Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Measurement of Clinical and Metabolic Parameters
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Effects of Vitamin D Supplementation on Serum 25(OH)D Levels and Serum 1,25-Dihydroxyvitamin D Levels and Biochemical Markers
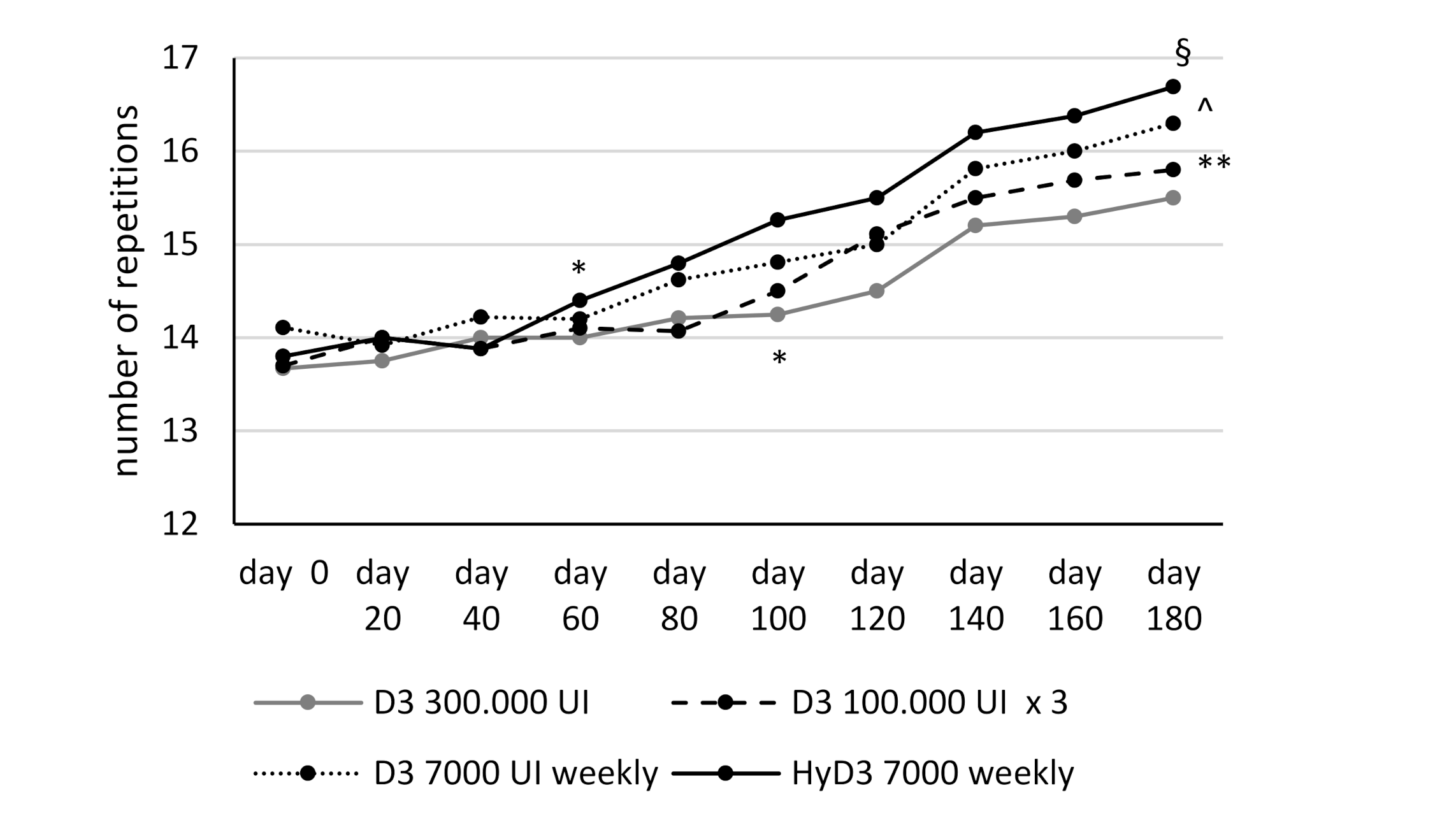
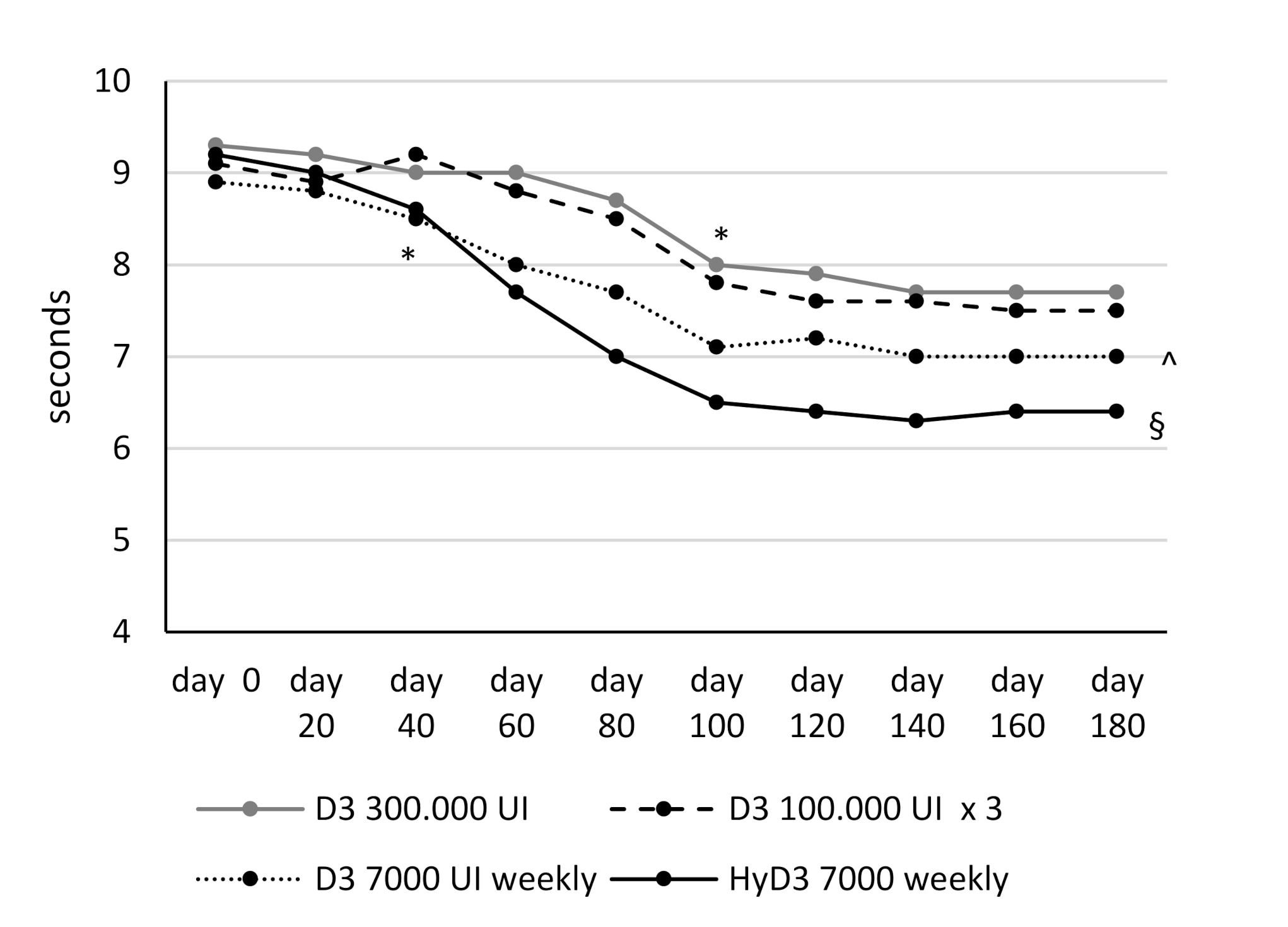
3.3. Effects of Vitamin D Supplementation on Muscular Lower Extremity Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 1997, 337, 670–676. [Google Scholar] [CrossRef]
- Ooms, M.E.; Roos, J.C.; Bezemer, P.D.; van der Vijgh, W.J.; Bouter, L.M.; Lips, P. Prevention of bone loss by vitamin D supplementation in elderly women: A randomized double- blind trial. J. Clin. Endocrinol. Metab. 1995, 80, 1052–1258. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28, Erratum in: Am. J. Clin. Nutr. 2006, 84, 1253. [Google Scholar] [CrossRef]
- Glerup, H.; Mikkelsen, K.; Poulsen, L.; Hass, E.; Overbeck, S.; Andersen, H.; Charles, P.; Eriksen, E.F. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif. Tissue Int. 2000, 66, 419–424. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [Green Version]
- Annweiler, C.; Henni, S.; Walrand, S.; Montero-Odasso, M.; Duque, G.; Duval, G.T. Vitamin D and walking speed in older adults: Systematic review and meta-analysis. Maturitas 2017, 106, 8–25. [Google Scholar] [CrossRef]
- Iolascon, G.; Mauro, G.L.; Fiore, P.; Cisari, C.; Benedetti, M.G.; Panella, L.; De Sire, A.; Calafiore, D.; Moretti, A.; Gimigliano, F. Can vitamin D deficiency influence muscle performance in postmenopausal women? A multicentre retrospective study. Eur. J. Phys. Rehabil. Med. 2018, 54, 676–682. