Urinary Titin N-Fragment Evaluation in a Randomized Controlled Trial of Beta-Hydroxy-Beta-Methylbutyrate for Acute Mild Trauma in Older Adults
Abstract
:1. Introduction
2. Materials and Methods
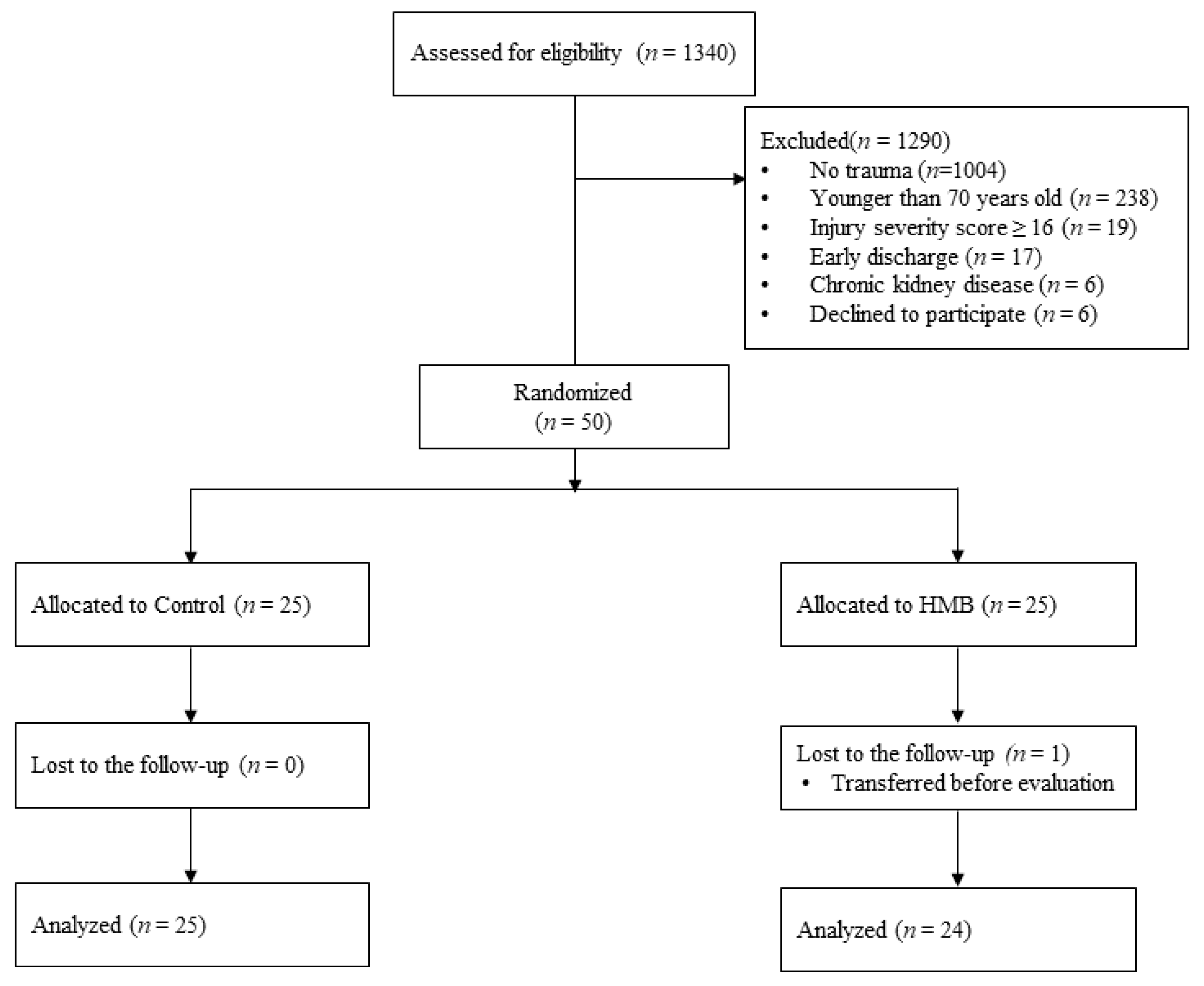
2.1. Patient Selection
2.2. Randomization and Allocation
2.3. Sample Size Estimation
2.4. Protocol
2.5. Outcome Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grossman, M.; Scaff, D.W.; Miller, D.; Reed, J.; Hoey, B.; Anderson, H.L. Functional outcomes in octogenarian trauma. J. Trauma 2003, 55, 26–32. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Azuhata, T.; Yokota, H.; Morimura, N. The swallowing problem after acute care in the elderly patients. J. Jpn. Assoc. Acute Med. 2019, 30, 103–109. [Google Scholar] [CrossRef]
- Matsuo, M.; Awano, H.; Maruyama, N.; Nishio, H. Titin fragment in urine: A noninvasive biomarker of muscle degradation. Adv. Clin. Chem. 2019, 90, 1–23. [Google Scholar] [CrossRef]
- Nakano, H.; Hashimoto, H.; Mochizuki, M.; Naraba, H.; Takahashi, Y.; Sonoo, T.; Matsubara, T.; Yamakawa, K.; Nakamura, K. Urine Titin N-fragment as a biomarker of muscle injury for critical illness myopathy. Am. J. Respir. Crit. Care Med. 2021, 203, 515–518, in press. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B.; et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The health, aging, and body composition (Health ABC) study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beasley, J.M.; LaCroix, A.Z.; Neuhouser, M.L.; Huang, Y.; Tinker, L.F.; Woods, N.F.A.; Michael, Y.L.; Curb, J.D.; Prentice, R.L. Protein intake and incident frailty in the Women’s Health Initiative observational study. J. Am. Geriatr. Soc. 2010, 58, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Koopman, R.; Verdijk, L.; Manders, R.J.; Gijsen, A.P.; Gorselink, M.; Pijpers, E.; Wagenmakers, A.J.M.; Van Loon, L.J.C. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am. J. Clin. Nutr. 2006, 84, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Wu, H.; Xia, Y.; Jiang, J.; Du, H.; Guo, X.; Liu, X.; Li, C.; Huang, G.; Niu, K. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [CrossRef]
