Abdominothoracic Postural Tone Influences the Sensations Induced by Meal Ingestion
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. Experimental Design
2.3. General Procedure
2.4. Probe Meal
2.5. Perception Measurements
2.6. Ancillary Validation Study
2.6.1. Abdominal Girth
2.6.2. Position of the Diaphragm
2.6.3. Intragastric Pressure and Respiratory Rate
2.6.4. Heart Rate and Heart Rate Variability (Vagal Tone)
2.6.5. Galvanic Skin Responses (Sympathetic Activity)
2.7. Statistical Analysis
3. Results
3.1. Demographics and Study Conduction
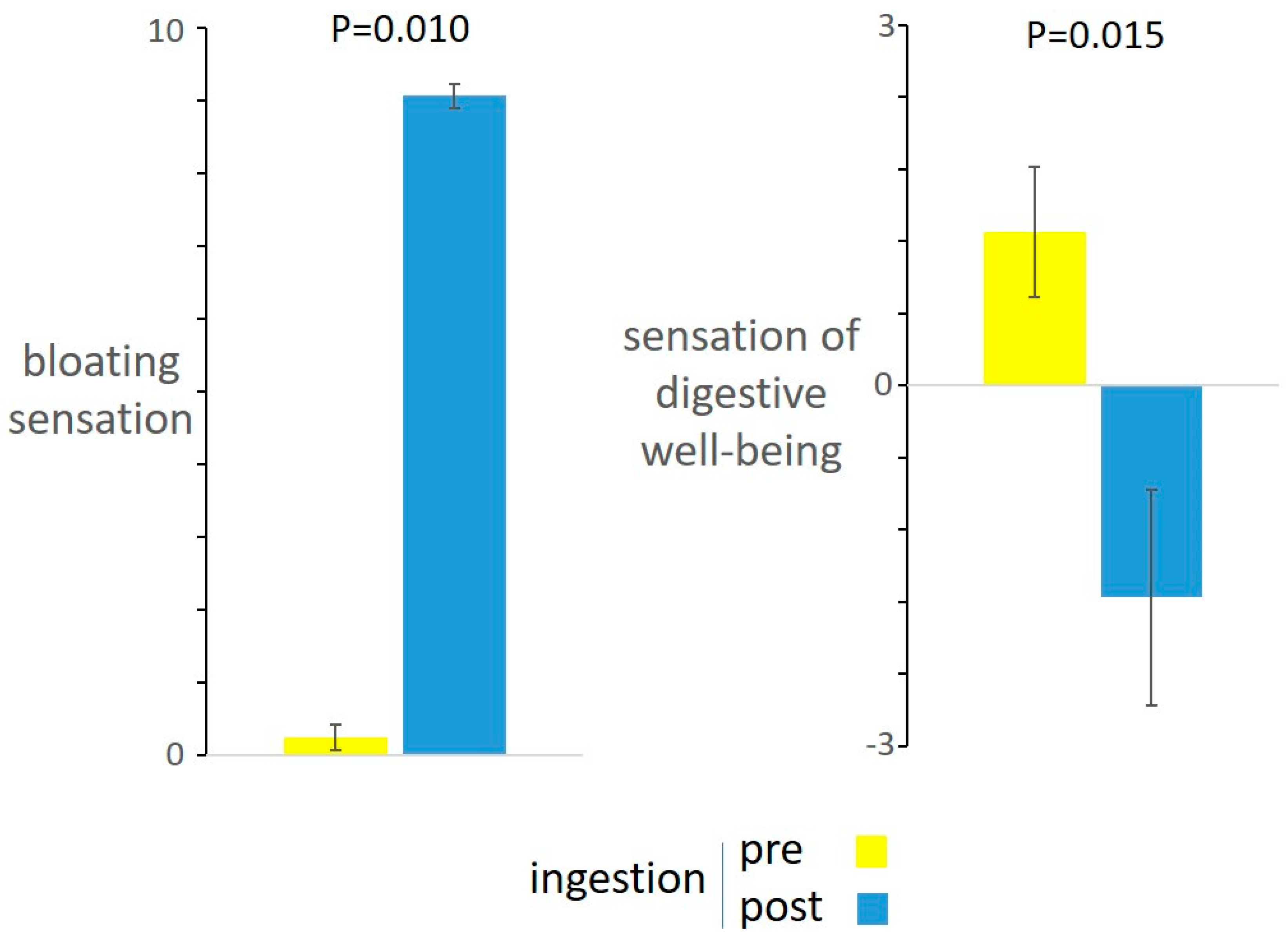
3.2. Responses to Meal Ingestion
3.3. Effect of Thoracoabdominal Postural Tone on Postprandial Sensations
3.4. Effect of Thoracoabdominal Postural Tone on Physiological Parameters (Validation Study)
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azpiroz, F.; Malagelada, J. Abdominal Bloating. Gastroenterology 2005, 129, 1060–1078. [Google Scholar] [CrossRef]
- Azpiroz, F. Intestinal gas. In Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management; Feldman, M.F.L., Brand, L.J., Eds.; Elsevier: Philadelphi, PA, USA, 2020. [Google Scholar]
- Caldarella, M.P.; Azpiroz, F.; Malagelada, J.-R. Selective effects of nutrients on gut sensitivity and reflexes. Gut 2007, 56, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burri, E.; Cisternas, D.; Villoria, A.; Accarino, A.; Soldevilla, A.; Malagelada, J.-R.; Azpiroz, F. Accommodation of the abdomen to its content: Integrated abdomino-thoracic response. Neurogastroenterol. Motil. 2012, 24, 312-e162. [Google Scholar] [CrossRef] [PubMed]
- Burri, E.; Cisternas, D.; Villoria, A.; Accarino, A.; Soldevilla, A.; Malagelada, J.-R.; Azpiroz, F. Abdominal accommodation induced by meal ingestion: Differential responses to gastric and colonic volume loads. Neurogastroenterol. Motil. 2013, 25, 339–e253. [Google Scholar] [CrossRef] [PubMed]
- Burri, E.; Barba, E.; Huaman, J.W.; Cisternas, D.; Accarino, A.; De, G.F.; Malagelada, J.-R.; Azpiroz, F. Mechanisms of postprandial abdominal bloating and distension in functional dyspepsia. Gut 2014, 63, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Azpiroz, F.; Boeckxstaens, G.; Elsenbruch, S.; Feinle-Bisset, C.; Holtmann, G.; Lackner, J.M.; Ronkainen, J.; Schemann, M.; Stengel, A.; et al. Functional dyspepsia. Nat. Rev. Dis. Primers 2017, 3, 17081. [Google Scholar] [CrossRef]
- Barba, E.; Burri, E.; Accarino, A.; Cisternas, D.; Quiroga, S.; Monclus, E.; Navazo, I.; Malagelada, J.R.; Azpiroz, F. Abdomino-thoracic mechanisms of functional abdominal distension and correction by biofeedback. Gastroenterology 2015, 148, 732–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba, E.; Accarino, A.; Azpiroz, F. Correction of Abdominal Distention by Biofeedback-Guided Control of Abdominothoracic Muscular Activity in a Randomized, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2017, 15, 1922–1929. [Google Scholar] [CrossRef] [Green Version]