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Bouvard, B.; Annweiler, C.; Sallé, A.; Beauchet, O.; Chappard, D.; Audran, M.; Legrand, E. Extraskeletal effects of vitamin D: Facts, uncertainties, and controversies. Jt. Bone Spine 2011, 78, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A. Which vitamin D oral supplement is best for postmenopausal women? Curr. Osteoporos. Rep. 2012, 10, 251–257. [Google Scholar] [CrossRef]
- Chao, Y.S.; Brunel, L.; Faris, P.; Veugelers, P.J. The importance of dose, frequency and duration of vitamin D supplementation for plasma 25-hydroxyvitamin D. Nutrients 2013, 5, 4067–4078. [Google Scholar] [CrossRef]
- Ish-Shalom, S.; Segal, E.; Salganik, T.; Raz, B.; Bromberg, I.L.; Vieth, R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J. Clin. Endocrinol. Metab. 2008, 93, 3430–3435. [Google Scholar] [CrossRef] [Green Version]
- Binkley, N.; Gemar, D.; Engelke, J.; Gangnon, R.; Ramamurthy, R.; Krueger, D.; Drezner, M.K. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J. Clin. Endocrinol. Metab. 2011, 96, 981–988. [Google Scholar] [CrossRef] [Green Version]
- Black, L.J.; Anderson, D.; Clarke, M.W.; Ponsonby, A.L.; Lucas, R.M. Ausimmune Investigator Group. Analytical Bias in the Measurement of Serum 25-Hydroxyvitamin D Concentrations Impairs Assessment of Vitamin D Status in Clinical and Research Settings. PLoS ONE 2015, 10, e0135478. [Google Scholar]
- Khant, N.; Dani, V.B.; Patel, P.; Rathod, R. Establishing the reference value for “timed up-and-go” test in healthy adults of Gujarat, India. J. Educ. Health Promot. 2018, 7, 62. [Google Scholar] [CrossRef]
- Stamp, T.C.; Haddad, J.G.; Twigg, C.A. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet 1977, 1, 1341–1343. [Google Scholar] [CrossRef]
