Translating Evidence-Based Guidelines into Practice—Are We Getting It Right? A Multi-Centre Prospective International Audit of Nutrition Care in Patients with Foregut Tumors (INFORM)
Abstract
1. Introduction
2. Materials and Methods
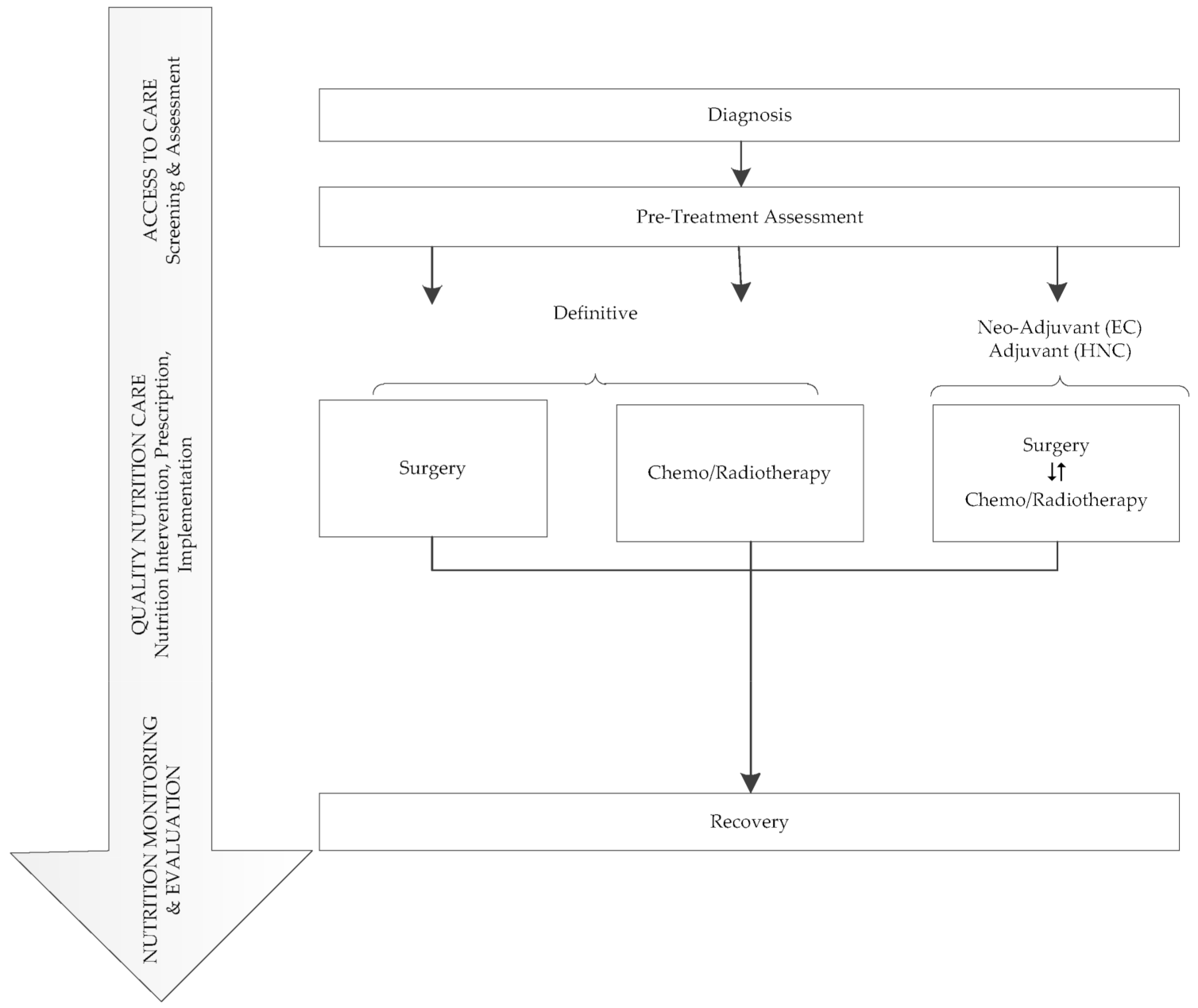
2.1. Study Design, Setting and Population
2.2. Outcomes
2.3. Data Collection
2.4. Statistical Analysis
2.5. Ethics Approval and Reporting
3. Results
3.1. Baseline Characteristics
3.2. Adherence to Evidence-Based Guideline Recommendations
3.3. Unplanned Admissions, Treatment Completion and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Bokhorst-de van der Schueren, M.A.; Van Leeuwen, P.A.; Kuik, D.J.; Klop, W.M.; Sauerwein, H.P.; Snow, G.B.; Quak, J.J. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer 1999, 86, 519–527. [Google Scholar] [CrossRef]
- Riccardi, D.; Allen, K. Nutritional Management of Patients With Esophageal and Esophagogastric Junction Cancer. Cancer Control. 1999, 6, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; De Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients With Cancer. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Arends, J. The causes and consequences of cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9, S51–S63. [Google Scholar] [CrossRef]
- Baracos, V.E. Cancer-associated malnutrition. Eur. J. Clin. Nutr. 2018, 72, 1255–1259. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Nature Reviews Disease Primers. Nat. Rev. Dis. Prim. 2019, 4, 17105. [Google Scholar] [CrossRef]
- Findlay, M.; Bauer, J.; Brown, T.; Davidson, W.; Isenring, E.; Kiss, N.; Kurmis, R.; Loeliger, J.; Sandison, A.; Talwar, B.; et al. Evidence-Based Practice Guidelines for the Nutritional Management of Adult Patients with Head and Neck Cancer; Cancer Council Australia: Sydney, Australia, 2009; Available online: http://wiki.cancer.org.au/australia/COSA:Head_and_neck_cancer_nutrition_guidelines (accessed on 4 June 2019).
