The Impact of Human Papillomavirus (HPV) Associated Oropharyngeal Squamous Cell Carcinoma (OPSCC) on Nutritional Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Inclusion Criteria
2.4. Primary Outcome
2.5. Secondary Outcomes
2.6. Loss of Weight
2.7. Depression
2.8. Quality of Life
2.9. Dietary Adequacy, NIS and Reactive NGT Insertions
2.10. Adverse Events
2.11. Sample Size
3. Results
3.1. Participants
3.2. Primary Outcome
3.3. Secondary Outcomes
3.3.1. Loss of Weight
3.3.2. Depression
3.3.3. Adverse Events
3.3.4. Reactive NGT Insertions, Dietary Adequacy and Nutrition Impact Symptoms
3.3.5. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Bokhorst-de van der, S.; van Leeuwen, P.A.; Kuik, D.J.; Klop, W.M.; Sauerwein, H.P.; Snow, G.B.; Quak, J.J. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer 1999, 86, 519–527. [Google Scholar] [CrossRef]
- Langius, J.A.E.; Bakker, S.; Rietveld, D.H.F.; Kruizenga, H.M.; Langendijk, J.A.; Weijs, P.J.M.; Leemans, C.R. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br. J. Cancer 2013, 109, 1093–1099. [Google Scholar] [CrossRef]
- Citak, E.; Tulek, Z.; Uzel, O. Nutritional status in patients with head and neck cancer undergoing radiotherapy: A longitudinal study. Supportive Care Cancer 2019, 27, 239–247. [Google Scholar] [CrossRef]
- Levendag, P.C.; Teguh, D.N.; Voet, P.; van der Est, H.; Noever, I.; de Kruijf, W.J.M.; Kolkman-Deurloo, I.-K.; Prevost, J.-B.; Poll, J.; Schmitz, P.I.M.; et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: A dose-effect relationship. Radiother. Oncol. 2007, 85, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Langius, J.A.E.; van Dijk, A.M.; Doornaert, P.; Kruizenga, H.M.; Langendijk, J.A.; Leemans, C.R.; Weijs, P.J.M.; Verdonck-de Leeuw, I.M. More Than 10% Weight Loss in Head and Neck Cancer Patients During Radiotherapy Is Independently Associated with Deterioration in Quality of Life. Nutr. Cancer 2013, 65, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Beaver, M.E.S.; Matheny, K.E.; Roberts, D.B.; Myers, J.N. Predictors of weight loss during radiation therapy. Otolaryngol. Head Neck Surg. 2001, 125, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Sturgis, E.M.; Burtness, B.; Ridge, J.A.; Ringash, J.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40. [Google Scholar] [CrossRef]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef] [Green Version]
- Hong, A.; Lee, C.S.; Jones, D.; Veillard, A.-S.; Zhang, M.; Zhang, X.; Smee, R.; Corry, J.; Porceddu, S.; Milross, C.; et al. Rising prevalence of human papillomavirus-related oropharyngeal cancer in Australia over the last 2 decades. Head Neck 2016, 38, 743–750. [Google Scholar] [CrossRef]
- MachczynDski, P.; Majchrzak, E.; Niewinski, P.; Marchlewska, J.; GolusinDski, W. A review of the 8th edition of the AJCC staging system for oropharyngeal cancer according to HPV status. Eur. Arch. Oto Rhino Laryngol. 2020, 277, 2407–2412. [Google Scholar] [CrossRef]
