Increased 1,25(OH)2-Vitamin D Concentrations after Energy Restriction Are Associated with Changes in Skeletal Muscle Phenotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Experimental Design
2.3. Muscle Sampling
2.4. Histological Staining and Image Analysis
2.5. Blood Biochemistry
2.6. RNA Extraction and Real-Time RT-PCR
2.7. Statistics
3. Results
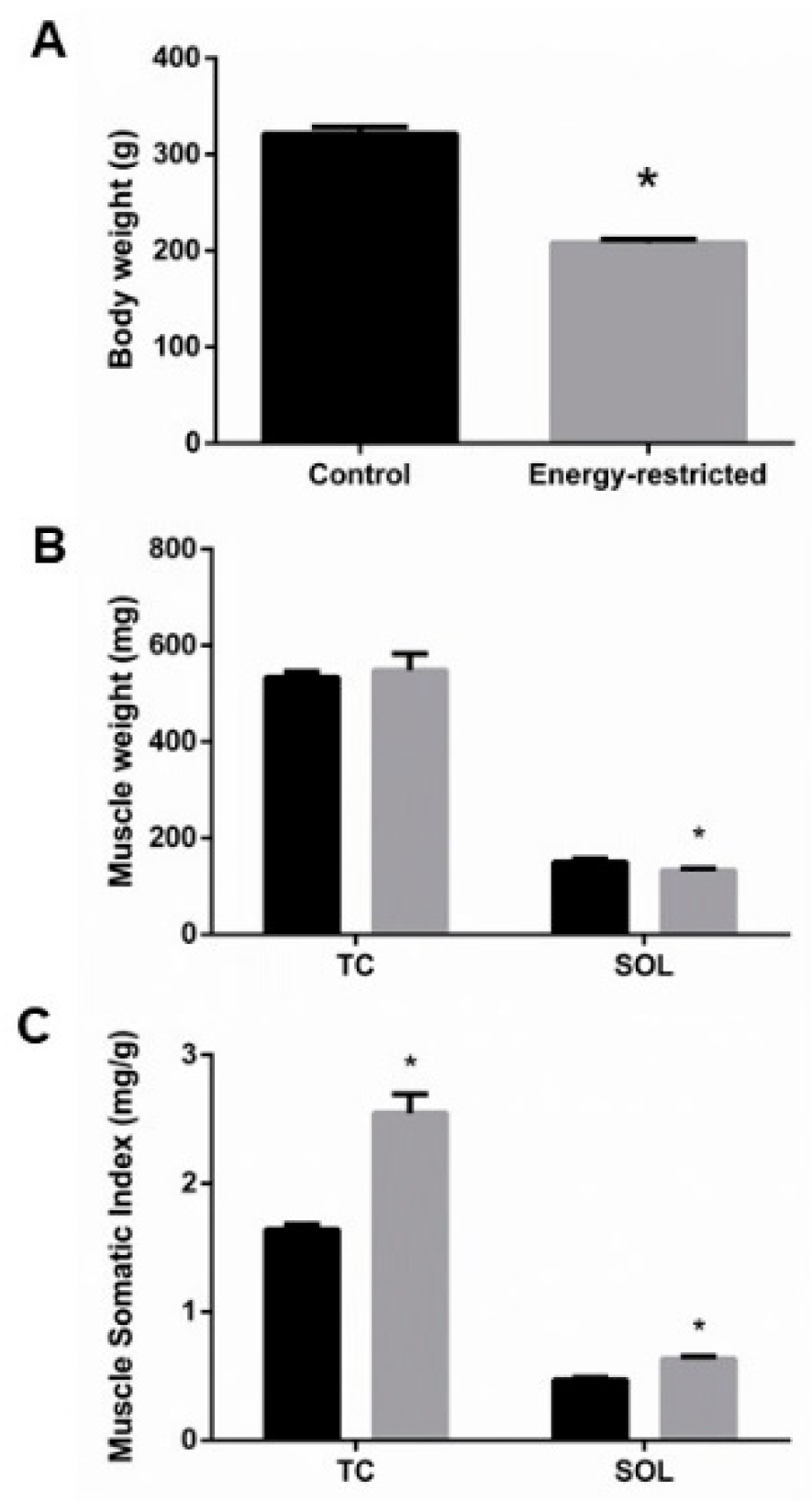
3.1. Energy Intake, Body Weight, Fat Mass, and Muscle Mass
3.2. Muscle Histomorphometry
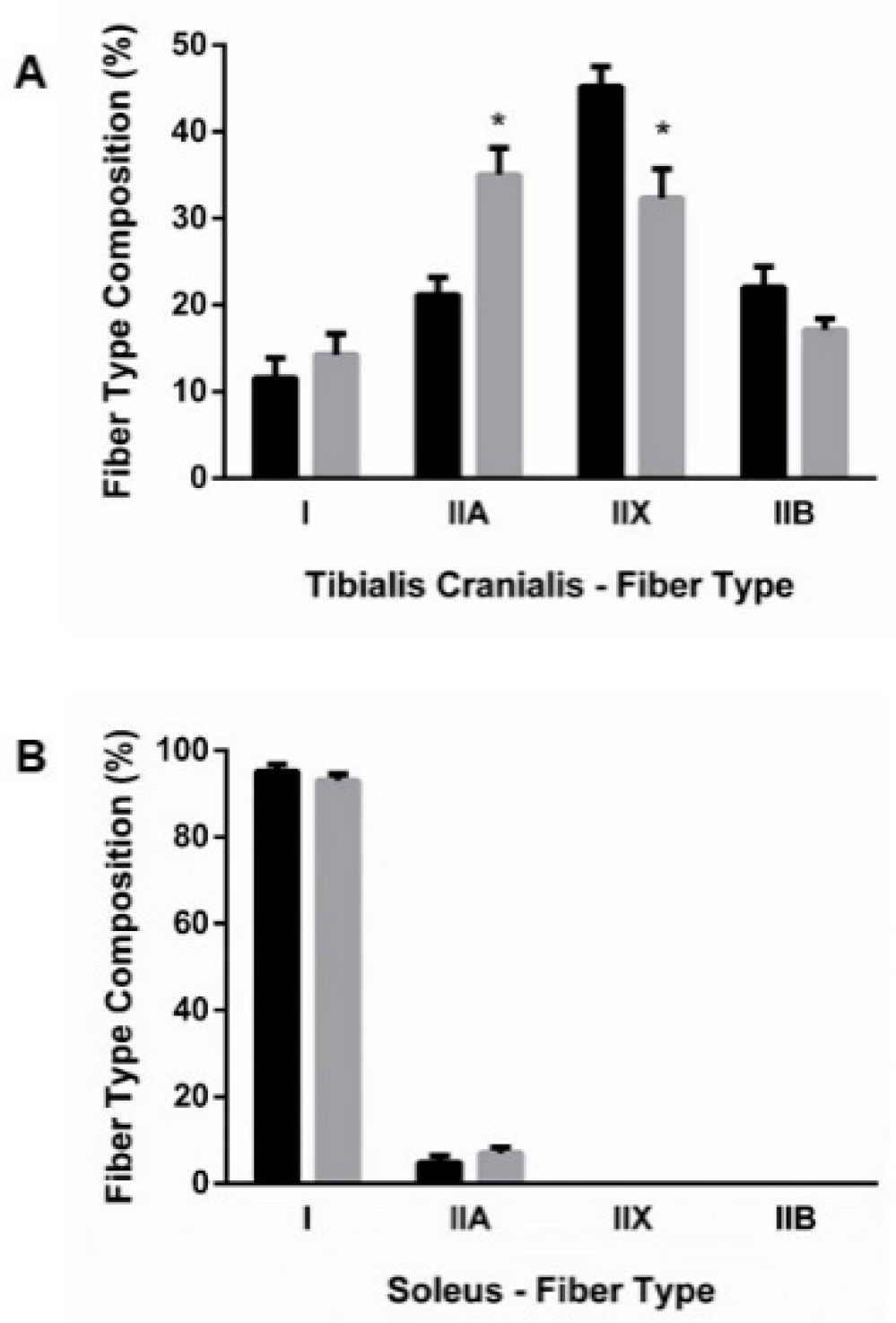
3.2.1. Fiber-Type Composition
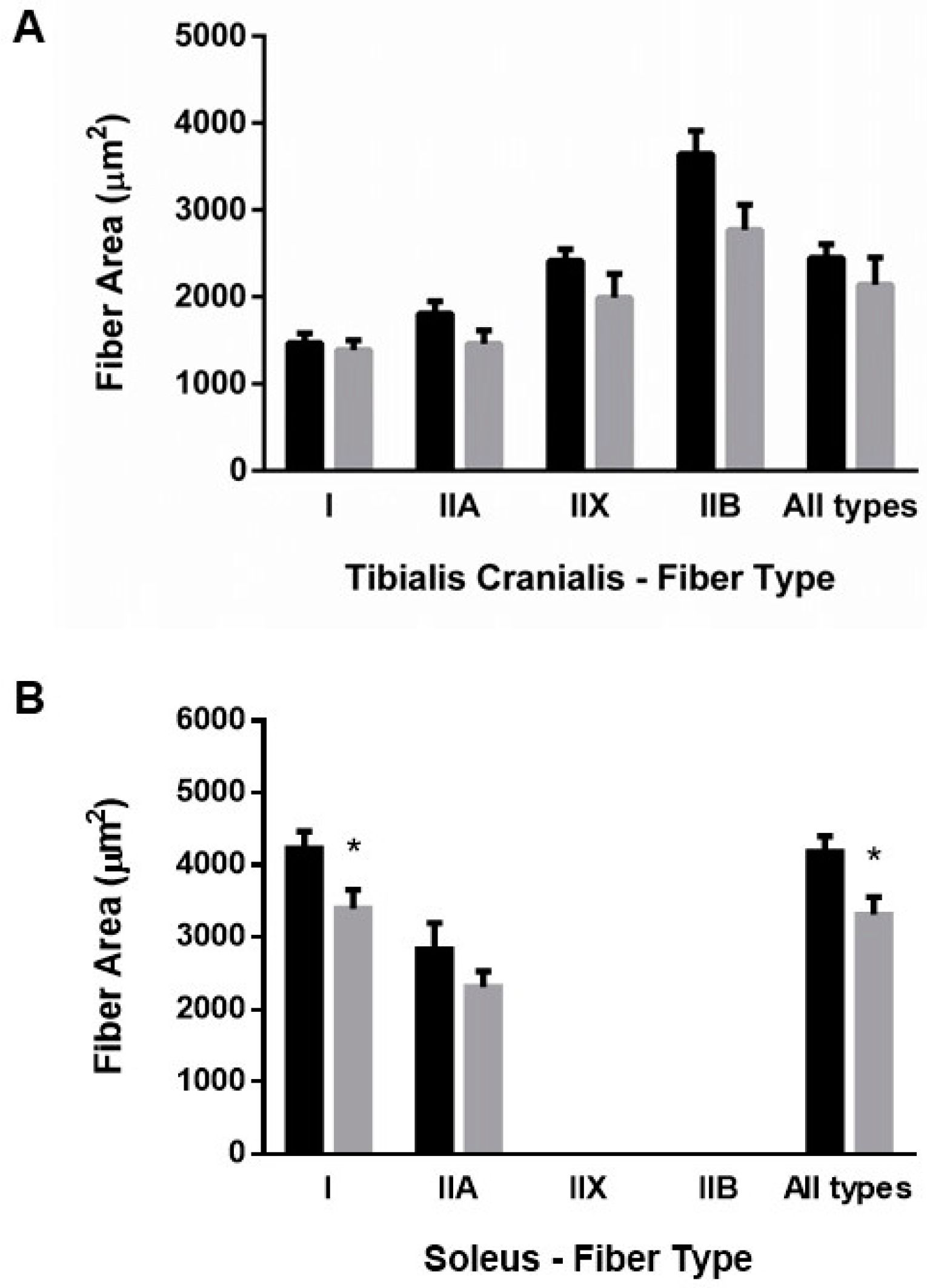
3.2.2. Muscle Fiber Size
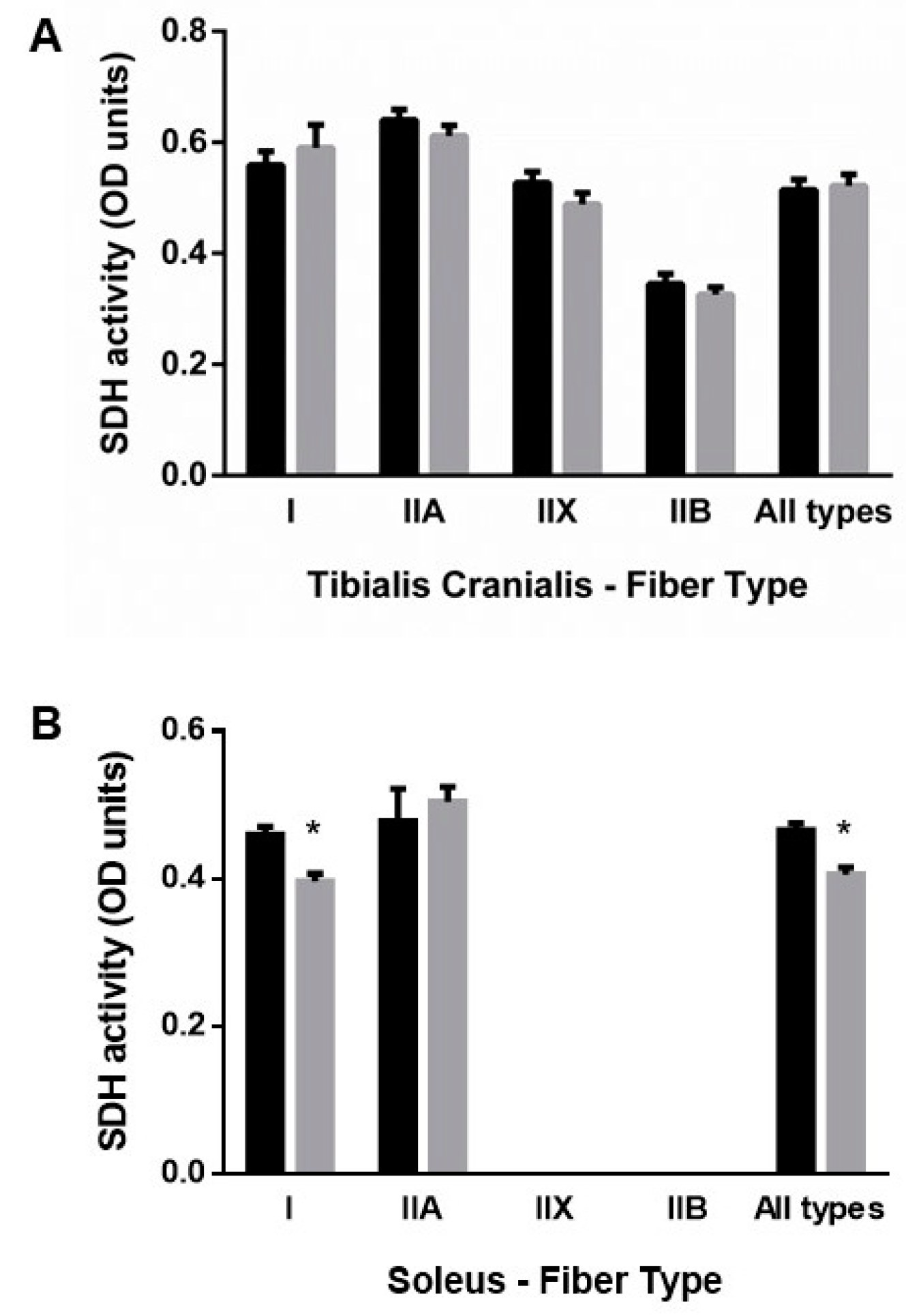
3.2.3. Muscle Oxidative Profile
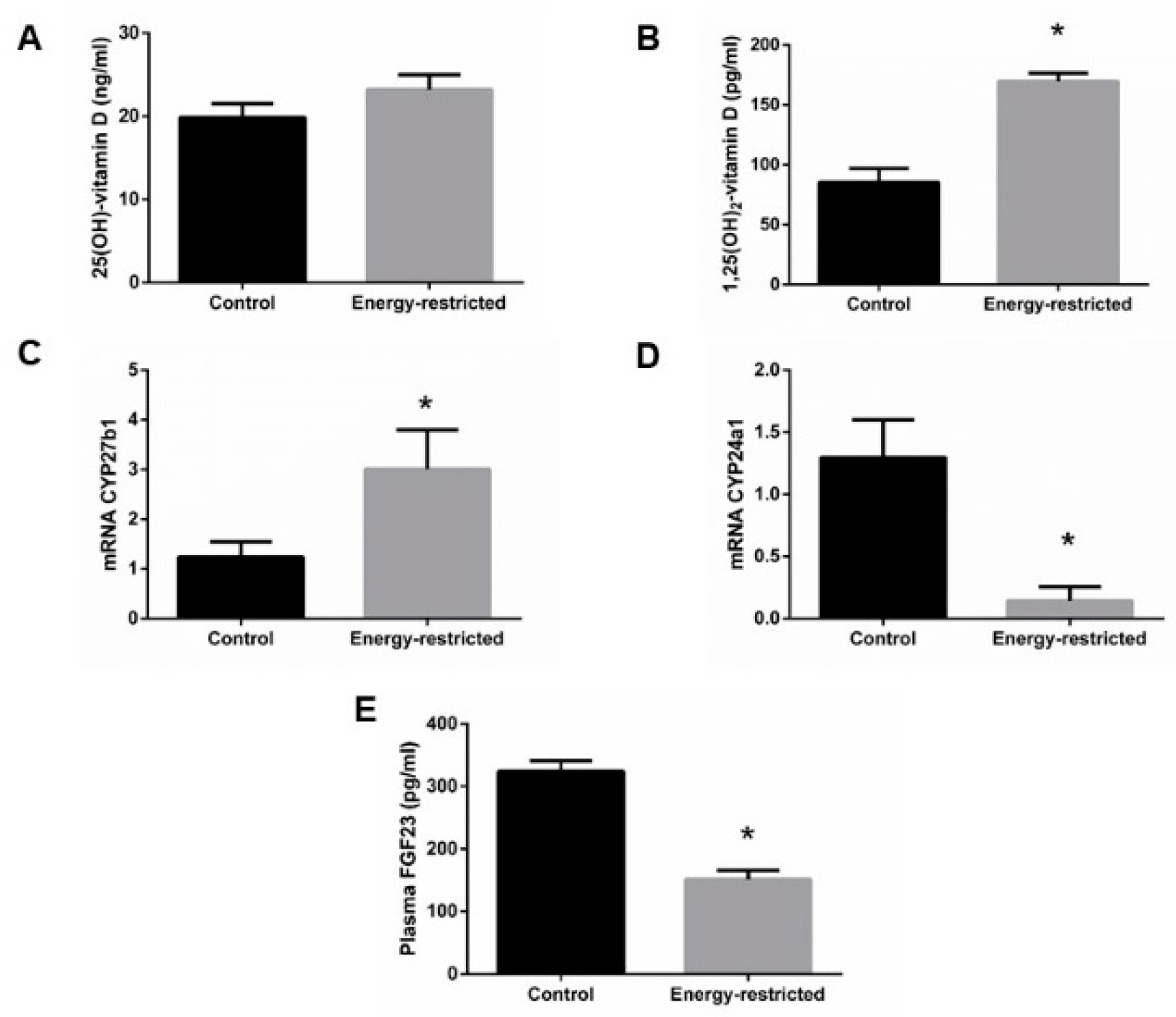
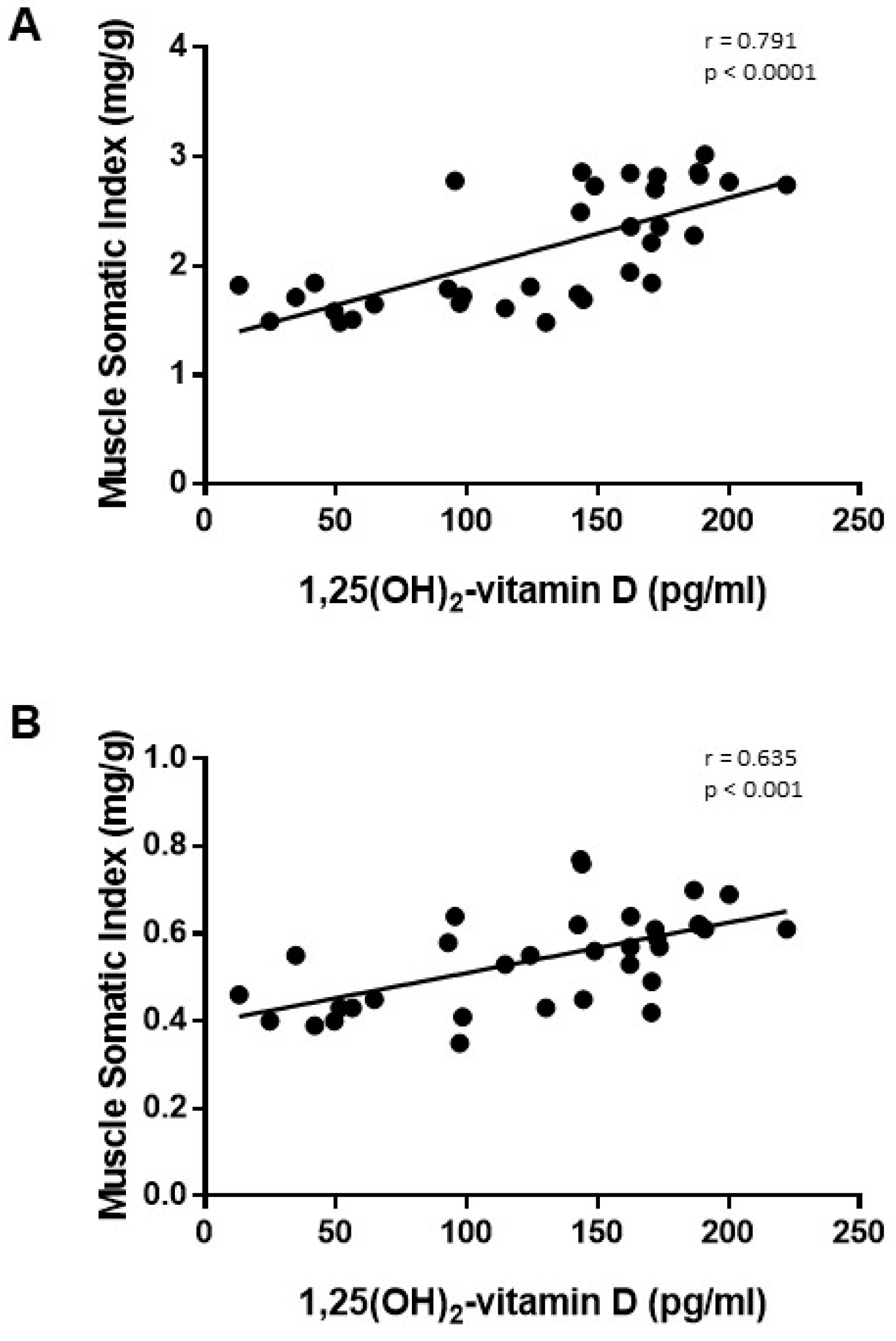
3.3. Studies on Vitamin D and Related Metabolites
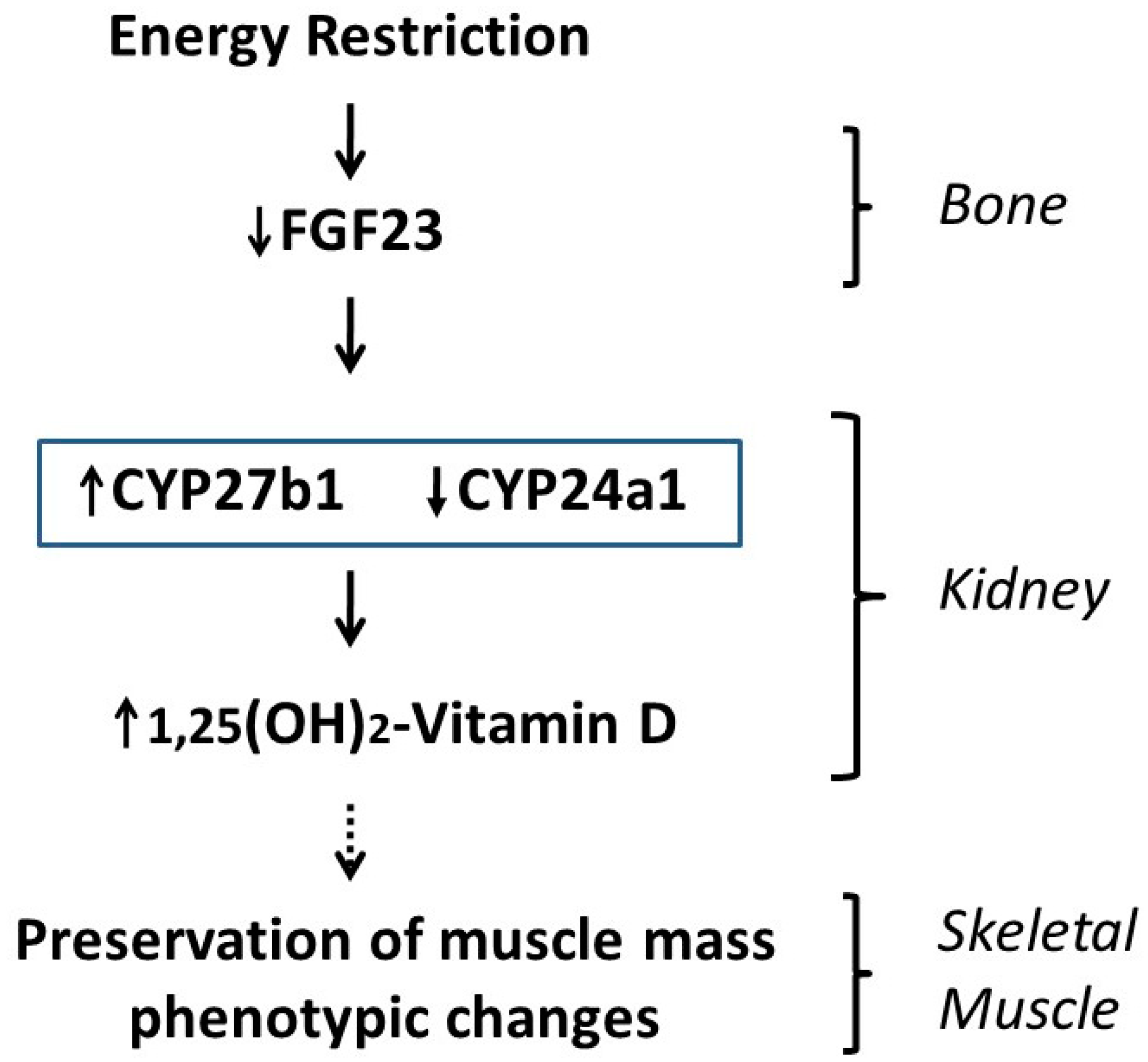
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omodei, D.; Fontana, L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011, 585, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Goldberg, A.L. SIRT1 Protein, by Blocking the Activities of Transcription Factors FoxO1 and FoxO3, Inhibits Muscle Atrophy and Promotes Muscle Growth. J. Biol. Chem. 2013, 288, 30515–30526. [Google Scholar] [CrossRef]
- Park, B.-S.; Henning, P.C.; Grant, S.C.; Lee, W.J.; Lee, S.-R.; Arjmandi, B.H.; Kim, J.-S. HMB attenuates muscle loss during sustained energy deficit induced by calorie restriction and endurance exercise. Metabolism 2013, 62, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Aspnes, L.E.; Lee, C.M.; Weindruch, R.; Chung, S.S.; Roecker, E.B.; Aiken, J.M. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1997, 11, 573–581. [Google Scholar] [CrossRef]
- Hepple, R.T.; Qin, M.; Nakamoto, H.; Goto, S. Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: Implications for sarcopenia. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R1231–R1237. [Google Scholar] [CrossRef]