- Isenring, E.A.; Zabel, R.; Bannister, M.; Brown, T.; Findlay, M.; Kiss, N.; Loeliger, J.; Johnstone, C.; Camilleri, B.; Davidson, W.; et al. Updated evidence-based practice guidelines for the nutritional management of patients receiving radiation therapy and/or chemotherapy. Nutr. Diet. 2013, 70, 312–324. [Google Scholar] [CrossRef]
- Dort, J.C.; Farwell, D.; Findlay, M.; Gerhard, F.H.; Paul, K.; Melissa, A.S.-B.; Christian, S.; Jeffrey, U.; David, Z.; Olle, L. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: A consensus review and recommendations from the enhanced recovery after surgery society. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 292–303. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Pertkiewicz, M.; Ljungqvist, O.; Soeters, P.; Fearon, K.; Weimann, A.; Bozzetti, F. ESPEN Guidelines on Parenteral Nutrition: Surgery. Clin. Nutr. 2009, 28, 378–386. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Harsanyi, L.; Laviano, A.; Ljungqvist, O.; Soeters, P.; Jauch, K.; Kemen, M.; Hiesmayr, J.; Horbach, T.; et al. ESPEN Guidelines on Enteral Nutrition: Surgery including Organ Transplantation. Clin. Nutr. 2006, 25, 224–244. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Thompson, K.L.; Elliott, L.; Fuchs-Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310.e47. [Google Scholar] [CrossRef] [PubMed]
- Talwar, B.; Donnelly, R.; Skelly, R.; Donaldson, M. Nutritional management in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Handu, D.; Moloney, L.; Wolfram, T.; Ziegler, P.; Acosta, A.; Steiber, A. Academy of Nutrition and Dietetics Methodology for Conducting Systematic Reviews for the Evidence Analysis Library. J. Acad. Nutr. Diet. 2016, 116, 311–318. [Google Scholar] [CrossRef]
- Scottish Intercollegiate Guidelines Network. A Guideline Developer’s Handbook; SIGN: Edinburgh, UK, 2014; Available online: http://www.sign.ac.uk (accessed on 19 September 2020).