- Barger-Lux, M.J.; Heaney, R.P.; Dowell, S.; Chen, T.C.; Holick, M.F. Vitamin D and its major metabolites: Serum levels after graded oral dosing in healthy men. Osteoporos. Int. 1998, 8, 222–230. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Stöcklin, E.; Sidelnikov, E.; Willett, W.C.; Edel, J.O.; Stähelin, H.B.; Wolfram, S.; Jetter, A.; Schwager, J.; et al. Oral supplementation with 25(OH)D3 versus vitamin D3: Effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J. Bone Miner. Res. 2012, 27, 160–169. [Google Scholar] [CrossRef]
- Mastaglia, S.R.; Mautalen, C.A.; Parisi, M.S.; Oliveri, B. Vitamin D2 dose required to rapidly increase 25(OH)D levels in osteoporotic women. Eur. J. Clin. Nutr 2006, 60, 681–687. [Google Scholar]
- Chel, V.; Wijnhoven, H.A.H.; Smit, J.H.; Ooms, M.; Lips, P.T.A.M. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos. Int. 2008, 19, 663–671. [Google Scholar]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Quesada-Gómez, J.M.; Bouillon, R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar]
- Jetter, A.; Egli, A.; Dawson-Hughes, B.; Staehelin, H.B.; Stoecklin, E.; Goessl, R.; Henschkowski, J.; Bischoff-Ferrari, H.A. Pharmacokinetics of oral vitamin D3 and calcifediol. Bone 2014, 59, 14–19. [Google Scholar]
- Rossini, M.; Viapiana, O.; Gatti, D.; James, G.; Girardello, S.; Adami, S. The long term correction of vitamin D deficiency: Comparison between di_erent treatments with vitamin D in clinical practice. Minerva Med. 2005, 96, 1–7. [Google Scholar]
- Navarro-Valverde, C.; Sosa-Henríquez, M.; Alhambra-Expósito, M.R.; Quesada-Gómez, J.M. Vitamin D3 and calcidiol are not equipotent. J. Steriod. Biochem. Mol. Biol. 2016, 164, 205–208. [Google Scholar]
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684. [Google Scholar] [CrossRef]