- Barba, E.; Sánchez, B.; Burri, E.; Accarino, A.; Monclus, E.; Navazo, I.; Guarner, F.; Margolles, A.; Azpiroz, F. Abdominal distension after eating lettuce: The role of intestinal gas evaluated in vitro and by abdominal CT imaging. Neurogastroenterol. Motil. 2019, 31, e13703. [Google Scholar] [CrossRef] [Green Version]
- Chassany, O.; Tugaut, B.; Marrel, A.; Guyonnet, D.; Arbuckle, R.; Duracinsky, M.; Whorwell, P.J.; Azpiroz, F. The Intestinal Gas Questionnaire: Development of a new instrument for measuring gas-related symptoms and their impact on daily life. Neurogastroenterol. Motil. 2015, 27, 885–898. [Google Scholar] [CrossRef]
- Huaman, J.-W.; Mego, M.; Manichanh, C.; Cañellas, N.; Cañueto, D.; Segurola, H.; Jansana, M.; Malagelada, C.; Accarino, A.; Vulevic, J.; et al. Effects of Prebiotics vs. a Diet Low in FODMAPs in Patients with Functional Gut Disorders. Gastroenterology 2018, 155, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Le Nevé, B.; De La Torre, A.M.; Tap, J.; Derrien, M.; Cotillard, A.; Barba, E.; Mego, M.; Ruiz, A.N.; Hernandez-Palet, L.; Dornic, Q.; et al. A Fermented Milk Product with B. lactis CNCM I-2494 and Lactic Acid Bacteria Improves Gastrointestinal Comfort in Response to a Challenge Diet Rich in Fermentable Residues in Healthy Subjects. Nutrients 2020, 12, 320. [Google Scholar] [CrossRef] [Green Version]
- Malagelada, C.; Accarino, A.; Molne, L.; Méndez, S.; Campos, E.; Gonzalez, A.; Malagelada, J.R.; Azpiroz, F. Digestive, cognitive and hedonic responses to a meal. Neurogastroenterol. Motil. 2015, 27, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Tremolaterra, F.; Villoria, A.; Azpiroz, F.; Serra, J.; Aguadé, S.; Malagelada, J. Impaired Viscerosomatic Reflexes and Abdominal-Wall Dystony Associated With Bloating. Gastroenterology 2006, 130, 1062–1068. [Google Scholar] [CrossRef]
- Passos, M.C.; Serra, J.; Azpiroz, F.; Tremolaterra, F.; Malagelada, J.-R. Impaired reflex control of intestinal gas transit in patients with abdominal bloating. Gut 2005, 54, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Hernando-Harder, A.C.; Serra, J.; Azpiroz, F.; Malagelada, J.-R. Sites of symptomatic gas retention during intestinal lipid perfusion in healthy subjects. Gut 2004, 53, 661–665. [Google Scholar] [CrossRef]
- Salvioli, B.; Serra, J.; Azpiroz, F.; Malagelada, J.-R. Impaired Small Bowel Gas Propulsion in Patients with Bloating During Intestinal Lipid Infusion. Am. J. Gastroenterol. 2006, 101, 1853–1857. [Google Scholar] [CrossRef]