- National Health and and Medical Research Council. NHMRC levels of evidence and grades for recommendations for guideline developers. In NHMRC Levels of Evidence and Grades for Recommendations; National Health and and Medical Research Council: Sydney, Australia, 2009. [Google Scholar]
- Schütz, T.; Herbst, B.; Koller, M. Methodology for the development of the ESPEN Guidelines on Enteral Nutrition. Clin. Nutr. 2006, 25, 203–209. [Google Scholar] [CrossRef]
- Bero, L.A.; Grilli, R.; Grimshaw, J.M.; Harvey, E.; Oxman, A.D.; Thomson, M.A. Getting research findings into practice: Closing the gap between research and practice: An overview of systematic reviews of interventions to promote the implementation of research findings. BMJ 1998, 317, 465–468. [Google Scholar] [CrossRef]
- Green, L.W.; Ottoson, J.M.; García, C.; Hiatt, R.A. Protected], [Email Diffusion Theory and Knowledge Dissemination, Utilization, and Integration in Public Health. Annu. Rev. Public Health 2009, 30, 151–174. [Google Scholar] [CrossRef]
- Balas, E.; Weingarten, S.; Garb, C.T.; Blumenthal, D.; A Boren, S.; Brown, G.D. Improving preventive care by prompting physicians. Arch. Intern. Med. 2000, 160, 301–308. [Google Scholar] [CrossRef]
- Balas, E.B.S. Managing clinical knowledge for health care improvement. In Yearbook of Medical Informatics 2000: Patient-Centered Systems; Bemmel, J.M.A., Ed.; Schattauer Verlagsgesellschaft mbH: Stuttgart, Germany, 2000; pp. 65–70. [Google Scholar]
- Grimshaw, J.; Eccles, M.; Tetroe, J. Implementing clinical guidelines: Current evidence and future implications. J. Contin. Educ. Health Prof. 2004, 24, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Hakel-Smith, N.; Lewis, N.M. A standardized nutrition care process and language are essential components of a conceptual model to guide and document nutrition care and patient outcomes. J. Am. Diet. Assoc. 2004, 104, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Lacey, K.; Pritchett, E. Nutrition Care Process and Model: ADA adopts road map to quality care and outcomes management. J. Am. Diet. Assoc. 2003, 103, 1061–1072. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; A Mendelson, R.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Ottery, F.D. Patient generated-subjective global assessment. In The Clinical Guide to Oncology Nutrition; McCallum, P., Polisena, C., Eds.; The American Dietetic Association: Chicago, CA, USA, 2000; pp. 11–23. [Google Scholar]
- Jager-Wittenaar, H.; Ottery, F.D. Assessing nutritional status in cancer: Role of the Patient-Generated Subjective Global Assessment. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Food and Nutrition Board; Health and Medicine Division; National Academies of Sciences, Engineer and Medicine. Examining Access to Nutrition Care in Outpatient Cancer Centers: Proceedings of a Workshop; The National Academies of Sciences, Engineering and Medicine: Washington, DC, USA, 2016.