- Ang, K.K.M.D.P.; Harris, J.M.S.; Wheeler, R.M.D.; Weber, R.M.D.; Rosenthal, D.I.M.D.; Nguyen-Tân, P.F.M.D.; Westra, W.H.M.D.; Chung, C.H.M.D.; Jordan, R.C.D.D.S.P.; Lu, C.M.D.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [Green Version]
- McIlwain, W.R.; Sood, A.J.; Nguyen, S.A.; Day, T.A. Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Vangelov, B.; Kotevski, D.P.; Williams, J.R.; Smee, R.I. The impact of HPV status on weight loss and feeding tube use in oropharyngeal carcinoma. Oral Oncol. 2018, 79, 33–39. [Google Scholar] [CrossRef]
- Vatca, M.; Lucas, J.T., Jr.; Laudadio, J.; D’Agostino, R.B.; Waltonen, J.D.; Sullivan, C.A.; Rouchard-Plasser, R.; Matsangou, M.; Browne, J.D.; Greven, K.M.; et al. Retrospective analysis of the impact of HPV status and smoking on mucositis in patients with oropharyngeal squamous cell carcinoma treated with concurrent chemotherapy and radiotherapy. Oral Oncol. 2014, 50, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Anderson, N.J.; Jackson, J.E.; Wada, M.; Schneider, M.; Poulsen, M.; Rolfo, M.; Fahandej, M.; Gan, H.; Khoo, V. The changing landscape of head and neck cancer radiotherapy patients: Is high-risk, prolonged feeding tube use indicative of on-treatment weight loss? J. Med. Radiat. Sci. 2019, 66, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marur, S.; Li, S.; Cmelak, A.J.; Gillison, M.L.; Zhao, W.J.; Ferris, R.L.; Westra, W.H.; Gilbert, J.; Bauman, J.E.; Wagner, L.I.; et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx-ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 2017, 35, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a Causal Association Between Human Papillomavirus and a Subset of Head and Neck Cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chasen, M.R.; Bhargava, R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support. Care Cancer 2009, 17, 1345–1351. [Google Scholar] [CrossRef]
- Frowen, J.; Cotton, S.; Corry, J.; Perry, A. Impact of demographics, tumor characteristics, and treatment factors on swallowing after (chemo)radiotherapy for head and neck cancer. Head Neck 2010, 32, 513–528. [Google Scholar] [CrossRef]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.; Ang, K.K. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef]
- Britton, B.; Clover, K.; Bateman, L.; Odelli, C.; Wenham, K.; Zeman, A.; Carter, G.L.; Britton, B.; Clover, K.; Bateman, L.; et al. Baseline depression predicts malnutrition in head and neck cancer patients undergoing radiotherapy. Support. Care Cancer 2012, 20, 335–342. [Google Scholar] [CrossRef]
- Langius, J.A.E.; Zandbergen, M.C.; Eerenstein, S.E.J.; van Tulder, M.W.; Leemans, C.R.; Kramer, M.H.H.; Weijs, P.J.M. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: A systematic review. Clin. Nutr. 2013, 32, 671–678. [Google Scholar] [CrossRef]