- McKiernan, S.H.; Colman, R.J.; Lopez, M.; Beasley, T.; Aiken, J.M.; Anderson, R.M.; Weindruch, R. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp. Gerontol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Phillips, T.; Leeuwenburgh, C. Muscle fiber-specific apoptosis and TNF-α signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005, 19, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, J.; Maxwell, N.; Shapiro, D.; Decabo, R.; Valdez, G. Caloric Restriction Mimetics Slow Aging of Neuromuscular Synapses and Muscle Fibers. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2017, 73, 21–28. [Google Scholar] [CrossRef]
- Yoshida, S.; Yamahara, K.; Kume, S.; Koya, D.; Yasuda-Yamahara, M.; Takeda, N.; Osawa, N.; Chin-Kanasaki, M.; Adachi, Y.; Nagao, K.; et al. Role of dietary amino acid balance in diet restriction-mediated lifespan extension, renoprotection, and muscle weakness in aged mice. Aging Cell 2018, 17, e12796. [Google Scholar] [CrossRef]
- Gutiérrez-Casado, E.; Khraiwesh, H.; López-Domínguez, J.A.; Montero-Guisado, J.; López-Lluch, G.; Navas, P.; De Cabo, R.; Ramsey, J.J.; González-Reyes, J.A.; Villalba, J.M. The Impact of Aging, Calorie Restriction and Dietary Fat on Autophagy Markers and Mitochondrial Ultrastructure and Dynamics in Mouse Skeletal Muscle. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 760–769. [Google Scholar] [CrossRef]
- Chen, Y.; Hagopian, K.; Bibus, D.; Villalba, J.M.; López-Lluch, G.; Navas, P.; Kim, K.; Ramsey, J.J. The Influence of Dietary Lipid Composition on Skeletal Muscle Mitochondria From Mice Following Eight Months of Calorie Restriction. Physiol. Res. 2014, 63, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.F.; Tahvilian, S.; Kang, J.H.; Svensson, K.; Hetrick, B.; Chick, W.S.; Schenk, S.; McCurdy, C.E. Calorie Restriction-Induced Increase in Skeletal Muscle Insulin Sensitivity Is Not Prevented by Overexpression of the p55α Subunit of Phosphoinositide 3-Kinase. Front. Physiol. 