- Corachán, A.; Ferrero, H.; Aguilar, A.; Garcia, N.; Monleon, J.; Faus, A.; Cervelló, I.; Pellicer, A. Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway. Fertil. Steril. 2019, 111, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Rezagholizadeh, F.; Keshavarz, S.A.; Djalali, M.; Rad, E.Y.; Alizadeh, S.; Javanbakht, M.H. Vitamin D3 supplementation improves serum SFRP5 and Wnt5a levels in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Int. J. Vitam. Nutr. Res. 2018, 88, 73–79. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y.; Xin, N.; Yuan, Y.; Zhang, Q.; Gong, P.; Wu, Y. 1α,25-Dihydroxyvitamin D3 promotes osteogenesis by promoting Wnt signaling pathway. J. Steroid Biochem. Mol. Biol. 2017, 174, 153–160. [Google Scholar] [CrossRef]
- Neve, A.; Cantatore, F.P.; Corrado, A.; Gaudio, A.; Ruggieri, S.; Ribatti, D. In vitro and in vivo angiogenic activity of osteoarthritic and osteoporotic osteoblasts is modulated by VEGF and vitamin D3 treatment. Regul. Pept. 2013, 184, 81–84. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin. Exp. Med. 2014, 14, 275–283. [Google Scholar] [CrossRef]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef] [Green Version]
- Skaria, J.; Katiyar, B.C.; Srivastava, T.P.; Dube, B. Myopathy and neuropathy associated with osteomalacia. Acta Neurol. Scand. 1975, 51, 37–58. [Google Scholar] [CrossRef]
- Young, A.; Edwards, R.H.T.; Jones, D.A.; Brenton, D.P. Quadriceps muscle strength and fibre size during the treatment of osteomalacia. In Stokes IAF, Mechanical Factors and the Skeleton; Libbey: London, UK, 1981; Volume 12, pp. 137–145. [Google Scholar]
- Glerup, H.; Eriksen, E.F. Osteomalacia and servere vitamin D deficiency—A review of the clinical and paraclinical findings and guidelines for the treatment with vitamin D. Ugeskr. Laeger. 1999, 161, 2515–2521. [Google Scholar]