- Stout, J.R.; Smith-Ryan, A.E.; Fukuda, D.H.; Kendall, K.L.; Moon, J.R.; Hoffman, J.R.; Wilson, J.M.; Oliver, J.S.; Mustad, V.A. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: A randomized, double-blind pilot trial. Exp. Gerontol. 2013, 48, 1303–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Kihata, A.; Naraba, H.; Kanda, N.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. β-Hydroxy-β-methylbutyrate, arginine, and glutamine complex on muscle volume loss in critically Ill patients: A randomized control trial. J. Parenter. Enter. Nutr. 2020, 44, 205–212. [Google Scholar] [CrossRef]
- Moreno, R.; Vincent, J.L.; Matos, R.; Mendonça, A.; Cantraine, F.; Thijs, L.; Takala, J.; Sprung, C.; Antonelli, M.; Bruining, H.; et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working group on sepsis related problems of the ESICM. Intensive Care Med. 1999, 25, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, N.; Asai, T.; Abe, C.; Inada, A.; Kawauchi, T.; Miyashita, K.; Maeda, M.; Matsuo, M.; Nabeshima, Y.-I. Establishment of a highly sensitive sandwich ELISA for the N-terminal fragment of titin in urine. Sci. Rep. 2016, 6, 39375. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Henriksen, K.; Karsdal, M.; Armbrecht, G.; Belavý, D.; Felsenberg, D.; Rittweger, J.; Wang, Y.; Zheng, Q.; Nedergaard, A. Measurement of a MMP-2 degraded Titin fragment in serum reflects changes in muscle turnover induced by atrophy. Exp. Gerontol. 2014, 58, 83–89. [Google Scholar] [CrossRef]
- Baier, S.; Johannsen, D.; Abumrad, N.; Rathmacher, J.A.; Nissen, S.; Flakoll, P. Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta-hydroxy-beta-methylbutyrate (HMB), L-arginine, and L-lysine. J. Parenter. Enter. Nutr. 2009, 33, 71–82. [Google Scholar] [CrossRef]