- Brown, T.; Findlay, M. Current issues in the nutritional management of patients with head and neck cancer in Australia. Cancer Forum 2011, 35, 92–96. [Google Scholar]
- Trujillo, E.B.; Dixon, S.W.; Claghorn, K.; Levin, R.M.; Mills, J.B.; Spees, C.K. Closing the Gap in Nutrition Care at Outpatient Cancer Centers: Ongoing Initiatives of the Oncology Nutrition Dietetic Practice Group. J. Acad. Nutr. Diet. 2018, 118, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hillner, B.E. Ensuring quality cancer care by the use of clinical practice guidelines and critical pathways. J. Clin. Oncol. 2001, 19, 2886–2897. [Google Scholar] [CrossRef]
- Martin, L.; de van der Schueren, M.A.E.; Blauwhoff-Buskermolen, S.; Baracos, V.; Gramlich, L. Identifying the Barriers and Enablers to Nutrition Care in Head and Neck and Esophageal Cancers. J. Parenter. Enter. Nutr. 2014, 40, 355–366. [Google Scholar] [CrossRef]
- Britton, B.; Baker, A.L.; Wolfenden, L.; Wratten, C.; Bauer, J.; Beck, A.K.; McCarter, K.; Harrowfield, J.; Isenring, E.; Tang, C.; et al. Eating As Treatment (EAT): A Stepped-Wedge, Randomized Controlled Trial of a Health Behavior Change Intervention Provided by Dietitians to Improve Nutrition in Patients With Head and Neck Cancer Undergoing Radiation Therapy (TROG 12.03). Int. J. Radiat. Oncol. 2019, 103, 353–362. [Google Scholar] [CrossRef]
- Findlay, M.; Rankin, N.M.; Shaw, T.; White, K.; Boyer, M.; Milross, C.G.; Lourenço, R.D.A.; Brown, C.; Collett, G.; Beale, P.; et al. Best Evidence to Best Practice: Implementing an Innovative Model of Nutrition Care for Patients with Head and Neck Cancer Improves Outcomes. Nutrients 2020, 12, 1465. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrients 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Kruizenga, H.M.; Seidell, J.; De Vet, H.C.W.; Wierdsma, N.; Schueren, M.A.E.V.B.-D.V.D. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ©). Clin. Nutr. 2005, 24, 75–82. [Google Scholar] [CrossRef]
- American Dietetic Association. Nutrition Diagnosis and Intervention: Standardized Language for the Nutrition Care Process.; American Dietetic Association: Chicago, IL, USA, 2007. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- McCarter, K.L.; Baker, A.L.; Britton, B.; Halpin, S.A.; Beck, A.; Carter, G.; Wratten, C.; Bauer, J.; Wolfenden, L.; Burchell, K.; et al. Head and neck cancer patient experience of a new dietitian-delivered health behaviour intervention: ‘You know you have to eat to survive’. Support. Care Cancer 2018, 26, 2167–2175. [Google Scholar] [CrossRef]
- Britton, B.; McCarter, K.; Baker, A.; Wolfenden, L.; Wratten, C.; Bauer, J.; Beck, A.; McElduff, P.; Halpin, S.; Carter, G. Eating As Treatment (EAT) study protocol: A stepped-wedge, randomised controlled trial of a health behaviour change intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiotherapy. BMJ Open 2015, 5, e008921. [Google Scholar] [CrossRef]
