. [Google Scholar] [CrossRef]
- Antoine, V.; Souid, M.; Andre, C.; Barthelemy, F.; Saint-Jean, O. Symptoms and quality of life of hemodialysis patients aged 75 and over. Nephrologie 2004, 25, 89–96. [Google Scholar] [PubMed]
- Ng, T.P.; Feng, L.; Nyunt, M.S.; Feng, L.; Gao, Q.; Lim, M.L.; Collinson, S.L.; Chong, M.S.; Lim, W.S.; Lee, T.S.; et al. Metabolic Syndrome and the Risk of Mild Cognitive Impairment and Progression to Dementia: Follow-up of the Singapore Longitudinal Ageing Study Cohort. JAMA Neurol. 2016, 73, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Bryant, S.E.; Humphreys, J.D.; Smith, G.E.; Ivnik, R.J.; Graff-Radford, N.R.; Petersen, R.C.; Lucas, J.A. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 2008, 65, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Suzuki, H.; Yasunaga, M.; Sugiyama, M.; Ijuin, M.; Sakuma, N.; Inagaki, H.; Iwasa, H.; Ura, C.; Yatomi, N.; et al. Brief screening tool for mild cognitive impairment in older Japanese: Validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr. Gerontol. Int. 2010, 10, 225–232. [Google Scholar] [CrossRef]
- Snowdon, D.A.; Greiner, L.H.; Mortimer, J.A.; Riley, K.P.; Greiner, P.A.; Markesbery, W.R. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 1997, 277, 813–817. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Erkinjuntti, T.; Reisberg, B.; Roman, G.; Sawada, T.; Pantoni, L.; Bowler, J.V.; Ballard, C.; DeCarli, C.; Gorelick, P.B.; et al. Vascular cognitive impairment. Lancet Neurol. 2003, 2, 89–98. [Google Scholar] [CrossRef]
- Yakushiji, Y.; Nishiyama, M.; Yakushiji, S.; Hirotsu, T.; Uchino, A.; Nakajima, J.; Eriguchi, M.; Nanri, Y.; Hara, M.; Horikawa, E.; et al. Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke 2008, 39, 3323–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Flier, W.M.; van Straaten, E.C.; Barkhof, F.; Verdelho, A.; Madureira, S.; Pantoni, L.; Inzitari, D.; Erkinjuntti, T.; Crisby, M.; Waldemar, G.; et al. Small vessel disease and general cognitive function in nondisabled elderly: The LADIS study. Stroke 2005, 36, 2116–2120. [Google Scholar] [CrossRef]
- Hata, R.; Matsumoto, M.; Handa, N.; Terakawa, H.; Sugitani, Y.; Kamada, T. Effects of hemodialysis on cerebral circulation evaluated by transcranial Doppler ultrasonography. Stroke 1994, 25, 408–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanai, H.; Hirakata, H.; Nakane, H.; Fujii, K.; Hirakata, E.; Ibayashi, S.; Kuwabara, Y. Depressed cerebral oxygen metabolism in patients with chronic renal failure: A positron emission tomography study. Am. J. Kidney Dis. 2001, 38, S129–S133. [Google Scholar] [CrossRef]
- Murray, A.M. Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv. Chronic Kidney Dis. 2008, 15, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Varjacic, A.; Mantini, D.; Demeyere, N.; Gillebert, C.R. Neural signatures of Trail Making Test performance: Evidence from lesion-mapping and neuroimaging studies. Neuropsychologia 2018, 115, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Arbuthnott, K.; Frank, J. Trail making test, part B as a measure of executive control: Validation using a set-switching paradigm. J. Clin. Exp. Neuropsychol. 2000, 22, 518–528. [Google Scholar] [CrossRef]