- Flakoll, P.; Sharp, R.; Baier, S.; Levenhagen, D.; Carr, C.; Nissen, S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004, 20, 445–451. [Google Scholar] [CrossRef]
- Bos, C.; Benamouzig, R.; Bruhat, A.; Roux, C.; Valensi, P.; Ferrière, F.; Tomé, D. Nutritional status after short-term dietary supplementation in hospitalized malnourished geriatric patients. Clin. Nutr. 2001, 20, 225–233. [Google Scholar] [CrossRef]
- Deutz, N.E.; Pereira, S.L.; Hays, N.P.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef]
- Hermans, G.; Casaer, M.P.; Clerckx, B.; Güiza, F.; Vanhullebusch, T.; Derde, S.; Meersseman, P.; Derese, I.; Mesotten, D.; Wouters, P.J.; et al. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: A subanalysis of the EPaNIC trial. Lancet Respir. Med. 2013, 1, 621–629. [Google Scholar] [CrossRef]
- Bertolini, G.; Iapichino, G.; Radrizzani, D.; Facchini, R.; Simini, B.; Bruzzone, P.; Zanforlin, G.; Tognoni, G. Early enteral immunonutrition in patients with severe sepsis: Results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med. 2003, 29, 834–840. [Google Scholar] [CrossRef]
- Bower, R.H.; Cerra, F.B.; Bershadsky, B.; Licari, J.J.; Hoyt, D.B.; Jensen, G.L.; Van Buren, C.T.; Rothkopf, M.M.; Daly, J.M.; Adelsberg, B.R. Early enteral administration of a formula (Impact) supplemented with arginine, nucleotides, and fish oil in intensive care unit patients: Results of a multicenter, prospective, randomized, clinical trial. Crit. Care Med. 1995, 23, 436–449. [Google Scholar] [CrossRef]
- Nowson, C.; O’Connell, S. Protein requirements and recommendations for older people: A review. Nutrients 2015, 7, 6874–6899. [Google Scholar] [CrossRef] [Green Version]