- McCarter, K.; Baker, A.L.; Britton, B.; Beck, A.K.; Carter, G.; Bauer, J.; Wratten, C.; A Halpin, S.; Holliday, E.; Oldmeadow, C.; et al. Effectiveness of clinical practice change strategies in improving dietitian care for head and neck cancer patients according to evidence-based clinical guidelines: A stepped-wedge, randomized controlled trial. Transl. Behav. Med. 2018, 8, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, P. Navigating Healthcare Reform—E-Book: An Insider’s Guide for Nurses and Allied Health Professionals, 1st ed.; Elselvier: Amsterdam, The Netherlands, 2017; p. 283. [Google Scholar]
- Findlay, M.; White, K.; Lai, M.; Luo, D.; Bauer, J.D. The Association Between Computed Tomography—Defined Sarcopenia and Outcomes in Adult Patients Undergoing Radiotherapy of Curative Intent for Head and Neck Cancer: A Systematic Review. J. Acad. Nutr. Diet. 2020, 120, 1330–1347.e8. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.; Brown, C.; Lourenço, R.D.A.; White, K.; Bauer, J. Sarcopenia and myosteatosis in patients undergoing curative radiotherapy for head and neck cancer: Impact on survival, treatment completion, hospital admission and cost. J. Hum. Nutr. Diet. 2020, 33, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Liu, S.; Liao, J.-F.; Wen, W.; Long, Z.-Q.; Lu, Z.-J.; Guo, L.; Lin, H.-X. When the Loss Costs Too Much: A Systematic Review and Meta-Analysis of Sarcopenia in Head and Neck Cancer. Front. Oncol. 2020, 9, 1561. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, C.; Martin, L.; Gramlich, L.; Scrimger, R.; Jha, N.; Debenham, B.; Chua, N.; Walker, J.; Baracos, V.E. Prevalence and prognostic significance of malnutrition in patients with cancers of the head and neck. Clin. Nutr. 2020, 39, 901–909. [Google Scholar] [CrossRef]
- Martin, L.; Gioulbasanis, I.; Senesse, P.; Baracos, V.E. Cancer-Associated Malnutrition and CT-Defined Sarcopenia and Myosteatosis Are Endemic in Overweight and Obese Patients. J. Parenter. Enter. Nutr. 2020, 44, 227–238. [Google Scholar] [CrossRef]
- Sealy, M.J.; Dechaphunkul, T.; van der Schans, C.P.; Krijnen, W.P.; Roodenburg, J.L.N.; Walker, J.; Jager-Wittenaar, H.; Baracos, V.E. Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clin. Nutr. 2020, 39, 501–509. [Google Scholar] [CrossRef]
- Wong, A.; Zhu, D.; Kraus, D.; Tham, T. Radiologically Defined Sarcopenia Affects Survival in Head and Neck Cancer: A Meta-Analysis. Laryngoscope 2020. [Google Scholar] [CrossRef]
- Saeed, S.D.; Fontaine, J.; Pena, L.; Hoffe, S.E.; Frakes, J.; Metha, R.; Gurd, E.; Pimiento, J.M. Prognostic value of nutritional status for esophageal cancer patients undergoing neoadjuvant therapy and resection. J. Clin. Oncol. 2019, 37, 133. [Google Scholar] [CrossRef]
- Simonsen, C.; de Heer, P.; Bjerre, E.D.; Suetta, C.; Hojman, P.; Pedersen, B.K.; Svendsen, L.B.; Christensen, J.F. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann. Surg. 2018, 268, 58–69. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Loeliger, J.; Findlay, M.; Isenring, E.; Baguley, B.J.; Boltong, A.; Butler, A.; Deftereos, I.; Eisenhuth, M.; Fraser, S.F.; et al. Clinical Oncology Society of Australia: Position statement on cancer-related malnutrition and sarcopenia. Nutr. Diet. 2020, 77, 416–425. [Google Scholar] [CrossRef]