2018, 9, 789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Naughton, D.P. Vitamin D in health and disease: Current perspectives. Nutr. J. 2010, 9, 65. [Google Scholar] [CrossRef]
- Arima, K.; Mizukami, S.; Nishimura, T.; Tomita, Y.; Nakashima, H.; Abe, Y.; Aoyagi, K. Epidemiology of the association between serum 25-hydroxyvitamin D levels and musculoskeletal conditions among elderly individuals: A literature review. J. Physiol. Anthr. 2020, 39, 1–6. [Google Scholar] [CrossRef]
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633. [Google Scholar] [CrossRef]
- Chiang, C.-M.; Ismaeel, A.; Griffis, R.B.; Weems, S. Effects of Vitamin D Supplementation on Muscle Strength in Athletes. J. Strength Cond. Res. 2017, 31, 566–574. [Google Scholar] [CrossRef]
- Yagüe, M.D.L.P.; Yurrita, L.C.; Cabañas, M.J.C.; Cenzual, M.A.C. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2018, 175, 177–189. [Google Scholar] [CrossRef]
- Bruyère, O.; Cavalier, E.; Reginster, J.-Y. Vitamin D and osteosarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 498–503. [Google Scholar] [CrossRef]
- Chang, W.-T.; Wu, C.-H.; Hsu, L.-W.; Chen, P.-W.; Yu, J.-R.; Chang, C.-S.; Tsai, W.-C.; Liu, P.-Y. Serum vitamin D, intact parathyroid hormone, and Fetuin A concentrations were associated with geriatric sarcopenia and cardiac hypertrophy. Sci. Rep. 2017, 7, 40996. [Google Scholar] [CrossRef]
- Hasegawa, H.; Nagano, N.; Urakawa, I.; Yamazaki, Y.; Iijima, K.; Fujita, T.; Yamashita, T.; Fukumoto, S.; Shimada, T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010, 78, 975–980. [Google Scholar] [CrossRef]