- Crossland, H.; Skirrow, S.; Puthucheary, Z.A.; Constantin-Teodosiu, D.; Greenhaff, P.L. The impact of immobilisation and inflammation on the regulation of muscle mass and insulin resistance: Different routes to similar end-points. J. Physiol. 2019, 597, 1259–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E. Nutrition therapy and critical illness: Practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit. Care. 2019, 23, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| HMB | Control | p | |
|---|---|---|---|
| n | 24 | 25 | |
| Age, years | 83.8 ± 5.2 | 84.9 ± 7.0 | 0.523 |
| Male, n (%) | 12 (50.0) | 10 (40.0) | 0.677 |
| Body height, cm | 152.2 ± 10.3 | 152.6 ± 8.3 | 0.866 |
| Body weight, kg | 50.4 ± 11.4 | 51.6 ± 8.8 | 0.708 |
| SOFA initial | 1.0 (0.8 to 3.0) | 2.0 (1.0 to 3.0) | 0.438 |
| SOFA maximum | 3.0 (2.0 to 5.2) | 4.0 (3.0 to 4.0) | 0.598 |
| APACHE II | 10.0 (8.0 to 11.2) | 10.0 (8.0 to 16.0) | 0.644 |
| ISS | 4.0 (4.0 to 9.0) | 9.0 (4.0 to 9.0) | 0.152 |
| CCI | 2.0 (1.0 to 3.2) | 1.0 (1.0 to 2.0) | 0.431 |
| Length of hospitalization, day | 19.5 (13.8 to 39.0) | 15.0 (5.0 to 25.0) | 0.055 |
| ICU admission, n (%) | 3 (12.5) | 0 (0.0) | 0.219 |
| Duration of ICU admission | 0.7 ± 2.2 | 0.0 ± 0.0 | 0.143 |
| Death, n (%) | 1 (4.2) | 1 (4.0) | 1 |
| MV use, n (%) | 3 (12.5) | 0 (0.0) | 0.219 |
| Duration of MV use, day | 0.4 ± 1.4 | 0.0 ± 0.0 | 0.199 |
| RRT use, n (%) | 1 (4.2) | 0 (0.0) | 0.984 |
| Calorie delivery, kcal * | 20.9 ± 10.3 | 19.5 ± 7.9 | 0.626 |
| Protein delivery, g * | 0.9 ± 0.4 | 0.8 ± 0.4 | 0.475 |
| HMB | Control | p | |
|---|---|---|---|
| n | 24 | 25 | |
| Follow-up evaluation day | 14.7 ± 3.1 | 15.1 ± 3.0 | 0.605 |
| RFCSA | |||
| pre, cm2 | 2.49 ± 0.77 | 2.28 ± 0.59 | 0.3 |
| post, cm2 | 2.41 ± 0.95 | 2.45 ± 1.09 | 0.887 |
| change, cm2 | −0.04 (−0.58 to 0.50) | 0.33 (−0.10 to 0.53) | 0.25 |
| Grip strength | |||
| pre, kg | 12.5 ± 7.3 | 10.4 ± 7.0 | 0.314 |
| post, kg | 13.3 ± 9.1 | 13.1 ± 8.9 | 0.946 |
| change, kg | 0.8 (−1.0 to 3.4) | 1.0 (0.0 to 3.0) | 0.508 |
| Barthel Index | |||
| pre | 100.0 (93.8 to 100.0) | 100.0 (95.0 to 100.0) | 0.71 |
| post | 20.0 (10.0 to 75.0) | 50.0 (20.0 to 90.0) | 0.404 |