- McCurdy, B.; Nejatinamini, S.; Debenham, B.; Álvarez-Camacho, M.; Kubrak, C.; Wismer, W.V.; Mazurak, V. Meeting Minimum ESPEN Energy Recommendations Is Not Enough to Maintain Muscle Mass in Head and Neck Cancer Patients. Nutrients 2019, 11, 2743. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Brown, T.E.; Banks, M.; Hughes, B.G.; Lin, C.; Kenny, L.; Bauer, J. Tube feeding during treatment for head and neck cancer—Adherence and patient reported barriers. Oral Oncol. 2017, 72, 140–149. [Google Scholar] [CrossRef]
| First Author, Year | Origin | Organisation | Guideline Focus | Appraisal Method |
|---|---|---|---|---|
| Arends, 2017 [14] | Europe | ESPEN a | Cancer | GRADE b |
| Dort, 2017 [10] | International | ERAS c Society | Surgery–HNC d | GRADE |
| Thompson, 2017 [15] | USA e | AND f | Cancer | AND EAL g |
| Weimann, 2017 [11] | Europe | ESPEN | Surgery | SIGN h, AHCPR i |
| Talwar, 2016 [16] | UK j | BAHNO k | HNC | ns l |
| Isenring, 2013 [9] | Australia | DAA m | RT n, CTx o | NHMRC p |
| Findlay, 2011 [8] | Australia * | COSA q | HNC | NHMRC |
| Braga, 2009 [12] ** | Europe | ESPEN | Surgery-PN r | ns |
| Weimann, 2006 [13] ** | Europe | ESPEN | Surgery | ESPEN |
| Nutrition Care Model [28,29] | Recommendation | Guideline | Evidence * | Adherence Criteria |
|---|---|---|---|---|
| ACCESS TO CARE Screening Assessment |
| COSA a [8], DAA b [9] ESPEN c, 2017 [14] AND d [15] ESPEN, 2017 [11] | B V Low/Strong Strong/Imperative Not specified |
|
| DAA, COSA | A |
| |
| DAA, COSA AND | B Strong/Imperative |
| |
| DAA, COSA AND | B Strong/Imperative |
| |
| QUALITY NUTRITION CARE Nutrition intervention, prescription, implementation | Intervention
| DAA, COSA | A |
|
Prescription
| DAA, COSA | C | Radiotherapy
| |
| COSA ESPEN 2017 [14] ESPEN 2009 [12] ESPEN 2009 [12] | C Low/Strong B B | Surgery
| |
Implementation
| COSA ERAS j [10] ESPEN 2017 [11] | A Mod/Strong A/GPP k |
| |
| NUTRITION MONITORING EVALUATION |
| DAA, COSA | A |
|
| DAA, COSA | A |
|
| Characteristic | Head and Neck (n = 119) n (%) | Esophageal (n = 51) n (%) | All (n = 170) n (%) |
|---|---|---|---|
| Centre (Country–City) | |||
| Australia–Brisbane | 10 (8.4%) | 0 (0.0%) | 10 (5.9%) |
| Australia–Sydney | 20 (16.8%) | 0 (0.0%) | 20 (11.8%) |
| Canada-Calgary * | 21 (17.6%) | 10 (19.6%) | 31 (18.2%) |
| Canada–Edmonton * | 20 (16.8%) | 18 (35.3%) | 38 (22.4%) |
| Italy–Rome | 19 (16.0%) | 0 (0.0%) | 19 (11.2%) |
| Netherlands-Amsterdam | 20 (16.8%) | 23 (45.1%) | 43 (25.3%) |
| USA-Sacramento | 9 (7.6%) | 0 (0.0%) | 9 (5.3%) |
| Age, years | |||
| median [Q1, Q3] | 62 [58, 69] | 65 [56, 71] | 63 [57, 70] |
| Sex | |||
| Male | 93 (78.2%) | 41 (80.4%) | 134 (78.8%) |
| Female | 26 (21.8%) | 10 (19.6%) | 36 (21.2%) |
| Ethnicity | |||
| Caucasian | 111 (93.3%) | 50 (98.0%) | 161 (94.7%) |
| First Nations | 1 (0.8%) | 0 (0.0%) | 1 (0.6%) |
| Hispanic | 2 (1.7%) | 0 (0.0%) | 2 (1.2%) |
| Asian | 3 (2.5%) | 1 (2.0%) | 4 (2.4%) |