- Young, A.; Brenton, D.P.; Edwards, R. Analysis of muscle weakness in osteomalacia. Clin. Sci. Mol. Med. 1978, 54, 31. [Google Scholar]
- Tanner, S.B.; Harwell, S.A. More than healthy bones: A review of vitamin D in muscle health. Ther. Adv. Musculoskelet. Dis. 2015, 7, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Annweiler, C.; Montero-Odasso, M.; Schott, A.M.; Berrut, G.; Fantino, B.; Beauchet, O. Fall prevention and vitamin D in the elderly: An overview of the key role of the non-bone effects. J. Neuroeng. Rehabil. 2010, 7, 50. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Staehelin, H.B.; Orav, J.E.; Stuck, A.E.; Theiler, R.; Wong, J.B.; Egli, A.; Kiel, D.P.; Henschkowski, J. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomized controlled trials. BMJ 2009, 339, b3692. [Google Scholar] [CrossRef] [Green Version]
- Murad, M.H.; Elamin, K.B.; Abu Elnour, N.O.; Elamin, M.B.; Alkatib, A.A.; Fatourechi, M.M.; Almandoz, J.P.; Mullan, R.J.; Lane, M.A.; Liu, H.; et al. Clinical review: The effect of vitamin D on falls: A systematic review and meta- analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2997–3006. [Google Scholar] [CrossRef] [Green Version]
- Annweiler, C.; Schott, A.M.; Berrut, G.; Fantino, B.; Beauchet, O. Vitamin D-related changes in physical performance: A systematic review. J. Nutr. Health Aging 2009, 13, 893–898. [Google Scholar] [CrossRef]
- Muir, S.W.; Montero-Odasso, M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: A systematic review and meta- analysis. J. Am. Geriatr. Soc. 2011, 59, 2291–2300. [Google Scholar] [CrossRef]
