Effect of Creatine Supplementation on Functional Capacity and Muscle Oxygen Saturation in Patients with Symptomatic Peripheral Arterial Disease: A Pilot Study of a Randomized, Double-Blind Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participant Recruitment and Screening
2.3. Clinical Characteristics
2.4. Creatine Supplementation Protocol and Blinding Procedure
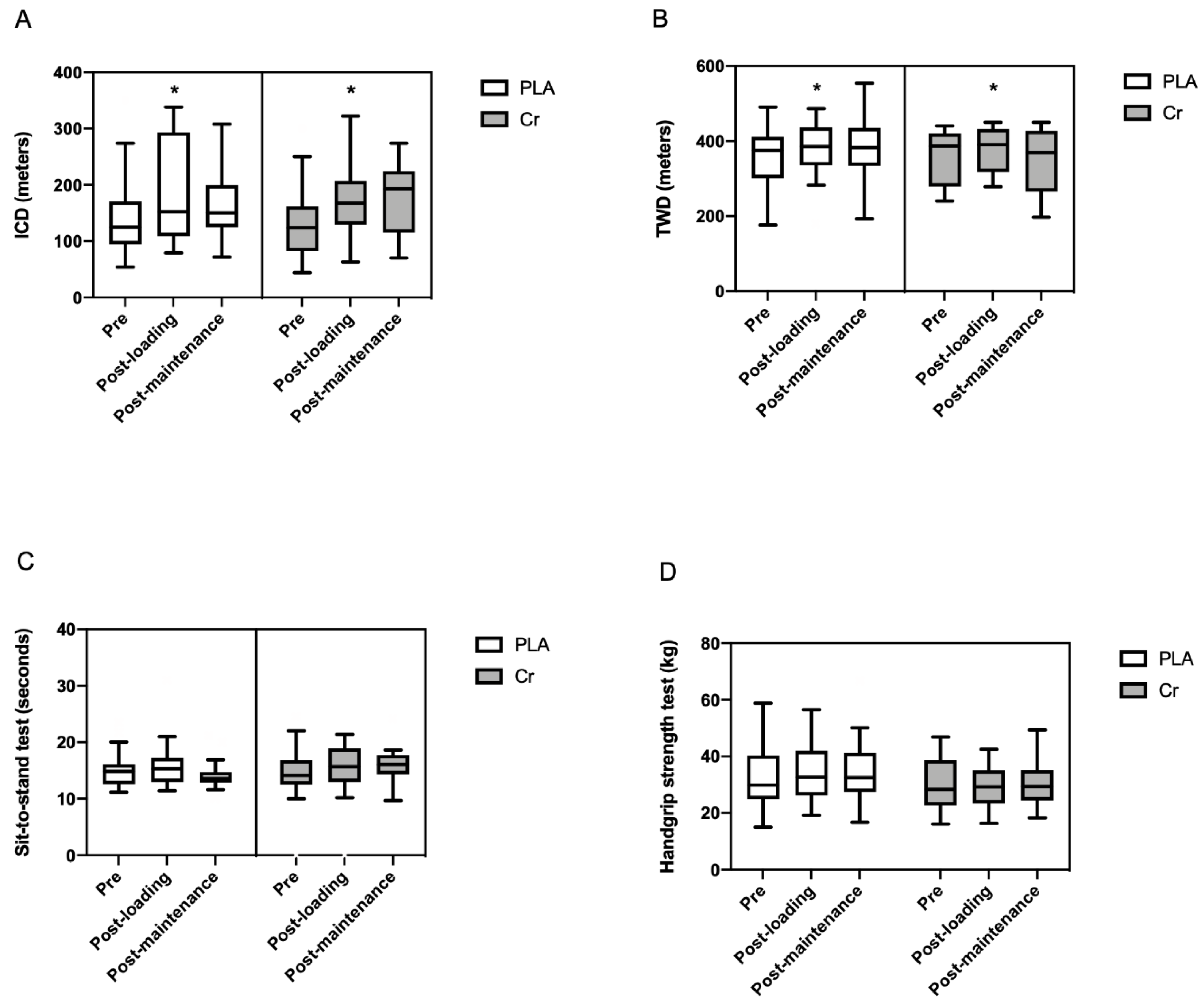
2.5. Primary Outcome—6 Min Walk Test (6MWT)
2.6. Secondary Outcomes
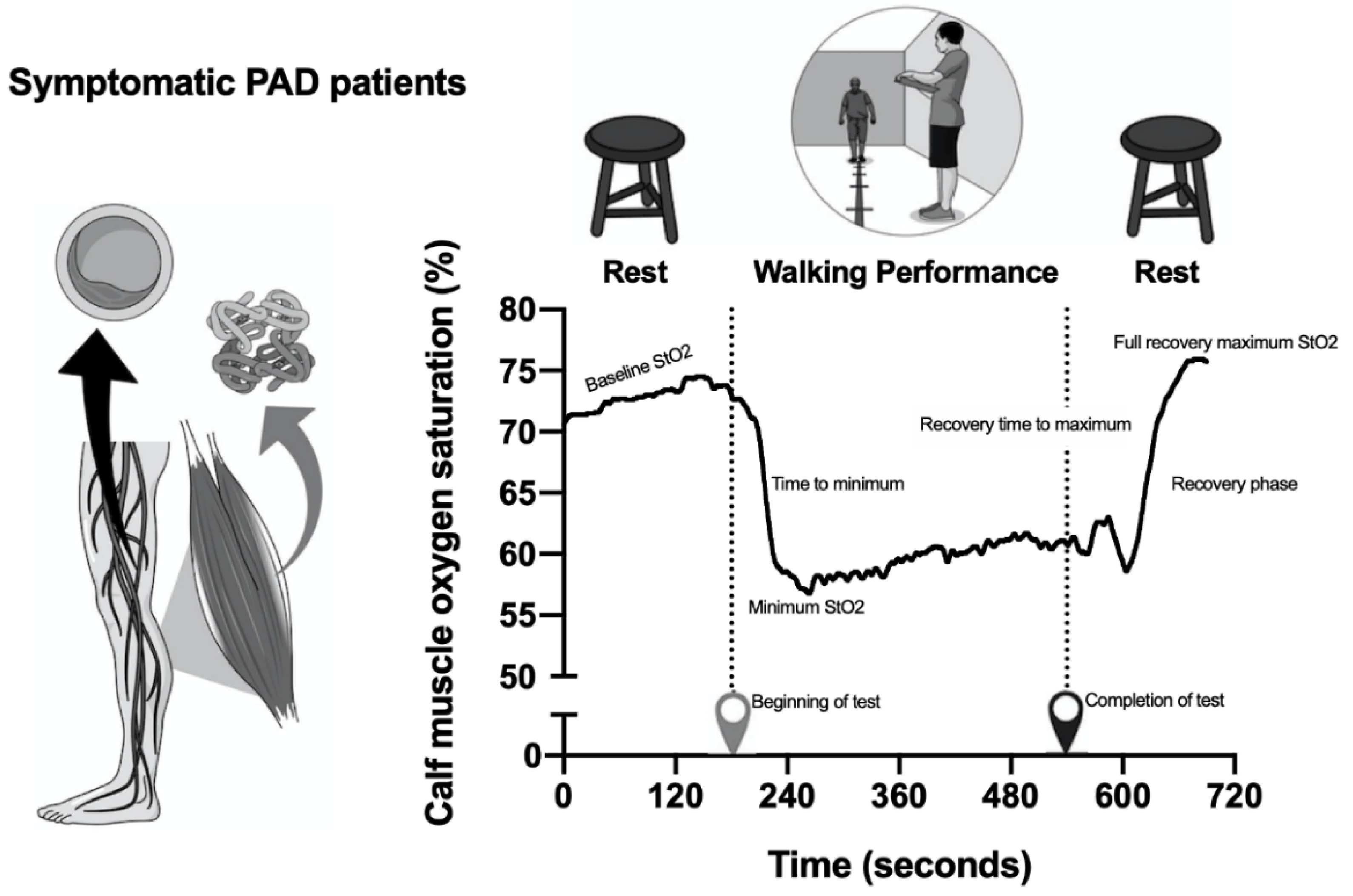
Calf Muscle Oxygen Saturation (Calf Muscle StO2)
2.7. Handgrip Strength Test
2.8. Sit-to-Stand Test
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbosa, J.; Farah, B.; Chehuen, M.; Cucato, G.; Farias Júnior, J.; Wolosker, N.; Forjaz, C.; Gardner, A.; Ritti-Dias, R. Barriers to Physical Activity in Patients with Intermittent Claudication. Int. J. Behav. Med. 2015, 22, 70–76. [Google Scholar] [CrossRef]
- Gerage, A.M.; de Correia, M.A.; de Oliveira, P.M.L.; Palmeira, A.C.; Domingues, W.J.R.; Zeratti, A.E.; Puech-Leão, P.; Wolosker, N.; Ritti-Dias, R.M.; Cucato, G.G. Physical Activity Levels in Peripheral Artery Disease Patients. Arq. Bras. Cardiol. 2019, 113, 410–416. [Google Scholar] [CrossRef]
- Germano-Soares, A.H.; Farah, B.Q.; Andrade-Lima, A.; Domingues, W.R.; Cavalcante, B.R.; de Almeida Correia, M.; Wolosker, N.; Cucato, G.G.; Ritti-Dias, R.M. Factors Associated to Arterial Stiffness in Patients With Symptomatic Peripheral Artery Disease. Ann. Vasc. Surg. 2019, 61, 78–82. [Google Scholar] [CrossRef]
- Germano-Soares, A.H.; Cucato, G.G.; Leicht, A.S.; Andrade-Lima, A.; Peçanha, T.; de Almeida Correia, M.; Zerati, A.E.; Wolosker, N.; Ritti-Dias, R.M. Cardiac Autonomic Modulation Is Associated with Arterial Stiffness in Patients with Symptomatic Peripheral Artery Disease. Ann. Vasc. Surg. 2019, 61, 72–77. [Google Scholar] [CrossRef]