- Britton, B.; Baker, A.L.; Wolfenden, L.; Wratten, C.; Bauer, J.; Beck, A.K.; McCarter, K.; Harrowfield, J.; Isenring, E.; Tang, C.; et al. Eating As Treatment (EAT): A Stepped-Wedge, Randomized Controlled Trial of a Health Behavior Change Intervention Provided by Dietitians to Improve Nutrition in Patients With Head and Neck Cancer Undergoing Radiation Therapy (TROG 12.03). Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottery, F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef]
- Head and Neck Guideline Steering Committee. Evidence-based practice guidelines for the nutritional management of adult patients with head and neck cancer. Available online: https://wiki.cancer.org.au/australia/COSA:Head_and_neck_cancer_nutrition_guidelines (accessed on 1 November 2020).
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; Haes, J.C.J.M.D.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- StataCorp LP. Stata Statistical Software. Release 13 [Software]; StataCorp LP: College Station, TX, USA, 2013. [Google Scholar]
- Langius, J.A.E.; Twisk, J.; Kampman, M.; Doornaert, P.; Kramer, M.H.H.; Weijs, P.J.M.; Leemans, C.R. Prediction model to predict critical weight loss in patients with head and neck cancer during (chemo)radiotherapy. Oral Oncol. 2016, 52, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Caudell, J.J.; Schaner, P.; Desmond, R.A. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int. J. Radiat Oncol. Biol. Phys. 2010, 76, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.K.; Krishnasamy, M.; Loeliger, J.; Granados, A.; Dutu, G.; Corry, J. A Dietitian-Led Clinic for Patients Receiving (Chemo)Radiotherapy for Head and Neck Cancer; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Gardine, R.L.; Kokal, W.A.; Beatty, J.D.; Riihimaki, D.U.; Wagman, L.D.; Terz, J.J. Predicting the need for prolonged enteral supplementation in the patient with head and neck cancer. Am. J. Surg. 1988, 156, 63–65. [Google Scholar] [CrossRef]
- McRackan, T.R.; Watkins, J.M.; Herrin, A.E.; Garrett-Mayer, E.M.; Sharma, A.K.; Day, T.A.; Gillespie, M.B. Effect of Body Mass Index on Chemoradiation Outcomes in Head and Neck Cancer. Laryngoscope 2008, 118, 1180–1185. [Google Scholar] [CrossRef]
- Wan, G.J.; Counte, M.A.; Cella, D.F. The influence of personal expectations on cancer patients’ reports of health-related quality of life. Psycho Oncol. 1997, 6, 1–11. [Google Scholar] [CrossRef]
- Hofman, M.; Morrow, G.R.; Roscoe, J.A.; Hickok, J.T.; Mustian, K.M.; Moore, D.F.; Wade, J.L.; Fitch, T.R. Cancer patients’ expectations of experiencing treatment-related side effects. Cancer 2004, 101, 851–857. [Google Scholar] [CrossRef] [PubMed]
- McCarter, K.; Baker, A.L.; Britton, B.; Beck, A.K.; Carter, G.; Bauer, J.; Wratten, C.; Halpin, S.A.; Holliday, E.; Oldmeadow, C.; et al. Effectiveness of clinical practice change strategies in improving dietitian care for head and neck cancer patients according to evidence-based clinical guidelines: A stepped-wedge, randomized controlled trial. Transl. Behav. Med. 2018, 8, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | HPV-Negative | HPV-Positive | |