- Glascher, J.; Adolphs, R.; Damasio, H.; Bechara, A.; Rudrauf, D.; Calamia, M.; Paul, L.K.; Tranel, D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2012, 109, 14681–14686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, B.; Rosser, N.; Tabeling, S.; Sturenburg, H.J.; de Haan, B.; Karnath, H.O.; Wessel, K. Errors on the Trail Making Test Are Associated with Right Hemispheric Frontal Lobe Damage in Stroke Patients. Behav. Neurol. 2015, 2015, 309235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miskin, N.; Thesen, T.; Barr, W.B.; Butler, T.; Wang, X.; Dugan, P.; Kuzniecky, R.; Doyle, W.; Devinsky, O.; Blackmon, K. Prefrontal lobe structural integrity and trail making test, part B: Converging findings from surface-based cortical thickness and voxel-based lesion symptom analyses. Brain Imaging Behav. 2016, 10, 675–685. [Google Scholar] [CrossRef] [PubMed]

| Duration of LCAR Treatment | p | |||
|---|---|---|---|---|
| Total | No or Short-Term | Long-Term | ||
| Characteristic | n = 14 | n = 7 | n = 7 | |
| Sociodemographic | ||||
| Age, years, mean ± SD | 70.8 ± 7.2 | 72.6 ± 9.7 | 69.0 ± 3.3 | 0.259 |
| Sex, male, no. (%) | 8 (57) | 4 (57) | 4 (57) | 0.589 |
| Final education | 0.431 | |||
| High school | 4 (29) | 1 (14) | 3 (43) | |
| Business school | 2 (14) | 1 (14) | 1 (14) | |
| Junior college | 3 (21) | 1 (14) | 2 (29) | |
| University | 5 (36) | 4 (57) | 1 (14) | |
| Body mass index | 20.6 ± 4.2 | 21.8 ± 5.3 | 19.3 ± 2.3 | 0.456 |
| Risk factors, no. (%) | ||||
| Hypertension | 13 (93) | 7 (100) | 6 (86) | 1 |
| Diabetes mellitus | 6 (43) | 3 (43) | 3 (43) | 0.589 |
| Dyslipidemia | 8 (57) | 3 (43) | 5 (71) | 0.589 |
| Current cigarette smoking | 1 (7) | 1 (14) | 0 (0) | 1 |
| Coronary artery disease | 2 (14) | 1 (14) | 1 (14) | 0.445 |
| Previous history of stroke | 3 (21) | 2 (29) | 1 (14) | 1 |
| Cause of renal failure | 0.718 | |||
| Glomerulonephritis | 3 (21) | 1 (14) | 2 (29) | |
| Diabetic nephropathy | 6 (43) | 3 (43) | 3 (43) | |
| Nephrosclerosis | 1 (7) | 1 (14) | 0 (0) | |
| Others | 4 (29) | 2 (29) | 2 (29) | |
| Total time of hemodialysis | 1217 ± 687 | 870 ± 750 | 1564 ± 405 | 0.097 |
| Total amount of LCAR, g | 496.4 ± 311.4 | 241.3 ± 233.1 | 751.4 ± 62.6 | 0.002 |
| Laboratory findings, mean ± SD | ||||
| Leukocyte count, 102/μL | 60.9 ± 16.6 | 64.1 ± 19.1 | 57.7 ± 13.4 | 0.413 |
| LDL-C, mg/dL | 84.5 ± 24.1 | 91.0 ± 28.7 | 78.0 ± 16.6 | 0.195 |
| HDL-C, mg/dL | 55.5 ± 15.1 | 51.0 ± 13.9 | 60.1 ± 15.1 | 0.082 |
| Triglycerides, mg/dL | 109.3 ± 43.7 | 112.2 ± 43.4 | 106.3 ± 43.4 | 0.772 |
| Glucose, mg/dL | 133.9 ± 45.5 | 135.4 ± 44.8 | 132.4 ± 47.2 | 0.782 |
| eGFR, mL/min | 4.7 ± 2.5 | 4.3 ± 1.8 | 5.2 ± 3.1 | 0.481 |
| Creatinine, mg/dL | 10.1 ± 2.6 | 10.2 ± 3.2 | 9.9 ± 2.0 | 0.93 |
| Hs-CRP, mg/dL | 0.41 ± 0.65 | 0.65 ± 0.68 | 0.17 ± 0.52 | <0.001 |
| Calcium, mg/dL | 9.0 ± 0.6 | 8.8 ± 0.8 | 9.2 ± 0.2 | 0.173 |
| Phosphate, mg/dL | 5.2 ± 1.4 | 5.5 ± 1.5 | 5.0 ± 1.3 | 0.191 |
| Conventional MRI | ||||
| PVH, grade 0–3 | 1.5 ± 0.7 | 1.4 ± 0.5 | 1.6 ± 0.8 | 0.902 |
| DSWMH, grade 0–3 | 1.1 ± 0.7 | 1.1 ±0.7 | 1.0 ±0.8 | 0.805 |
| Number of CMBs | 2.9 ± 6.4 | 5.1 ± 8.7 | 0.7 ± 1.0 | 0.318 |
| Cognitive function test | ||||