- Merletti, R.; Hermens, H.J. Detection and conditioning of the surface EMG signal. In Electromyography—Physiology, Engineering, and Noninvasive Applications, 1st ed.; Merletti, R., Parker, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 107–131. [Google Scholar]
- Ng, J.K.; Kippers, V.; Richardson, C.A. Muscle fibre orientation of abdominal muscles and suggested surface EMG electrode positions. Electromyogr. Clin. Neurophysiol. 1998, 38, 51–58. [Google Scholar] [PubMed]
- Caldarella, M.P.; Serra, J.; Azpiroz, F.; Malagelada, J.-R. Prokinetic effects in patients with intestinal gas retention. Gastroenterology 2002, 122, 1748–1755. [Google Scholar] [CrossRef]
- Salvioli, B.; Serra, J.; Azpiroz, F.; Lorenzo, C.; Aguade, S.; Castell, J.; Malagelada, J.-R. Origin of gas retention and symptoms in patients with bloating. Gastroenterology 2005, 128, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Harder, H.; Serra, J.; Azpiroz, F.; Passos, M.C.; Aguadé, S.; Malagelada, J.-R. Intestinal gas distribution determines abdominal symptoms. Gut 2003, 52, 1708–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, V.; Von Rosenberg, W.; Montaldo, P.; Adjei, T.; Mendoza, J.; Shivamurthappa, V.; Mandic, D.; Thayyil, S. Early Postnatal Heart Rate Variability in Healthy Newborn Infants. Front. Physiol. 2019, 10, 922. [Google Scholar] [CrossRef] [Green Version]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Salahuddin, L.; Cho, J.; Jeong, M.G.; Kim, D. Ultra Short Term Analysis of Heart Rate Variability for Monitoring Mental Stress in Mobile Settings. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 4656–4659. [Google Scholar]
- Castaldo, R.; Montesinos, L.; Melillo, P.; James, C.; Pecchia, L. Ultra-short term HRV features as surrogates of short term HRV: A case study on mental stress detection in real life. BMC Med. Inform. Decis. Mak. 2019, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Esco, M.R.; Flatt, A.A. Ultra-Short-Term Heart Rate Variability Indexes at Rest and Post-Exercise in Athletes: Evaluating the Agreement with Accepted Recommendations. J. Sports Sci. Med. 2014, 13, 535–541. [Google Scholar]
- Munoz, M.L.; Van Roon, A.; Riese, H.; Thio, C.; Oostenbroek, E.; Westrik, I.; De Geus, E.J.C.; Gansevoort, R.; Lefrandt, J.; Nolte, I.M.; et al. Validity of (Ultra-)Short Recordings for Heart Rate Variability Measurements. PLoS ONE 2015, 10, e0138921. [Google Scholar] [CrossRef] [Green Version]
- Hoshikawa, Y.; Fitzke, H.; Sweis, R.; Fikree, A.; Saverymuttu, S.; Kadirkamanathan, S.; Iwakiri, K.; Yazaki, E.; Aziz, Q.; Sifrim, D. Rumination syndrome: Assessment of vagal tone during and after meals and during diaphragmatic breathing. Neurogastroenterol. Motil. 2020, 32, e13873. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Critchley, H.D. Review: Electrodermal Responses: What Happens in the Brain. Neuroscientist 2002, 8, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Livovsky, D.M.; Pribic, T.; Azpiroz, F. Food, Eating, and the Gastrointestinal Tract. Nutrients 2020, 12, 986. [Google Scholar] [CrossRef] [Green Version]