|---|---|---|---|
| n = 13 (16%) | n = 70 (84%) | ||
| Categorical variables, n (%) | |||
| Male | 12 (92) | 61 (87) | |
| Aboriginal or Torres Strait Islander | 0 | 2 (3) | |
| Non-English speaker at home | 1 (8) | 1 (1) | |
| Marital status | |||
| Married/Defacto | 9 (69) | 44 (63) | |
| Separated/Divorced/Widowed | 2 (15) | 17 (24) | |
| Single/Never married | 2 (15) | 9 (13) | |
| Highest level of education | |||
| Primary school | 6 (46) | 6 (9) | |
| High school | 3 (23) | 23 (33) | |
| University/Vocational college | 4 (31) | 41 (59) | |
| Tumor stage (AJCC 7) | |||
| I | 0 | 2 (3) | |
| II | 2 (15) | 10 (14) | |
| III | 0 | 14 (20) | |
| IV | 11 (85) | 44 (63) | |
| Concurrent chemotherapy | 13 (100) | 61 (87) | |
| Post-operative radiotherapy | 0 (0) | 9 (13) | |
| Prophylactic PEG | 4 (31) | 22 (31) | |
| Prophylactic NGT | 2 (15) | 2 (3) | |
| Received EAT intervention | 7 (54) | 42 (60) | |
| Substance use | |||
| Harmful Alcohol Use and Likely Dependence (AUDIT ≥8) | 5 (38) | 18 (26) | |
| Reported current smoking | 3 (23) | 8 (11) | |
| Nicotine Dependence (AUDIT ≥8) | 6 (46) | 3 (4) | |
| Continuous variables, mean (s.d) | |||
| Age in years | 56 (9.4) | 57 (7.4) | |
| Prescribed radiation (Gy) | 69 (2.8) | 69 (8.3) | |
| Depression (PHQ-9) | 4.1 (4.1) | 3.4 (3.7) | |
| Baseline Nutrition Variables | |||
| PG-SGA score mean (s.d) | 8.1 (6.9) | 4.1 (3.9) * | |
| PG-SGA category (B/C) n (%) # | 6 (46) | 8 (12) * | |
| BMI in Kg/m2 mean (s.d) | 24.5 (5.3) | 29.7 (6.2) * | |
| Variable | HPV-Negative n = 13 | HPV-Positive n = 70 | Statistic | p-Value | 95% CI | |
|---|---|---|---|---|---|---|
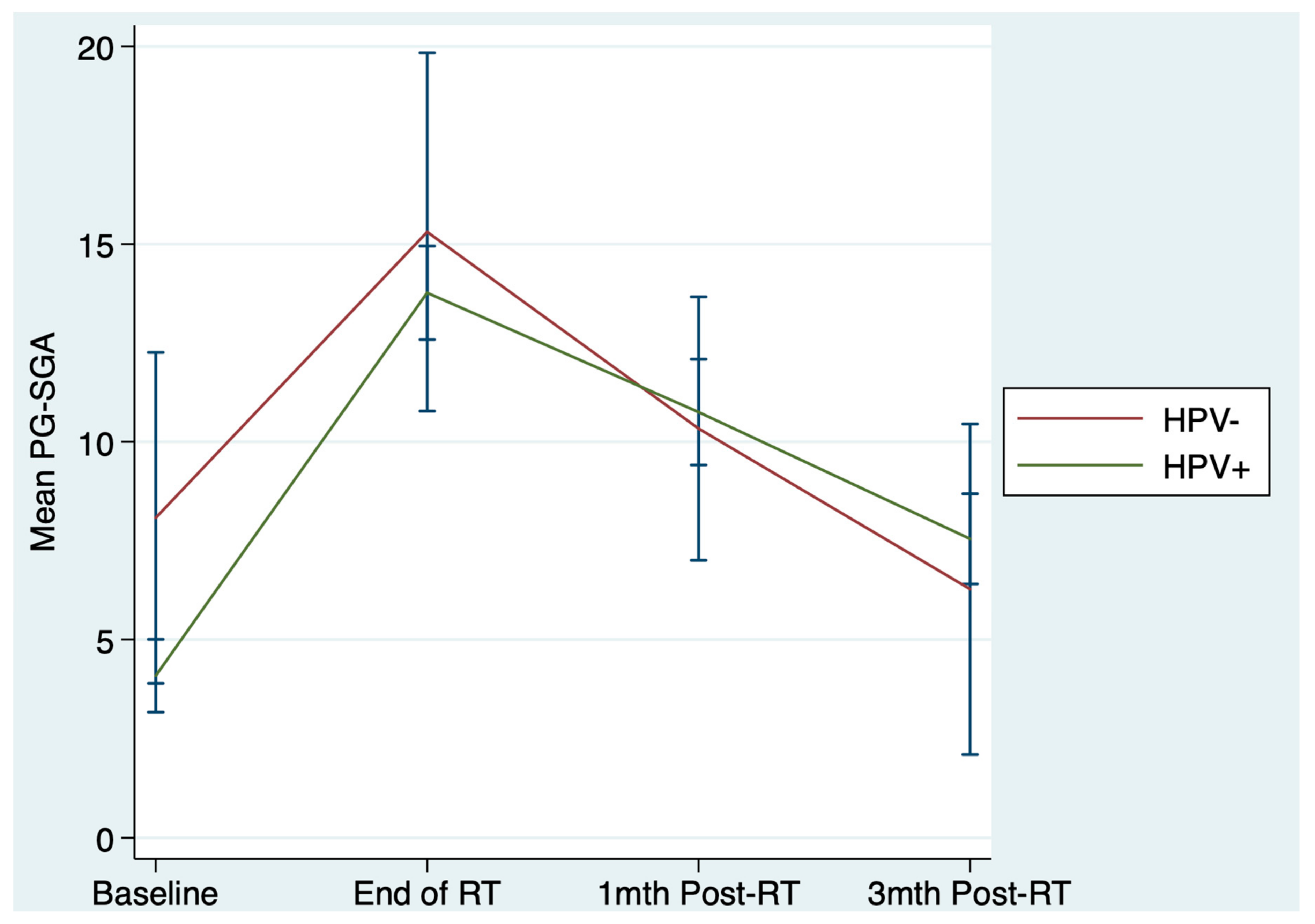
| PG-SGA score, mean (s.d) * | β = 0.80 | 0.44 | −1.22, 2.82 | |||
| Last wk treatment | 15.3 (7.5) | 13.8 (4.9) | ||||
| 1-mth post-treatment | 10.3 (5.2) | 10.8 (5.5) | ||||
| 3-mth post-treatment | 6.3 (6.2) | 7.5 (4.7) | ||||
| PG-SGA category B/C, n (%) # | OR = 0.50 | 0.30 | 0.14, 1.83 | |||
| Last wk treatment | 11 (85) | 62 (89) | ||||
| 1-mth post-treatment | 8 (62) | 50 (71) | ||||
| 3-mth post-treatment | 5 (38) | 30 (43) | ||||
| Variable | HPV-Negative n = 13 | HPV-Positive n = 70 | Statistic | p-Value | 95% CI | ||
|---|---|---|---|---|---|---|---|
| % LOW, mean (s.d) | β = −1.93 | 0.15 | −4.51, 0.66 | ||||
| Last wk treatment | 8.5 (5.4) | 7.1 (4.5) | |||||
| 1-mth post-treatment | 11.2 (5.9) | 10.2 (6.8) | |||||
| 3-mth post-treatment | 9.9 (9.9) | 12.8 (7.6) | |||||
| >5% LOW, n (%) | |||||||
| Last wk treatment | 9 (69) | 48 (69) | OR = 1.12 | 0.81 | 0.46, 2.70 | ||
| 1-mth post-treatment | 10 (77) | 56 (80) | |||||
| 3-mth post-treatment | 10 (77) | 61 (87) | |||||
| >10% LOW, n (%) | OR = 0.68 | 0.29 | 0.05, 9.31 | ||||
| Last wk treatment | 4 (31) | 19 (27) | |||||
| 1-mth post-treatment | 7 (54) | 37 (53) | |||||
| 3-mth post-treatment | 4 (31) | 47(67) | OR = 49.68 | <0.01 | 2.7, 912.86 | ||
| Depression, mean (s.d) | β = 0.49 | 0.66 | −1.72, 2.70 | ||||