| MMSE | 25.4 ± 3.5 | 26.0 ± 2.6 | 24.9 ± 4.3 | 0.71 |
| HDS-R | 26.3 ± 2.3 | 27.1 ± 1.5 | 25.4 ± 2.8 | 0.259 |
| FAB | 14.9 ± 3.2 | 15.0 ± 4.2 | 14.7 ± 2.0 | 0.318 |
| MoCA-J | 23.9 ± 3.2 | 23.9 ± 3.8 | 23.9 ± 2.8 | 0.71 |
| Modality | Contrast | Cluster Size | Anatomical Region | Peak t-Value | Peak MNI Coordinates (X, Y, Z) |
|---|---|---|---|---|---|
| DTI | |||||
| FA | HC > NSTLC | 48,825 | Bilateral ATR, corticospinal tract, CCG, forceps minor and major, IFOF, ILF, SLF, SLF temporal part, medial lemniscus, CP, ALIC, PLIC, retrolenticular part of IC, ACR, SCR, PCR, PTR, SS, external capsule, fornix stria terminalis, SFOF, tapetum; left UF, corticospinal tract, ICP, UF; right SCP; MCP, pontine crossing tract, genu, body and splenium of CC, fornix | 6.74 | (74, 69, 105) |
| AD | HC < NSTLC | 10,699 | Bilateral ATR, corticospinal tract, IFOF, SLF, ALIC, PLIC, retrolenticular part of IC, ACR, SCR, PCR, PTR, external capsule, fornix stria terminalis, SFOF; left ILF, SS, tapetum; forceps minor, UF, genu, body and splenium of CC, fornix | 5.85 | (50, 125, 100) |
| RD | HC < NSTLC | 49,556 | Bilateral ATR, corticospinal tract, CCG, IFOF, ILF, SLF, UF, SLF temporal part, CP, ALIC, PLIC, retrolenticular part of IC, ACR, SCR, PCR, PTR, SS, external capsule, fornix stria terminalis, SFOF, tapetum; forceps minor and major, genu, body and splenium of CC, fornix | 7.43 | (140, 117, 50) |
| MD | HC < NSTLC | 43,676 | Bilateral ATR, corticospinal tract, CCG, IFOF, ILF, SLF, UF, SLF temporal part, ALIC, PLIC, retrolenticular part of IC, ACR, SCR, PCR, PTR, SS, external capsule, fornix stria terminalis, SFOF, tapetum; left CHp; forceps minor and major, genu, body and splenium of CC, fornix | 7.10 | (53, 104, 105) |
| DKI | |||||
| AK | HC > NSTLC | 15,653 | Bilateral corticospinal tract, IFOF, ILF, SLF, PLIC, retrolenticular part of IC, ACR, SCR, PCR, PTR, external capsule, fornix stria terminalis, tapetum; right ATR, cingulum hippocampus, UF, SS, CCG, CHp; forceps minor and major, body and splenium of CC | 6.03 | (53, 65, 61) |
| HC > LTLC | 16,138 | Bilateral corticospinal tract, CHp, IFOF, ILF, SLF, SLF temporal part, corticospinal tract, medial lemniscus, SCP, CP, PLIC, retrolenticular part of IC, SCR, PCR, PTR, SS, fornix stria terminalis, tapetum; left ATR, UF, ALIC, ACR, external capsule, SFOF; forceps major, MCP, pontine crossing tract, body and splenium of CC | 6.19 | (55, 92, 82) | |
| RK | HC > NSTLC | 45,239 | Bilateral ATR, corticospinal tract, CCG, IFOF, ILF, SLF, UF, SLF temporal part, ALIC, retrolenticular part of IC, ACR, SCR, PCR, PTR, SS, external capsule, fornix stria terminalis, SFOF; right UF, tapetum; forceps minor and major, genu, body and splenium of CC | 6.11 | (98, 172, 110) |
| HC > LTLC | 69 | forceps minor | 4.89 | (79, 177, 101) | |
| MK | HC > NSTLC | 28,546 | Bilateral ATR, IFOF, ILF, SLF, ALIC, ACR, SCR, PCR, PTR, SS, external capsule, SFOF; left corticospinal tract, UF, retrolenticular part of IC; forceps minor and major, genu, body and splenium of CC | 6.82 | (97, 141, 131) |
| NODDI | |||||