| East Indian | 1 (0.8%) | 0 (0.0%) | 1 (0.6%) |
| Other | 1 (0.8%) | 0 (0.0%) | 1 (0.6%) |
| Current Smoker | |||
| Yes | 44 (37.0%) | 13 (25.5%) | 57 (33.5%) |
| No | 75 (63.0%) | 38 (74.5%) | 113 (66.5%) |
| Alcohol use | |||
| Yes | 47 (39.5%) | 9 (17.6%) | 56 (32.9%) |
| No | 72 (60.5%) | 42 (82.4%) | 114 (67.1%) |
| ECOG a Performance Status | |||
| 0 | 78 (65.5%) | 32 (62.7%) | 110 (64.7%) |
| 1 | 32 (26.9%) | 16 (31.4%) | 48 (28.2%) |
| 2 | 6 (5.0%) | 3 (5.9%) | 9 (5.3%) |
| 3 | 3 (2.5%) | 0 (0.0%) | 3 (1.8%) |
| Clinical Stage | |||
| 1 | 5 (4.2%) | 4 (7.8%) | 9 (5.3%) |
| 2 | 8 (6.7%) | 13 (25.5%) | 21 (12.4%) |
| 3 | 18 (15.1%) | 15 (29.4%) | 33 (19.4%) |
| 4 (Any) | 74 (62.2%) | 4 (7.8%) | 78 (45.9%) |
| Could not assess stage | 7 (5.9%) | 11 (21.6%) | 16 (10.6%) |
| Not staged | 7 (5.9%) | 4 (7.8%) | 11 (6.5%) |
| Tumor Site–Head and Neck | |||
| Primary unknown | 3 (2.5%) | 3 (1.8%) | |
| Hypopharynx | 10 (8.4%) | 10 (5.9%) | |
| Larynx | 22 (18.5%) | 22 (12.9%) | |
| Nasopharynx | 5 (4.2%) | 5 (2.9%) | |
| Oral cavity | 32 (26.9%) | 32 (18.8%) | |
| Oropharynx | 39 (32.8%) | 39 (22.9%) | |
| Other | 3(2.5%) | 3 (1.8%) | |
| Salivary gland | 5 (4.2%) | 5 (2.9%) | |
| Treatment Modality b | |||
| None | 5 (5.0%) | 5 (9.8%) | 10 (6.6%) |
| Chemotherapy-definitive | 0 (0.0%) | 2 (3.9%) | 2 (1.3%) |
| Chemotherapy-adjuvant | 0 (0.0%) | 1 (2.0%) | 1(0.7%) |
| Radiotherapy-definitive | 15 (15.0%) | 0 (0.0%) | 15 (9.9%) |
| Surgery | 10 (10.0%) | 2 (3.9%) | 12 (7.9%) |
| Chemoradiotherapy-definitive | 54 (54.0%) | 6 (11.8%) | 60 (39.7%) |
| Surgery + adj c/neoadj d RT e | 7 (7.0%) | 0 (0.0%) | 7 (4.6%) |
| Surgery + adj/neoadj CRT f | 9 (9.0%) | 35 (68.6%) | 44 (29.1%) |
| Nutritional Characteristics | Head and Neck (n = 119) n (%) | Esophageal (n = 51) n (%) | All (n = 170) n (%) | |
|---|---|---|---|---|
| Weight (kg) | ||||
| median [Q1, Q3] | 77 [67, 91] | 85 [75, 95] | 81 [70, 91] | |
| Height (cm) | ||||
| median [Q1, Q3] | 173 [168, 178] | 176 [170, 182] | 174 [169, 179] | |
| BMI a (kg/m2) | ||||
| median [Q1, Q3] | 26 [23, 30] | 27 [25, 31] | 26 [23, 30] | |
| Was a nutrition diagnosis made? | ||||
| Yes | 52 (43.7%) | 33 (64.7%) | 85 (50.0%) | |
| No | 27 (22.7%) | 1 (2.0%) | 28 (16.5%) | |
| No data | 40 (33.6%) | 17 (33.3%) | 57 (33.5%) | |
| Nutritional Risk, PG-SGA b Short Form Score | ||||
| (n) median [Q1, Q3] | (116) 5 [1, 10] | (47) 8 [4, 11] | 6 [2, 11] | |
| Location patient first introduced to cancer care setting | ||||
| Inpatient | 14 (11.8%) | 4 (7.8%) | 18 (10.6%) | |
| Outpatient | 105 (88.2%) | 47 (92.2%) | 152 (89.4%) | |
| Did patient receive EN c or PN d within the last month prior to study enrollment? | ||||
| Yes | 16 (13.4%) | 6 (11.8%) | 22 (12.9%) | |
| No | 103 (86.6%) | 45 (88.2%) | 148 (87.1%) | |
| Nutrition Care Model [27,28] | Measure–System Level (Usual Practice in Centre at Study Enrollment) | N (n = 11) | % | ||
|---|---|---|---|---|---|
| Access to Care | Screening | ||||
| Malnutrition screening routinely performed. | 11 | 100% | |||