- Monrroy, H.; Pribic, T.; Galan, C.; Nieto, A.; Amigo, N.; Accarino, A.; Correig, X.; Azpiroz, F. Meal Enjoyment and Tolerance in Women and Men. Nutrients 2019, 11, 119. [Google Scholar] [CrossRef] [Green Version]
- Ciccantelli, B.; Pribic, T.; Malagelada, C.; Accarino, A.; Azpiroz, F. Relation between cognitive and hedonic responses to a meal. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Villoria, A.; Azpiroz, F.; Soldevilla, A.; Perez, F.; Malagelada, J.-R. Abdominal Accommodation: A Coordinated Adaptation of the Abdominal Wall to Its Content. Am. J. Gastroenterol. 2008, 103, 2807–2815. [Google Scholar] [CrossRef]
- Barba, E.; Quiroga, S.; Accarino, A.; Lahoya, E.M.; Malagelada, C.; Burri, E.; Navazo, I.; Malagelada, J.-R.; Azpiroz, F. Mechanisms of abdominal distension in severe intestinal dysmotility: Abdomino-thoracic response to gut retention. Neurogastroenterol. Motil. 2013, 25. [Google Scholar] [CrossRef]
- Villoria, A.; Azpiroz, F.; Burri, E.; Cisternas, D.; Soldevilla, A.; Malagelada, J.R. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am. J. Gastroenterol. 2011, 106, 815–819. [Google Scholar] [CrossRef]
- Iovino, P.; Azpiroz, F.; Domingo, E.; Malagelada, J.R. The sympathetic nervous system modulates perception and reflex responses to gut distension in humans. Gastroenterology 1995, 108, 680–686. [Google Scholar] [CrossRef]
- Monrroy, H.; Borghi, G.; Pribic, T.; Galan, C.; Nieto, A.; Amigo, N.; Accarino, A.; Correig, X.; Azpiroz, F. Biological Response to Meal Ingestion: Gender Differences. Nutrients 2019, 11, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masihy, M.; Monrroy, H.; Borghi, G.; Pribic, T.; Galan, C.; Nieto, A.; Accarino, A.; Azpiroz, F. Influence of Eating Schedule on the Postprandial Response: Gender Differences. Nutrients 2019, 11, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livovsky, D.M.; Barber, C.; Barba, E.; Accarino, A.; Azpiroz, F. Abdominothoracic Postural Tone Influences the Sensations Induced by Meal Ingestion. Nutrients 2021, 13, 658. https://doi.org/10.3390/nu13020658
Livovsky DM, Barber C, Barba E, Accarino A, Azpiroz F. Abdominothoracic Postural Tone Influences the Sensations Induced by Meal Ingestion. Nutrients. 2021; 13(2):658. https://doi.org/10.3390/nu13020658
Chicago/Turabian StyleLivovsky, Dan M., Claudia Barber, Elizabeth Barba, Anna Accarino, and Fernando Azpiroz. 2021. "Abdominothoracic Postural Tone Influences the Sensations Induced by Meal Ingestion" Nutrients 13, no. 2: 658. https://doi.org/10.3390/nu13020658