| First wk treatment | 4.2 (4.1) | 3.4 (3.7) | |||||
| Last wk treatment | 7.1 (3.2) | 9.9 (5.5) | |||||
| 1-mth post-treatment | 6.3 (5.1) | 6.3 (4.9) | |||||
| 3-mth post-treatment | 7.7 (6.5) | 4.6 (4.9) | |||||
| PG-SGA Box 2 mean (s.d) | β = 0.33 | 0.14 | −0.11, 0.78 | ||||
| First wk treatment | 0.9 (1.1) | 0.4 (0.8) | |||||
| Last wk treatment | 2.0 (1.6) | 1.7 (1.2) | |||||
| 1-mth post-treatment | 0.6 (1.2) | 1.3 (1.2) | |||||
| 3-mth post-treatment | 0.6 (1.2) | 0.9 (0.9) | |||||
| PG-SGA Box 3 mean (s.d) | β = 0.53 | 0.41 | −0.74, 1.79 | ||||
| First wk treatment | 2.6 (2.8) | 1.1 (2.2) | |||||
| Last wk treatment | 4.8 (4.0) | 4.7 (3.9) | |||||
| 1-mth post-treatment | 2.8 (4.0) | 3.4 (3.7) | |||||
| 3-mth post-treatment | 1.9 (2.9) | 2.3 (2.9) | |||||
| Unplanned admissions, n | 6 | 64 | OR = 3.00 | 0.03 | 1.13, 8.02 | ||
| LOS in days, n | 26 | 253 | β = −1.70 | 0.34 | −1.81, 5.19 | ||
| RT interruptions, n (%) | 3 (25) | 5 (7) | OR = 0.24 | 0.09 | 0.5, 1.25 | ||
| Reactive NGT, n (%) | 3 (23) | 21 (30) | OR = 0.75 | 0.65 | 0.22, 0.26 | ||
| Mortality at 2-yrs n (%) | 4(31) | 5(7) | β = −1.58 | <0.01 | −2.27, −0.89 | ||
| Variable | β Statistic | p-Value | 95% CI | |
|---|---|---|---|---|
| Total HRQOL score * | 2.63 | 0.50 | 0.26, 0.68 | |
| Global Health * | 2.83 | 0.53 | −6.17, 11.83 | |
| Functional Outcomes * | ||||
| Role functioning | −2.61 | 0.70 | 16.23, 10.99 | |
| Physical Functioning | 2.91 | 0.54 | −6.54, 12.37 | |
| Emotional Functioning | 0.47 | 0.91 | −7.50, 8.43 | |
| Cognitive Functioning | −14.34 | <0.01 | −75, −5.04 | |
| Social Functioning | −3.03 | 0.58 | −13.81, 7.77 | |
| Symptom Scales # | ||||
| Fatigue | −3.44 | 0.53 | −14.06, 7.18 | |
| Nausea & Vomiting | −5.69 | 0.33 | −17.94, 6.56 | |
| Pain | 9.59 | 0.11 | −2.26, 21.44 | |
| Dyspnea | −5.69 | 0.21 | −14.57, 3.20 | |
| Insomnia | 0.82 | 0.89 | −11.27, 12.91 | |
| Appetite Loss | −10.91 | 0.19 | −27.33, 5.50 | |
| Constipation | 4.28 | 0.47 | −7.42, 15.99 | |
| Diarrhea | −2.99 | 0.48 | −11.29, 5.29 | |
| Financial difficulties | 2.48 | 0.75 | −12.73, 17.69 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrowfield, J.; Isenring, E.; Kiss, N.; Laing, E.; Lipson-Smith, R.; Britton, B. The Impact of Human Papillomavirus (HPV) Associated Oropharyngeal Squamous Cell Carcinoma (OPSCC) on Nutritional Outcomes. Nutrients 2021, 13, 514. https://doi.org/10.3390/nu13020514
Harrowfield J, Isenring E, Kiss N, Laing E, Lipson-Smith R, Britton B. The Impact of Human Papillomavirus (HPV) Associated Oropharyngeal Squamous Cell Carcinoma (OPSCC) on Nutritional Outcomes. Nutrients. 2021; 13(2):514. https://doi.org/10.3390/nu13020514
Chicago/Turabian StyleHarrowfield, Jane, Elizabeth Isenring, Nicole Kiss, Erin Laing, Ruby Lipson-Smith, and Ben Britton. 2021. "The Impact of Human Papillomavirus (HPV) Associated Oropharyngeal Squamous Cell Carcinoma (OPSCC) on Nutritional Outcomes" Nutrients 13, no. 2: 514. https://doi.org/10.3390/nu13020514