| change | −42.5 (−81.2 to −23.8) | −35.0 (−60.0 to −10.0) | 0.311 |
| N-titin/Cre | |||
| Day 1, pmol/mgCre | 22.3 (15.9 to 29.7) | 20.6 (12.7 to 34.2) | 0.801 |
| Day 3, pmol/mgCre | 27.3 (19.1 to 39.1) | 18.2 (12.1 to 25.4) | 0.046 * |
| change, pmol/mgCre | 5.9 (−0.4 to 16.5) | 0.3 (−4.3 to 10.2) | 0.12 |
| HMB | Control | p | |
|---|---|---|---|
| n | 19 | 23 | |
| Age, years | 84.1 ± 4.8 | 84.7 ± 7.1 | 0.776 |
| Male, n (%) | 8 (42.1) | 10 (43.5) | 1 |
| SOFA maximum | 2.0 (2.0 to 3.0) | 3.0 (2.5 to 4.0) | 0.091 |
| APACHE II | 9.0 (8.0 to 11.0) | 10.0 (8.0 to 12.5) | 0.359 |
| ISS | 4.0 (4.0 to 9.0) | 9.0 (5.0 to 9.0) | 0.089 |
| CCI | 2.0 (1.0 to 3.5) | 1.0 (1.0 to 2.5) | 0.315 |
| Length of hospitalization, day | 19.0 (13.5 to 32.0) | 15.0 (4.5 to 25.0) | 0.069 |
| ICU admission, n (%) | 0.0 (0.0) | 0.0 (0.0) | NA |
| Death, n (%) | 0.0 (0.0) | 0.0 (0.0) | NA |
| Follow-up evaluation day | 14.1 ± 2.5 | 15.1 ± 2.9 | 0.257 |
| RFCSA | |||
| pre, cm2 | 2.38 ± 0.75 | 2.33 ± 0.59 | 0.807 |
| post, cm2 | 2.32 ± 0.88 | 2.56 ± 1.06 | 0.427 |
| change, cm2 | 0.05 (−0.52 to 0.46) | 0.33 (−0.09 to 0.57) | 0.216 |
| Grip strength | |||
| pre, kg | 13.2 ± 7.2 | 10.5 ± 6.8 | 0.222 |
| post, kg | 14.8 ± 8.8 | 13.5 ± 8.8 | 0.641 |
| change, kg | 1.0 (−0.7 to 3.5) | 1.4 (0.0 to 3.5) | 0.63 |
| Barthel Index | |||
| pre | 100.0 (90.0 to 100.0) | 100.0 (95.0 to 100.0) | 0.701 |
| post | 30.0 (15.0 to 75.0) | 55.0 (25.0 to 92.5) | 0.543 |
| change | −35.0 (−77.5 to −22.5) | −35.0 (−60.0 to −7.5) | 0.751 |
| N-titin/Cre | |||
| Day 1, pmol/mgCre | 23.3 (15.8 to 37.5) | 20.6 (12.8 to 30.0) | 0.693 |
| Day 3, pmol/mgCre | 25.4 (18.8 to 37.5) | 17.0 (11.5 to 23.9) | 0.077 |
| change, pmol/mgCre | 5.7 (−1.1 to 11.5) | −0.5 (−5.0 to 4.6) | 0.135 |
| MAX SOFA < 7 | MAX SOFA ≥ 7 | p | |
|---|---|---|---|
| n | 42 | 7 | |
| Age, years | 84.4 ± 6.1 | 83.9 ± 6.6 | 0.829 |
| Male, n (%) | 18 (42.9) | 4 (57.1) | 0.769 |