- Vidal, A.; Rios, R.; Pineda, C.; Lopez, I.; Muñoz-Castañeda, J.R.; Rodriguez, M.; Aguilera-Tejero, E.; Raya, A.I. Direct regulation of fibroblast growth factor 23 by energy intake through mTOR. Sci. Rep. 2020, 10, 1795. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, L.M.; López, I.; Peralta-Ramírez, A.; Pineda, C.; Chamizo, V.E.; Rodríguez, M.; Aguilera-Tejero, E.; Rivero, J.-L.L. High-phosphorus diet maximizes and low-dose calcitriol attenuates skeletal muscle changes in long-term uremic rats. J. Appl. Physiol. 2016, 120, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.-L.L.; Talmadge, R.J.; Edgerton, V.R. Interrelationships of myofibrillar ATPase activity and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. Histochem. Cell Biol. 1999, 111, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; De Cabo, R. Calorie restriction in rodents: Caveats to consider. Ageing Res. Rev. 2017, 39, 15–28. [Google Scholar] [CrossRef]
- Xie, K.; Neff, F.; Markert, A.; Rozman, J.; Aguilar-Pimentel, J.A.; Amarie, O.V.; Becker, L.; Brommage, R.; Garrett, L.; Henzel, K.S.; et al. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat. Commun. 2017, 8, 1–19. [Google Scholar] [CrossRef]
- Agarwal, E.; Miller, M.W.; Yaxley, A.; Isenring, E. Malnutrition in the elderly: A narrative review. Maturitas 2013, 76, 296–302. [Google Scholar] [CrossRef]
- Prescod, A.L.V.; Halliday, W.C.; Taylor, C.G. Protein deficiency, but not zinc deficiency, reduces recovery of type 1 and type 2 muscle fibre diameters in the gastrocnemius muscle of growing rats. Br. J. Nutr. 2011, 106, 675–682. [Google Scholar] [CrossRef]
- Walrand, S.; Zangarelli, A.; Guillet, C.; Salles, J.; Soulier, K.; Giraudet, C.; Patrac, V.; Boirie, Y. Effect of fast dietary proteins on muscle protein synthesis rate and muscle strength in ad libitum-fed and energy-restricted old rats. Br. J. Nutr. 2011, 106, 1683–1690. [Google Scholar] [CrossRef]