- Cea Soriano, L.; Fowkes, F.G.R.; Johansson, S.; Allum, A.M.; García Rodriguez, L.A. Cardiovascular outcomes for patients with symptomatic peripheral artery disease: A cohort study in The Health Improvement Network (THIN) in the UK. Eur. J. Prev. Cardiol. 2017, 24, 1927–1937. [Google Scholar] [CrossRef]
- Morris, D.R.; Rodriguez, A.J.; Moxon, J.V.; Cunningham, M.A.; McDermott, M.M.; Myers, J.; Leeper, N.J.; Jones, R.E.; Golledge, J. Association of lower extremity performance with cardiovascular and all-cause mortality in patients with peripheral artery disease: A systematic review and meta-analysis. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- De Araujo Bonetti De Poli, R.; Roncada, L.H.; De Souza Malta, E.; Artioli, G.G.; Bertuzzi, R.; Zagatto, A.M. Creatine supplementation improves phosphagen energy pathway during supramaximal effort, but does not improve anaerobic capacity or performance. Front. Physiol. 2019, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.C.; Söderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Mielgo-Ayuso, J.; Calleja-Gonzalez, J.; Marqués-Jiménez, D.; Caballero-García, A.; Córdova, A.; Fernández-Lázaro, D. Effects of creatine supplementation on athletic performance in soccer players: A systematic review and meta-analysis. Nutrients 2019, 11, 757. [Google Scholar] [CrossRef] [Green Version]
- Sculthorpe, N.; Grace, F.; Jones, P.; Fletcher, I. The effect of short-term creatine loading on active range of movement. Appl. Physiol. Nutr. Metab. 2010, 35, 507–511. [Google Scholar] [CrossRef]
- Rawson, E.S.; Wehnert, M.L.; Clarkson, P.M. Effects of 30 days of creatine ingestion in older men. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 139–144. [Google Scholar] [CrossRef]
- Cañete, S.; San Juan, A.F.; Pérez, M.; Gómez-Gallego, F.; López-Mojares, L.M.; Earnest, C.P.; Fleck, S.J.; Lucia, A. Does creatine supplementation improve functional capacity in elderly women? J. Strength Cond. Res. 2006, 20, 22–28. [Google Scholar]
- Pinto, C.L.; Botelho, P.B.; Pimentel, G.D.; Campos-Ferraz, P.L.; Mota, J.F. Creatine supplementation and glycemic control: A systematic review. Amino Acids 2016, 48, 2103–2129. [Google Scholar] [CrossRef]