| SOFA maximum | 3.0 (2.0 to 4.0) | 8.0 (7.5 to 10.5) | <0.001 * |
| APACHE II | 10.0 (8.0 to 11.8) | 18.0 (14.0 to 23.0) | 0.001 * |
| ISS | 8.5 (4.0 to 9.0) | 9.0 (4.0 to 9.0) | 0.917 |
| CCI | 2.0 (1.0 to 3.0) | 1.0 (0.5 to 1.5) | 0.249 |
| Length of hospitalization, day | 15.5 (7.2 to 27.0) | 25.0 (20.0 to 60.5) | 0.109 |
| ICU admission, n (%) | 0 (0.0) | 3 (42.9) | <0.001 * |
| Death, n (%) | 0 (0.0) | 2 (28.6) | 0.012 * |
| Follow-up evaluation day | 14.6 ± 2.8 | 16.4 ± 4.2 | 0.15 |
| RFCSA | |||
| pre, cm2 | 2.35 ± 0.66 | 2.58 ± 0.85 | 0.411 |
| post, cm2 | 2.45 ± 0.98 | 2.33 ± 1.29 | 0.778 |
| change, cm2 | 0.15 (−0.35 to 0.55) | −0.37 (−0.84 to 0.43) | 0.338 |
| Grip strength | |||
| pre, kg | 11.7 ± 7.1 | 9.3 ± 8.2 | 0.41 |
| post, kg | 14.1 ± 8.7 | 7.7 ± 8.3 | 0.079 |
| change, kg | 1.2 (−0.3 to 3.7) | 0.0 (−4.0 to 0.5) | 0.133 |
| Barthel Index | |||
| pre | 100.0 (91.2 to 100.0) | 100.0 (100.0 to 100.0) | 0.391 |
| post | 52.5 (15.0 to 78.8) | 5.0 (0.0 to 30.0) | 0.048 * |
| change | −35.0 (−67.5 to −12.5) | −85.0 (−97.5 to −35.0) | 0.091 |
| N-titin/Cre | |||
| Day 1, pmol/mgCre | 22.1 (15.4 to 33.1) | 18.4 (16.7 to 26.1) | 0.702 |
| Day 3, pmol/mgCre | 19.1 (13.6 to 28.9) | 38.1 (29.2 to 46.8) | 0.03 * |
| change, pmol/mgCre | 1.3 (−4.8 to 8.7) | 18.7 (13.9 to 22.8) | 0.013 * |
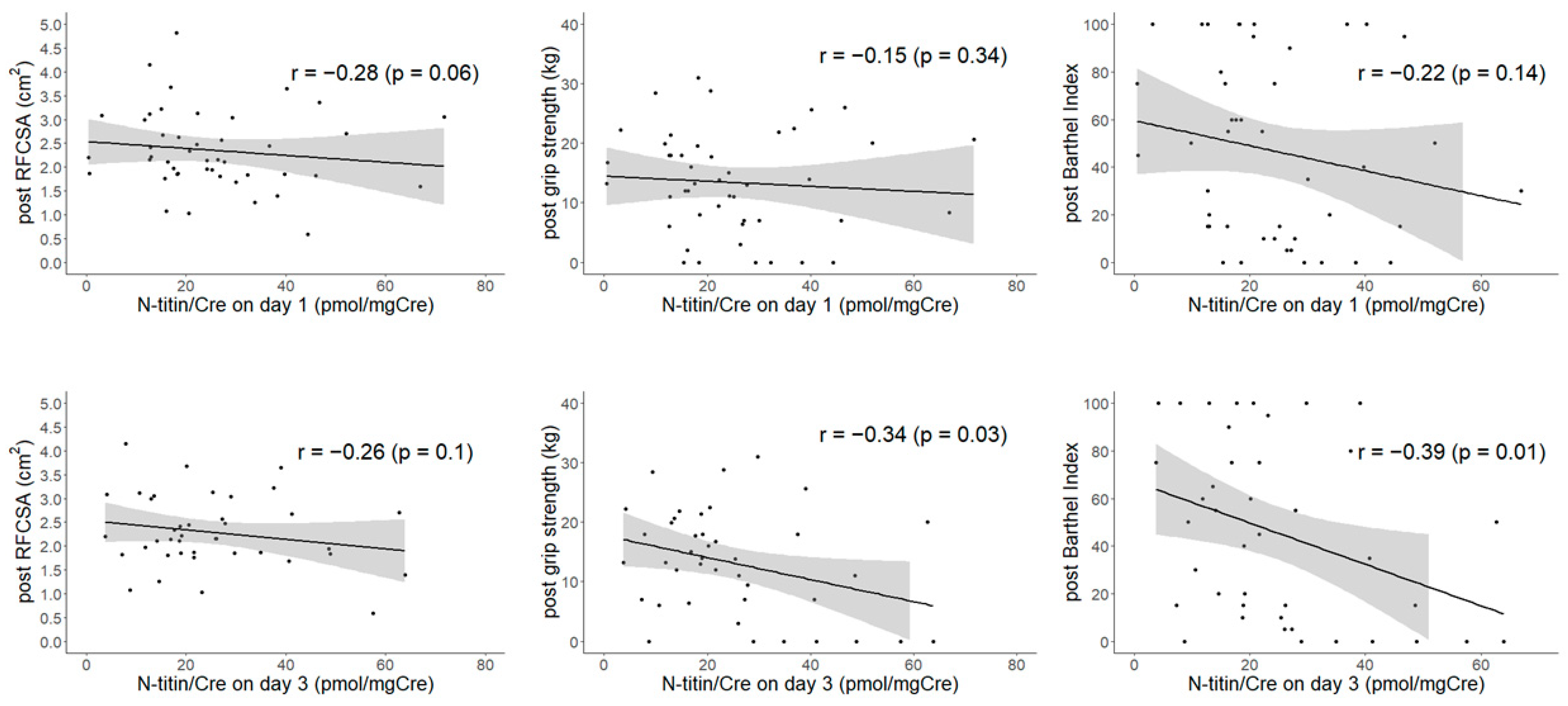
| N-Titin/Cre on Day 1 | N-Titin/Cre on Day 3 | N-Titin/Cre Change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Outpatient | Inpatient | Overall | Outpatient | Inpatient | Overall | Outpatient | Inpatient | |
| RFCSA | |||||||||
| pre, cm2 | −0.12 | −0.34 | −0.04 | −0.08 | −0.47 | 0.09 | 0.03 | −0.11 | 0.07 |
| post, cm2 | −0.28 | −0.31 | −0.29 | −0.26 | −0.38 | −0.21 | −0.24 | −0.45 | −0.19 |
| change, cm2 | −0.25 | −0.3 | −0.23 | −0.23 | −0.29 | −0.18 | −0.22 | −0.4 | −0.17 |
| Grip strength | |||||||||
| pre, kg | −0.17 | 0.4 | −0.3 | −0.26 | 0.3 | −0.39 | −0.31 | −0.21 | −0.25 |