- Chomentowski, P.; Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Zhu, S.; Toledo, F.G.; Goodpaster, B.H. Moderate Exercise Attenuates the Loss of Skeletal Muscle Mass That Occurs With Intentional Caloric Restriction-Induced Weight Loss in Older, Overweight to Obese Adults. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2009, 64, 575–580. [Google Scholar] [CrossRef]
- Lu, Y.; Bradley, J.S.; McCoski, S.R.; Gonzalez, J.M.; Ealy, A.D.; Johnson, S.E. Reduced skeletal muscle fiber size following caloric restriction is associated with calpain-mediated proteolysis and attenuation of IGF-1 signaling. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R806–R815. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, S.H.; Bua, E.; McGorray, J.; Aiken, J. Early-onset calorie restriction conserves fiber number in aging rat skeletal muscle. FASEB J. 2004, 18, 580–581. [Google Scholar] [CrossRef]
- Hoek, A.M.V.D.; Zondag, G.C.; Verschuren, L.; De Ruiter, C.; Attema, J.; De Wit, E.C.; Schwerk, A.M.; Guigas, B.; Lek, S.; Rietman, A.; et al. A novel nutritional supplement prevents muscle loss and accelerates muscle mass recovery in caloric-restricted mice. Metabolism 2019, 97, 57–67. [Google Scholar] [CrossRef]
- Boldrin, L.; Ross, J.A.; Whitmore, C.; Doreste, B.; Beaver, C.; Eddaoudi, A.; Pearce, D.J.; Morgan, J.E. The effect of calorie restriction on mouse skeletal muscle is sex, strain and time-dependent. Sci. Rep. 2017, 7, 5160. [Google Scholar] [CrossRef]
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef]
- Galusca, B.; Verney, J.; Meugnier, E.; Ling, Y.; Edouard, P.; Feasson, L.; Ravelojaona, M.; Vidal, H.; Estour, B.; Germain, N. Reduced fibre size, capillary supply and mitochondrial activity in constitutional thinness’ skeletal muscle. Acta Physiol. 2018, 224, e13097. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Flück, M. Normal mammalian skeletal muscle and its phenotypic plasticity. J. Exp. Biol. 2002, 205, 2143–2152. [Google Scholar]