- Eijnde, B.O.; Richter, E.A.; Henquin, J.C.; Kiens, B.; Hespel, P. Effect of creatine supplementation on creatine and glycogen content in rat skeletal muscle. Acta Physiol. Scand. 2001, 171, 169–176. [Google Scholar] [CrossRef]
- De Benedetto, F.; Pastorelli, R.; Ferrario, M.; de Blasio, F.; Marinari, S.; Brunelli, L.; Wouters, E.F.M.; Polverino, F.; Celli, B.R. Supplementation with Qter® and Creatine improves functional performance in COPD patients on long term oxygen therapy. Respir. Med. 2018, 142, 86–93. [Google Scholar] [CrossRef]
- Clarke, H.; Kim, D.H.; Meza, C.A.; Ormsbee, M.J.; Hickner, R.C. The evolving applications of creatine supplementation: Could creatine improve vascular health? Nutrients 2020, 12, 2834. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [Green Version]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Lookstein, R.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease. Circulation 2017, 21, 726–779. [Google Scholar]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.C.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic). Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [Green Version]
- Buchberger, W.; Ferdig, M. Improved high-performance liquid chromatographic determination of guanidino compounds by pre-column dervatization with ninhydrin and fluorescence detection. J. Sep. Sci. 2004, 27, 1309–1312. [Google Scholar] [CrossRef]
- Boeselt, T.; Spielmanns, M.; Nell, C.; Storre, J.H.; Windisch, W.; Magerhans, L.; Beutel, B.; Kenn, K.; Greulich, T.; Alter, P.; et al. Validity and usability of physical activity monitoring in patients with Chronic Obstructive Pulmonary Disease (COPD). PLoS ONE 2016, 11, e0157229. [Google Scholar] [CrossRef]
- Montgomery, P.S.; Gardner, A.W. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J. Am. Geriatr. Soc. 1998, 46, 706–711. [Google Scholar] [CrossRef]
