Oral Sucrosomial Iron Is as Effective as Intravenous Ferric Carboxy-Maltose in Treating Anemia in Patients with Ulcerative Colitis
Abstract
:1. Introduction
2. Methods
Statistical Analysis
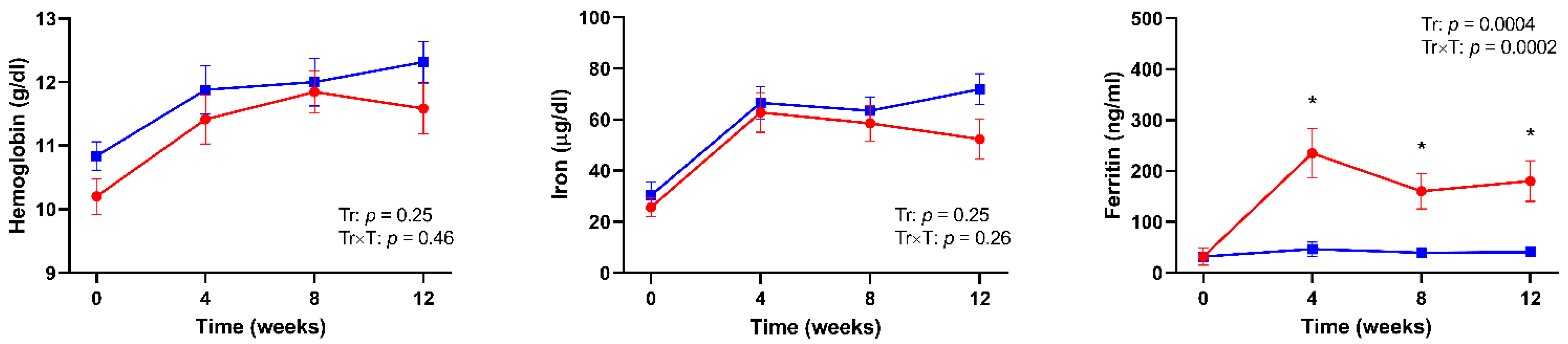
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2001, 96, 1116–1122. [Google Scholar] [CrossRef]
- Wilson, A.; Reyes, E.; Ofman, J. Prevalence and outcomes of anemia in inflammatory bowel disease: A systematic review of the literature. Am. J. Med. 2004, 116, 44S–49S. [Google Scholar] [CrossRef] [PubMed]
- Gomollon, F.; Gisbert, J.P. Anemia and inflammatory bowel diseases. World J. Gastroenterol. 2009, 15, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Di Sabatino, A.; Albertini, R.; Ardizzone, S.; Biancheri, P.; Bonetti, E.; Cassinotti, A.; Cazzola, P.; Markopoulos, K.; Massari, A.; et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica 2010, 95, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, C.; Henriksson, I.; Brus, O.; Zhulina, Y.; Nyhlin, N.; Tysk, C.; Montgomery, S.; Halfvarson, J. Incidence, prevalence and clinical outcome of anaemia in inflammatory bowel disease: A population-based cohort study. Aliment. Pharmacol. Ther. 2018, 48, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Vegh, Z.; Katsanos, K.H.; Christodoulou, D.K.; Lazar, D.; Goldis, A.; O’Morain, C.; Fernandez, A.; Pereira, S.; Myers, S.; et al. Occurrence of anaemia in the first year of inflammatory bowel disease in a European population-based inception cohort—An ECCO-EpiCom study. J. Crohn’s Colitis 2017, 11, 1213–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, J.; Hartmann, F.; Dignass, A.U. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg, S.; Stoinov, S.; Simanenkov, V.; Dudar, L.V.; Karnafel, W.; Garcia, L.C.; Sambuelli, A.M.; D’Haens, G.; Gasche, C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: The ferric carboxymaltose (FERINJECT) randomized controlled trial. Am. J. Gastroenterol. 2008, 103, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Evstatiev, R.; Marteau, P.; Iqbal, T.; Khalif, I.L.; Stein, J.; Bokemeyer, B.; Chopey, I.V.; Gutzwiller, F.S.; Riopel, L.; Gasche, C.; et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011, 141, 846–853.e2. [Google Scholar] [CrossRef]
- Befrits, R.; Wikman, O.; Blomquist, L.; Hjortswang, H.; Hammarlund, P.; Bajor, A.; Klintman, D.; Blom, H. Anemia and iron deficiency in inflammatory bowel disease: An open, prospective, observational study on diagnosis, treatment with ferric carboxymaltose and quality of life. Scand. J. Gastroenterol. 2013, 48, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Ferric carboxymaltose: A review in iron deficiency. Drugs 2018, 78, 479–493. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dibb, M.; Subramanian, S. Anaemia in inflammatory bowel disease. Frontline Gastroenterol. 2014, 5, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ramirez, S.; Brilli, E.; Tarantino, G.; Munoz, M. Sucrosomial((R)) iron: A new generation iron for improving oral supplementation. Pharmaceuticals 2018, 11, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbati, G.; Incerti, F.; Boarini, C.; Pileri, F.; Bocchi, D.; Ventura, P.; Buzzetti, E.; Pietrangelo, A. Safety and efficacy of sucrosomial iron in inflammatory bowel disease patients with iron deficiency anemia. Intern. Emerg. Med. 2019, 14, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Mumolo, M.G.; Tapete, G.; Albano, E.; Baiano Svizzero, G.; Zanzi, F.; Ceccarelli, L.; Bellini, M.; Marchi, S.; Costa, F. Fecal calprotectin: Current and future perspectives for inflammatory bowel disease treatment. Eur. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- WHO; UNICEF; UNU. Iron Deficiency Anemia: Assessment, Prevention and Control; Report of a Joint WHO/UNICEF/UNU consultation; 1998. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Vernero, M.; Boano, V.; Ribaldone, D.G.; Pellicano, R.; Astegiano, M. Oral iron supplementation with Feralgine(R) in inflammatory bowel disease: A retrospective observational study. Minerva Gastroenterol. E Dietol. 2019, 65, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Egberg, M.D.; Mitchell, P.D.; Galanko, J.A.; Rufo, P.A. Effectiveness of oral iron supplementation in treatment of anemia associated with pediatric ulcerative colitis flare. Am. J. Hematol. 2018, 93, E404–E406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makrides, M.; Crowther, C.A.; Gibson, R.A.; Gibson, R.S.; Skeaff, C.M. Efficacy and tolerability of low-dose iron supplements during pregnancy: A randomized controlled trial. Am. J. Clin. Nutr. 2003, 78, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Rimon, E.; Kagansky, N.; Kagansky, M.; Mechnick, L.; Mashiah, T.; Namir, M.; Levy, S. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am. J. Med. 2005, 118, 1142–1147. [Google Scholar] [CrossRef]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegard, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohn’s Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Schoepfer, A.M.; Safroneeva, E.; Rogler, G.; Schwenkglenks, M.; Achermann, R. A shift from oral to intravenous iron supplementation therapy is observed over time in a large swiss cohort of patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Bastida, G. Efficacy and tolerability of Sucrosomial iron supplementation in IBD patients with iron deficiency anemia and intolerance to iron oral salts. Expert Rev. Hematol. 2016, 9 (Suppl. 1). [Google Scholar] [CrossRef] [Green Version]
- Stein, J.; Aksan, A.; Farrag, K.; Dignass, A.; Radeke, H.H. Management of inflammatory bowel disease-related anemia and iron deficiency with specific reference to the role of intravenous iron in current practice. Expert Opin. Pharmacother. 2017, 18, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, S.; Wikman, O.; Befrits, R.; Blom, H.; Eriksson, A.; Granno, C.; Ung, K.A.; Hjortswang, H.; Lindgren, A.; Unge, P. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: A randomized, controlled, evaluator-blind, multicentre study. Scand. J. Gastroenterol. 2009, 44, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Yoon, H.; Shin, C.M.; Koh, S.J.; Im, J.P.; Kim, B.G.; Kim, J.S.; Jung, H.C. Comparison of the efficacies of parenteral iron sucrose and oral iron sulfate for anemic patients with inflammatory bowel disease in Korea. Gut Liver 2016, 10, 562–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroder, O.; Mickisch, O.; Seidler, U.; de Weerth, A.; Dignass, A.U.; Herfarth, H.; Reinshagen, M.; Schreiber, S.; Junge, U.; Schrott, M.; et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease—A randomized, controlled, open-label, multicenter study. Am. J. Gastroenterol. 2005, 100, 2503–2509. [Google Scholar] [CrossRef]