- Faitg, J.; Leduc-Gaudet, J.-P.; Reynaud, O.; Ferland, G.; Gaudreau, P.; Gouspillou, G. Effects of Aging and Caloric Restriction on Fiber Type Composition, Mitochondrial Morphology and Dynamics in Rat Oxidative and Glycolytic Muscles. Front. Physiol. 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Collino, M.; Mastrocola, R.; Nigro, D.; Chiazza, F.; Aragno, M.; D’Antona, G.; Minetto, M.A. Variability in Myosteatosis and Insulin Resistance Induced by High-Fat Diet in Mouse Skeletal Muscles. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Hearris, M.A.; Hammond, K.M.; Fell, J.M.; Morton, J.P. Regulation of Muscle Glycogen Metabolism during Exercise: Implications for Endurance Performance and Training Adaptations. Nutrients 2018, 10, 298. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Astin, R.; McPhail, M.J.W.; Saeed, S.; Pasha, Y.; Bear, D.E.; Constantin, D.; Velloso, C.; Manning, S.; Calvert, L.; et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax 2018, 73, 926–935. [Google Scholar] [CrossRef]
- Mujika, I.; Rønnestad, B.R.; Martin, D.T. Effects of Increased Muscle Strength and Muscle Mass on Endurance-Cycling Performance. Int. J. Sports Physiol. Perform. 2016, 11, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Pons, V.; Riera, J.; Capó, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Drobnic, F.; Pons, A. Calorie restriction regime enhances physical performance of trained athletes. J. Int. Soc. Sports Nutr. 2018, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- St-Jean-Pelletier, F.; Pion, C.H.; Leduc-Gaudet, J.-P.; Sgarioto, N.; Zovilé, I.; Barbat-Artigas, S.; Reynaud, O.; Alkaterji, F.; Lemieux, F.C.; Grenon, A.; et al. The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J. Cachex Sarcopenia Muscle 2017, 8, 213–228. [Google Scholar] [CrossRef]