| ICVF | HC > NSTLC | 67,959 | Bilateral ATR, corticospinal tract, CCG, IFOF, ILF, SLF, UF, SLF temporal part, CP, ALIC, PLIC, retrolenticular part of IC, ACR, SCR, PCR, PTR, SS, external capsule, CHp, fornix stria terminalis, SFOF, tapetum; left UF; right CHp; forceps minor and major, genu, body and splenium of CC | 8.15 | (106, 82, 84) |
| HC > LTLC | 38,021 | Bilateral ATR, corticospinal tract, CCG, IFOF, ILF, SLF, UF, SLF temporal part, ALIC, PLIC, retrolenticular part of IC, ACR, SCR, PCR, PTR, SS, external capsule, CHp, fornix stria terminalis, SFOF; left tapetum; right CHp, CP; forceps minor and major, genu, body and splenium of CC | 6.22 | (113, 63, 103) | |
| ISO | HC > LTLC | 3978 | right ATR, Inferior fronto-occipital fasciculus, ILF, UF, retrolenticular part of IC, ACR, SCR, PTR, SS; forceps minor and major | 5.88 | (48, 116, 49) |
| NSTLC > LTLC | 8312 | Bilateral corticospinal tract, IFOF, ILF, SLF, UF, SCP, CP, retrolenticular part of IC, PTR, SS; right ATR, CHp, SLF temporal part, ALIC, PLIC, external capsule, fornix stria terminalis; forceps minor and major, MCP, pontine crossing tract | 7.52 | (129, 83, 68) | |
| Region | FA | AD | RD | MD | AK | RK | MK | ICVF | ISO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p | R | p | |
| Whole | 0.628 | 0.016 | 0.604 | 0.022 | −0.741 | 0.002 * | −0.591 | 0.026 | −0.609 | 0.021 | −0.547 | 0.043 | ||||||
| Lcc | −0.543 | 0.045 | −0.745 | 0.002 * | ||||||||||||||
| Rcc | −0.609 | 0.021 | 0.669 | 0.009 | 0.591 | 0.026 | −0.565 | 0.035 | ||||||||||
| Latr | 0.679 | 0.008 | 0.6 | 0.023 | −0.771 | 0.001 * | −0.613 | 0.02 | ||||||||||
| Ratr | 0.793 | 0.001 * | 0.554 | 0.04 | 0.648 | 0.012 | −0.248 | 0.392 | 0.609 | 0.021 | ||||||||
| Lcs | 0.644 | 0.013 | ||||||||||||||||
| Rcs | 0.569 | 0.034 | 0.559 | 0.038 | ||||||||||||||
| fm | 0.569 | 0.034 | 0.678 | 0.008 | −0.789 | 0.001 * | ||||||||||||
| fmi | −0.574 | 0.032 | −0.644 | 0.013 | −0.534 | 0.049 | ||||||||||||
| Lifof | −0.613 | 0.02 | −0.582 | 0.029 | ||||||||||||||
| Rifof | −0.648 | 0.012 | −0.591 | 0.026 | ||||||||||||||
| Lslf | −0.613 | 0.02 | ||||||||||||||||
| Rslf | −0.758 | 0.002 * | −0.574 | 0.032 | ||||||||||||||
| Lilf | −0.556 | 0.039 | ||||||||||||||||
| Rilf | −0.578 | 0.03 | ||||||||||||||||
| Rslftemp | −0.587 | 0.027 | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, Y.; Saito, A.; Nakata, J.; Kamagata, K.; Taniguchi, D.; Motoi, Y.; Io, H.; Andica, C.; Shindo, A.; Shiina, K.; et al. Possible Neuroprotective Effects of l-Carnitine on White-Matter Microstructural Damage and Cognitive Decline in Hemodialysis Patients. Nutrients 2021, 13, 1292. https://doi.org/10.3390/nu13041292
Ueno Y, Saito A, Nakata J, Kamagata K, Taniguchi D, Motoi Y, Io H, Andica C, Shindo A, Shiina K, et al. Possible Neuroprotective Effects of l-Carnitine on White-Matter Microstructural Damage and Cognitive Decline in Hemodialysis Patients. Nutrients. 2021; 13(4):1292. https://doi.org/10.3390/nu13041292
Chicago/Turabian StyleUeno, Yuji, Asami Saito, Junichiro Nakata, Koji Kamagata, Daisuke Taniguchi, Yumiko Motoi, Hiroaki Io, Christina Andica, Atsuhiko Shindo, Kenta Shiina, and et al. 2021. "Possible Neuroprotective Effects of l-Carnitine on White-Matter Microstructural Damage and Cognitive Decline in Hemodialysis Patients" Nutrients 13, no. 4: 1292. https://doi.org/10.3390/nu13041292