| post, kg | −0.15 | −0.04 | −0.14 | −0.34 * | 0.29 | −0.47 * | −0.34 * | 0.17 | −0.34 |
| change, kg | −0.04 | −0.48 | 0.16 | −0.13 | 0.02 | −0.05 | −0.07 | 0.31 | −0.05 |
| Barthel Index | |||||||||
| pre | −0.21 | −0.23 | −0.22 | −0.31 * | −0.08 | −0.35 | −0.32 * | −0.51 | −0.22 |
| post | −0.22 | −0.19 | −0.28 | −0.39 * | −0.02 | −0.47 * | −0.38 * | −0.34 | −0.27 |
| change | −0.08 | −0.19 | −0.03 | −0.27 | −0.11 | −0.12 | −0.22 | −0.29 | −0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, H.; Hashimoto, H.; Mochizuki, M.; Naraba, H.; Takahashi, Y.; Sonoo, T.; Matsuishi, Y.; Ogawa, Y.; Shimojo, N.; Inoue, Y.; et al. Urinary Titin N-Fragment Evaluation in a Randomized Controlled Trial of Beta-Hydroxy-Beta-Methylbutyrate for Acute Mild Trauma in Older Adults. Nutrients 2021, 13, 899. https://doi.org/10.3390/nu13030899
Nakano H, Hashimoto H, Mochizuki M, Naraba H, Takahashi Y, Sonoo T, Matsuishi Y, Ogawa Y, Shimojo N, Inoue Y, et al. Urinary Titin N-Fragment Evaluation in a Randomized Controlled Trial of Beta-Hydroxy-Beta-Methylbutyrate for Acute Mild Trauma in Older Adults. Nutrients. 2021; 13(3):899. https://doi.org/10.3390/nu13030899
Chicago/Turabian StyleNakano, Hidehiko, Hideki Hashimoto, Masaki Mochizuki, Hiromu Naraba, Yuji Takahashi, Tomohiro Sonoo, Yujiro Matsuishi, Yasuhiro Ogawa, Nobutake Shimojo, Yoshiaki Inoue, and et al. 2021. "Urinary Titin N-Fragment Evaluation in a Randomized Controlled Trial of Beta-Hydroxy-Beta-Methylbutyrate for Acute Mild Trauma in Older Adults" Nutrients 13, no. 3: 899. https://doi.org/10.3390/nu13030899
APA StyleNakano, H., Hashimoto, H., Mochizuki, M., Naraba, H., Takahashi, Y., Sonoo, T., Matsuishi, Y., Ogawa, Y., Shimojo, N., Inoue, Y., & Nakamura, K. (2021). Urinary Titin N-Fragment Evaluation in a Randomized Controlled Trial of Beta-Hydroxy-Beta-Methylbutyrate for Acute Mild Trauma in Older Adults. Nutrients, 13(3), 899. https://doi.org/10.3390/nu13030899