- Zierath, J.R.; Hawley, J.A. Skeletal Muscle Fiber Type: Influence on Contractile and Metabolic Properties. PLoS Biol. 2004, 2, e348. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B. Vitamin D and muscle function. J. Steroid Biochem. Mol. Biol. 2017, 173, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, Skeletal Muscle Function and Athletic Performance in Athletes—A Narrative Review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef]
- Montenegro, K.R.; Cruzat, V.; Carlessi, R.; Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr. Res. Rev. 2019, 32, 192–204. [Google Scholar] [CrossRef]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrients 2019, 60, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol. Metab. 2020, 42, 101059. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Kazi, A.A.; Deane, C.S.; Nakhuda, A.; Ashcroft, S.P.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; et al. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J. Physiol. 2021, 599, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Bollen, S.E.; Atherton, P.J. Myogenic, genomic and non-genomic influences of the vitamin D axis in skeletal muscle. Cell Biochem. Funct. 2021, 39, 48–59. [Google Scholar] [CrossRef]
- Raya, A.I.; Rios, R.; Pineda, C.; Rodriguez-Ortiz, M.E.; Diez, E.; Almaden, Y.; Muñoz-Castañeda, J.R.; Rodriguez, M.; Aguilera-Tejero, E.; Lopez, I. Energy-dense diets increase FGF23, lead to phosphorus retention and promote vascular calcifications in rats. Sci. Rep. 2016, 6, 36881. [Google Scholar] [CrossRef]
- Murayama, A.; Takeyama, K.-I.; Kitanaka, S.; Kodera, Y.; Kawaguchi, Y.; Hosoya, T.; Kato, S. Positive and Negative Regulations of the Renal 25-Hydroxyvitamin D3 1α-Hydroxylase Gene by Parathyroid Hormone, Calcitonin, and 1α,25(OH)2D3 in Intact Animals*. Endocrinology 1999, 140, 2224–2231. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef]