- Rejnmark, L. Effects of vitamin d on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic. Dis. 2011, 2, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Rosendahl-Riise, H.; Spielau, U.; Ranhoff, A.H.; Gudbrandsen, O.A.; Dierkes, J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Stockton, K.A.; Mengersen, K.; Paratz, J.D.; Kandiah, D.; Bennell, K.L. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos. Int. 2011, 22, 859–871. [Google Scholar] [CrossRef]

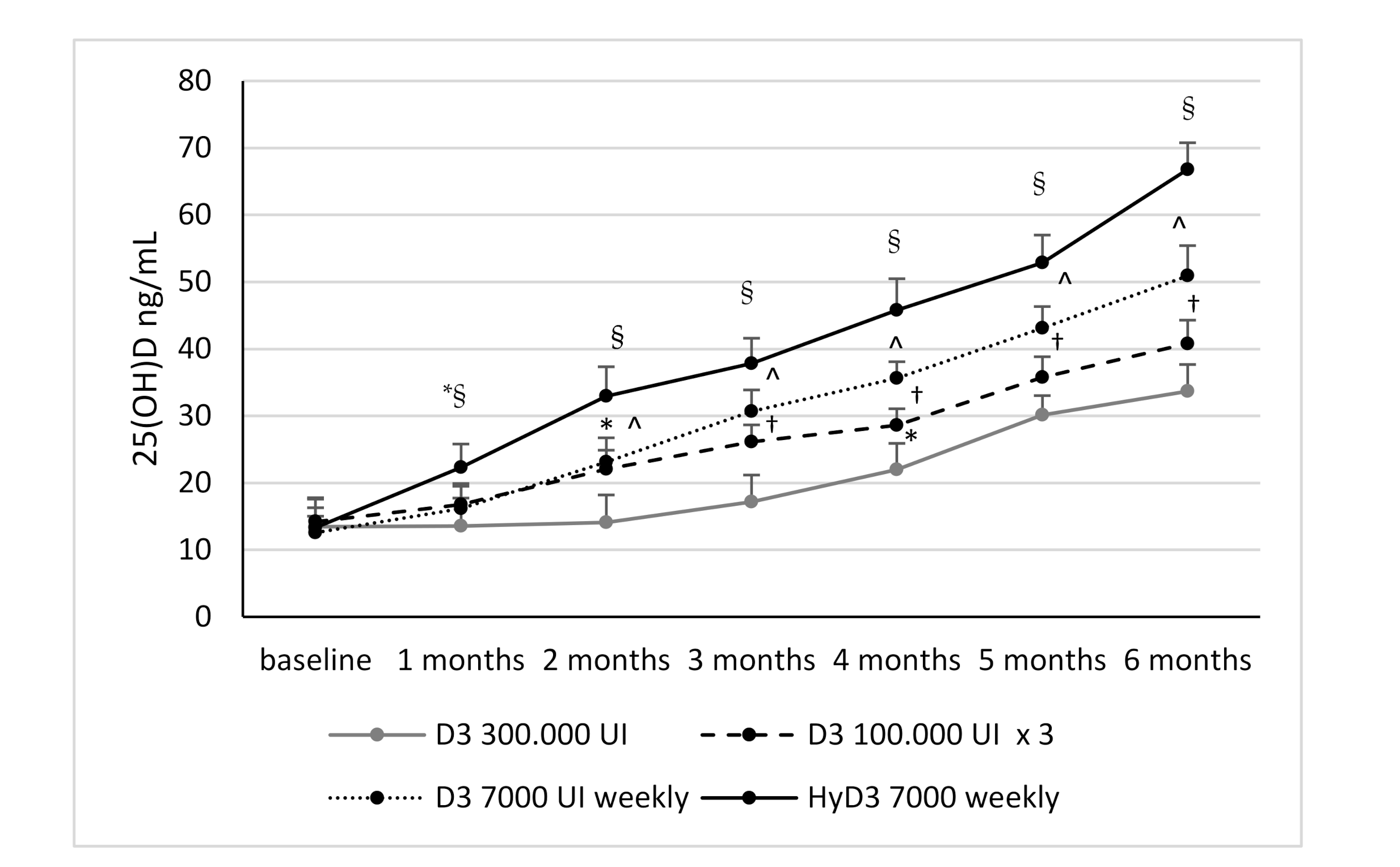
| Group 1 (28) D3 300,000 IU Single Dose | Group 2 (26) D3 100,000 IU Monthly | Group 3 (27) D3 7000 IU Weekly | Group 4 (26) HyD3 7000 IU Weekly | p | |
|---|---|---|---|---|---|
| Age | 60.51 ± 5.1 | 58.3 ± 7.4 | 63.4 ± 5.5 | 60.9 ± 8.1 | ns |
| BMI | 24.8 ± 2.3 | 25.9 ± 0.45 | 23.8 ± 1.5 | 23.3 ± 1.2 | ns |
| SST (n) | 13.6 ± 1.07 | 13.7 ± 1.19 | 14.1 ± 0.8 | 13.8 ± 1.14 | ns |
| TUG (seconds) | 9.36 ± 1 | 9.19 ± 1.03 | 8.9 ± 1.01 | 9.2 ± 1.04 | ns |
| Serum 25(OH)D | 13.46 ± 4.3 | 14.2 ± 3.3 | 12.5 ± 2.46 | 13.3 ± 2.9 | ns |
| Serum Ca | 9.9 (0.93) | 9.9 (0.60) | 9.4 (1.8) | 9.1 (1.5) | ns |
| Serum P | 3.35 (0.3) | 3.3 (0.7) | 3.3 (0.28) | 3.6 (0.35) | ns |
| PTH | 33.5 (18.28) | 37 (6.27) | 35.5(4.1) | 28.3 (8.08) * | 0.0001 |
| ALP | 50.5 (12.17) | 50.1 (8.45) | 73.2 (9.4) ** | 70.5 (7.3) ** | 0.0001 |
| Baseline | 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | ||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Ca | 9.9 (0.9) | 9.7 (0.8) | 9.6 (0.8) | 9.7 (0.5) | 9.6 (0.6) | 9.6 (0.6) | 9.4 (0.5) |
| P | 3.3 (0.3) | 3.5 (0.4) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.5) | 3.6 (0.5) | 3.5 (0.4) | |
| PTH | 33.5 (18.3) | 30.6 (9.7) | 30 (6.3) | 33.1 (11.7) | 32.1 (8.4) | 33.4 (7.8) | 32.8 (10.4) | |
| ALP | 50.5 (12.2) | 66.8 (12.8) | 67 (8.2) | 67.3 (5.1) | 69.2 (6.4) | 69.2 (9.7) | 70.3 (10.3) | |
| Group 2 | Ca | 9.9 (0.6) | 9.7 (0.5) | 9.4 (0.5) | 9.4 (0.6) | 9.6 (0.6) | 9.5 (0.4) | 9.5 (0.5) |