| Validated nutrition screening tool used. | 7 | 64% | |||
| Assessment | |||||
| Nutrition assessment routinely performed. | 11 | 100% | |||
| Validated nutrition assessment tool used. | 8 | 73% | |||
| Quality Nutrition Care | Measure—Patient Level | HNC | EC | ||
| Surgery | (n = 26) | (n = 38) | |||
| n | % | n | % | ||
| Nutrition assessment | |||||
| Pre-operative | 6 | 23% | 37 | 97% | |
| Post-operative | 24 | 92% | 36 | 95% | |
| Nutrition support-tube feeding commenced | |||||
| Pre-operative | 1 | 4% | 8 | 21% | |
| Post-operative | |||||
| within 24 h | 5 | 19% | 2 | 5% | |
| within 1 to 7 days | 12 | 46% | 17 | 44% | |
| Nutrition prescription, median [Q1, Q3] | |||||
| Energy prescription (kcal/kg/d) | 30.8 [29.6, 32.7] | 26.3 [23.2, 28.1] | |||
| Protein prescription (g/kg/d) | 1.3 [1.2, 1.5] | 1.4 [1.2, 1.5] | |||
| Radiotherapy/Chemoradiotherapy | (n = 85) | (n = 41) | |||
| n# | % | n# | % | ||
| Nutrition prescription, median [Q1, Q3] | |||||
| Energy prescription (kcal/kg/d) | 30.4 [26.7, 33.4] | 26.7 [23.3, 28.8] | |||
| Protein prescription (g/kg/d) | 1.3 [1.2, 1.5] | 1.4 [1.2, 1.5] | |||
| Nutrition Monitoring and Evaluation | Received recommended dietitian consultation: | ||||
| Pre-treatment (Access to Care) | 54/85 | 64% | 27/41 | 66% | |
| Weekly during treatment | 36/85 | 42% | 10/41 | 24% | |
| Fortnightly for 6 weeks post-treatment | 11/85 | 13% | 17/41 | 17% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Findlay, M.; Bauer, J.D.; Dhaliwal, R.; de van der Schueren, M.; Laviano, A.; Widaman, A.; Martin, L.; Day, A.G.; Gramlich, L.M. Translating Evidence-Based Guidelines into Practice—Are We Getting It Right? A Multi-Centre Prospective International Audit of Nutrition Care in Patients with Foregut Tumors (INFORM). Nutrients 2020, 12, 3808. https://doi.org/10.3390/nu12123808
Findlay M, Bauer JD, Dhaliwal R, de van der Schueren M, Laviano A, Widaman A, Martin L, Day AG, Gramlich LM. Translating Evidence-Based Guidelines into Practice—Are We Getting It Right? A Multi-Centre Prospective International Audit of Nutrition Care in Patients with Foregut Tumors (INFORM). Nutrients. 2020; 12(12):3808. https://doi.org/10.3390/nu12123808
Chicago/Turabian StyleFindlay, Merran, Judith D. Bauer, Rupinder Dhaliwal, Marian de van der Schueren, Alessandro Laviano, Adrianne Widaman, Lisa Martin, Andrew G. Day, and Leah M. Gramlich. 2020. "Translating Evidence-Based Guidelines into Practice—Are We Getting It Right? A Multi-Centre Prospective International Audit of Nutrition Care in Patients with Foregut Tumors (INFORM)" Nutrients 12, no. 12: 3808. https://doi.org/10.3390/nu12123808
APA StyleFindlay, M., Bauer, J. D., Dhaliwal, R., de van der Schueren, M., Laviano, A., Widaman, A., Martin, L., Day, A. G., & Gramlich, L. M. (2020). Translating Evidence-Based Guidelines into Practice—Are We Getting It Right? A Multi-Centre Prospective International Audit of Nutrition Care in Patients with Foregut Tumors (INFORM). Nutrients, 12(12), 3808. https://doi.org/10.3390/nu12123808