| Control | Energy Restriction | |
|---|---|---|
| Crude Nutrients (%) | ||
| Nitrogen free extractives | 60 | 24.3 |
| Crude protein | 20.7 | 17.1 |
| Crude fat | 4 | 2 |
| Crude fiber | 3.1 | 44 |
| Crude ash | 4.3 | 5.5 |
| Moisture | 7.9 | 7.1 |
| Metabolized Energy (%) | ||
| Carbohydrates | 66 | 34 |
| Protein | 24 | 52 |
| Fat | 10 | 14 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | AGGGCTGCCTTCTCTTGTGAC | TGGGTAGAATCATACTGGAACATGTAG |
| CYP27b1 | AAGAGTGATGACTACTGGG | ATAGTATCAAATAGCCGGGG |
| CYP24a1 | AAGTGTGCCATTTACAACTC | GTTAACACTGTTCCTTTGGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, A.; Rios, R.; Pineda, C.; Lopez, I.; Raya, A.I.; Aguilera-Tejero, E.; Rivero, J.-L.L. Increased 1,25(OH)2-Vitamin D Concentrations after Energy Restriction Are Associated with Changes in Skeletal Muscle Phenotype. Nutrients 2021, 13, 607. https://doi.org/10.3390/nu13020607
Vidal A, Rios R, Pineda C, Lopez I, Raya AI, Aguilera-Tejero E, Rivero J-LL. Increased 1,25(OH)2-Vitamin D Concentrations after Energy Restriction Are Associated with Changes in Skeletal Muscle Phenotype. Nutrients. 2021; 13(2):607. https://doi.org/10.3390/nu13020607
Chicago/Turabian StyleVidal, Angela, Rafael Rios, Carmen Pineda, Ignacio Lopez, Ana I. Raya, Escolastico Aguilera-Tejero, and Jose-Luis L. Rivero. 2021. "Increased 1,25(OH)2-Vitamin D Concentrations after Energy Restriction Are Associated with Changes in Skeletal Muscle Phenotype" Nutrients 13, no. 2: 607. https://doi.org/10.3390/nu13020607
APA StyleVidal, A., Rios, R., Pineda, C., Lopez, I., Raya, A. I., Aguilera-Tejero, E., & Rivero, J.-L. L. (2021). Increased 1,25(OH)2-Vitamin D Concentrations after Energy Restriction Are Associated with Changes in Skeletal Muscle Phenotype. Nutrients, 13(2), 607. https://doi.org/10.3390/nu13020607