- Erichsen, K.; Ulvik, R.J.; Nysaeter, G.; Johansen, J.; Ostborg, J.; Berstad, A.; Berge, R.K.; Hausken, T. Oral ferrous fumarate or intravenous iron sucrose for patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2005, 40, 1058–1065. [Google Scholar] [CrossRef]
- Constante, M.; Fragoso, G.; Lupien-Meilleur, J.; Calve, A.; Santos, M.M. Iron supplements modulate colon microbiota composition and potentiate the protective effects of probiotics in dextran sodium sulfate-induced colitis. Inflamm. Bowel Dis. 2017, 23, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Brilli, E.; Fogli, S.; Beconcini, D.; Carpi, S.; Tarantino, G.; Zambito, Y. Sucrosomial(R) iron absorption studied by in vitro and ex-vivo models. Eur. J. Pharm. Sci. 2018, 111, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Brilli, E.; Mattii, L.; Testai, L.; Moscato, S.; Citi, V.; Tarantino, G.; Zambito, Y. Ex vivo and in vivo study of sucrosomial® iron intestinal absorption and bioavailability. Int. J. Pharm. Sci. 2018, 19, 2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghishan, F.K.; Kiela, P.R. Vitamins and minerals in inflammatory bowel disease. Gastroenterol. Clin. North Am. 2017, 46, 797–808. [Google Scholar] [CrossRef]
- Schreiner, P.; Martinho-Grueber, M.; Studerus, D.; Vavricka, S.R.; Tilg, H.; Biedermann, L.; on behalf of Swiss Ibdnet, an official working group of the Swiss Society of Gastroenterology. Nutrition in inflammatory bowel disease. Digestion 2020, 101, 120–135. [Google Scholar] [CrossRef]
| FCM | SI | p | |
|---|---|---|---|
| Number | 20 | 20 | - |
| Age (years) | 43.5 [31.5–66.8] | 42.0 [29.5–60.8] | 0.65 |
| Sex (n, %) | 0.99 | ||
| Men | 12 (60.0) | 11 (55.0) | |
| Women | 8 (40.0) | 9 (65.0) | |
| Hemoglobin (g/dl) | |||
| Baseline | 10.3 [9.0–11.0] | 11.1 [9.9–11.6] | 0.10 |
| 12 weeks | 11.8 [10.7–12.7] | 12.2 [11.5–13.3] | 0.17 |
| Mean change from baseline | 1.3 [0.7–2.2] | 1.1 [0.4–2.1] | 0.56 |
| Iron (μg/dl) | |||
| Baseline | 22 [14–38] | 23 [12–39] | 0.78 |
| 12 weeks | 36 [26–72] | 71 [50–97] | 0.03 |
| Mean change from baseline | 26 [9–60] | 47 [7–56] | 0.51 |
| Ferritin (ng/mL) | |||
| Baseline | 10 [5–13] | 16 [9–25] | 0.09 |
| 12 weeks | 131 [90–225] | 26 [15–44] | 0.001 |
| Mean change from baseline | 137 [61–231] | 9 [3–17] | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertani, L.; Tricò, D.; Zanzi, F.; Baiano Svizzero, G.; Coppini, F.; de Bortoli, N.; Bellini, M.; Antonioli, L.; Blandizzi, C.; Marchi, S. Oral Sucrosomial Iron Is as Effective as Intravenous Ferric Carboxy-Maltose in Treating Anemia in Patients with Ulcerative Colitis. Nutrients 2021, 13, 608. https://doi.org/10.3390/nu13020608
Bertani L, Tricò D, Zanzi F, Baiano Svizzero G, Coppini F, de Bortoli N, Bellini M, Antonioli L, Blandizzi C, Marchi S. Oral Sucrosomial Iron Is as Effective as Intravenous Ferric Carboxy-Maltose in Treating Anemia in Patients with Ulcerative Colitis. Nutrients. 2021; 13(2):608. https://doi.org/10.3390/nu13020608
Chicago/Turabian StyleBertani, Lorenzo, Domenico Tricò, Federico Zanzi, Giovanni Baiano Svizzero, Francesca Coppini, Nicola de Bortoli, Massimo Bellini, Luca Antonioli, Corrado Blandizzi, and Santino Marchi. 2021. "Oral Sucrosomial Iron Is as Effective as Intravenous Ferric Carboxy-Maltose in Treating Anemia in Patients with Ulcerative Colitis" Nutrients 13, no. 2: 608. https://doi.org/10.3390/nu13020608