| P | 3.3 (0.7) | 3.3 (0.5) | 3.6 (0.2) | 3.5 (0.3) | 3.5 (0.4) | 3.6 (0.4) | 3.6 (0.6) | |
| PTH | 37 (6.3) | 40.1 (7.7) | 35.7 (7.5) | 39.5 (8.3) | 44.4 (7.8) | 41.4 (6.3) | 42.7 (13.1) | |
| ALP | 50.1 (8.4) | 71.4 (11.1) | 64.8 (9.2) | 69.7 (9.9) | 62.2 (8.1) | 61.3 (7.6) | 66.4 (7.5) | |
| Group 3 | Ca | 9.4 (1.8) | 9.4 (0.7) | 9.5 (0.7) | 9.6 (0.4) | 9.4 (0.6) | 9.6 (0.7) | 9.5 (0.5) |
| P | 3.3 (0.4) | 3.4 (0.5) | 3.5 (0.6) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.5) | |
| PTH | 35.5 (4.1) | 40.2 (8.3) | 40.2 (8.3) | 40 (12) | 41.3 (7.5) | 38.4 (5.8) | 34.2 (7.7) | |
| ALP | 73.2 (9.4) | 68.9 (13.9) | 70.5 (9.4) | 71.2 (8.9) | 71.9 (6.3) | 70.2 (6.6) | 70.2 (8.5) | |
| Group 4 | Ca | 9.1 (1.5) | 9.4 (0.7) | 9.1 (0.8) | 9.2 (0.5) | 9.1 (0.7) | 9.4 (0.5) | 9.3 (0.6) |
| P | 3.6 (0.4) | 3.6 (0.5) | 3.6 (0.6) | 3.7 (0.8) | 3.7 (0.7) | 3.6 (0.7) | 3.7 (0.9) | |
| PTH | 28.3 (8.1) | 30.2 (7.3) | 30.1 (9) | 30 (6.2) | 32.9 (7.1) | 33.5 (8.2) | 31.2 (6.7) | |
| ALP | 70.5 (7.3) | 75.9 (10.3) | 69.5 (6.1) | 70.1 (9.1) | 70.1 (4.8) | 70.3 (12.4) | 69.5 (12.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrado, A.; Rotondo, C.; Cici, D.; Berardi, S.; Cantatore, F.P. Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study. Nutrients 2021, 13, 380. https://doi.org/10.3390/nu13020380
Corrado A, Rotondo C, Cici D, Berardi S, Cantatore FP. Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study. Nutrients. 2021; 13(2):380. https://doi.org/10.3390/nu13020380
Chicago/Turabian StyleCorrado, Addolorata, Cinzia Rotondo, Daniela Cici, Stefano Berardi, and Francesco Paolo Cantatore. 2021. "Effects of Different Vitamin D Supplementation Schemes in Post-Menopausal Women: A Monocentric Open-Label Randomized Study" Nutrients 13, no. 2: 380. https://doi.org/10.3390/nu13020380