- Andrade-Lima, A.; Cucato, G.G.; Domingues, W.J.R.; Germano-Soares, A.H.; Cavalcante, B.R.; Correia, M.A.; Saes, G.F.; Wolosker, N.; Gardner, A.W.; Zerati, A.E.; et al. Calf Muscle Oxygen Saturation during 6-min Walk Test and Its Relationship with Walking Impairment in Symptomatic Peripheral Artery Disease. Ann. Vasc. Surg. 2018, 52, 147–152. [Google Scholar] [CrossRef]
- Jones, S.E.; Kon, S.S.C.; Canavan, J.L.; Patel, M.S.; Clark, A.L.; Nolan, C.M.; Polkey, M.I.; Man, W.D.C. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax 2013, 68, 1015–1020. [Google Scholar] [CrossRef] [Green Version]
- De Andrade Nemezio, K.M.; Bertuzzi, R.; Correia-Oliveira, C.R.; Gualano, B.; Bishop, D.J.; Lima-Silva, A.E. Effect of Creatine Loading on Oxygen Uptake during a 1-km Cycling Time Trial. Med. Sci. Sports Exerc. 2015, 47, 2660–2668. [Google Scholar] [CrossRef]
- Van Loon, L.J.C.; Oosterlaar, A.M.; Hartgens, F.; Hesselink, M.K.C.; Snow, R.J.; Wagenmakers, A.J.M. Effects of creatine loading and prolonged creatine supplementation on body composition, fuel selection, sprint and endurance performance in humans. Clin. Sci. 2003, 104, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Howden, D.; Boekenoogen, C.; Wagoner, J.; Miller, S.; Mendez-Guajardo, B. The Effects of Creatine Supplementation on Muscular Strength and Endurance in Mice. 2015 NCUR 2015. [Google Scholar]
- Acosta, M.J.; Vazquez Fonseca, L.; Desbats, M.A.; Cerqua, C.; Zordan, R.; Trevisson, E.; Salviati, L. Coenzyme Q biosynthesis in health and disease. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1079–1085. [Google Scholar] [CrossRef]
- Cracowski, J.-L.; Gaillard-Bigot, F.; Cracowski, C.; Sors, C.; Roustit, M.; Millet, C. Involvement of cytochrome epoxygenase metabolites in cutaneous postocclusive hyperemia in humans. J. Appl. Physiol. 2013, 114, 245–251. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, R.; Van Bavel, D.; De Moraes, B.S.; Tibiriçá, E. Effects of dietary creatine supplementation on systemic microvascular density and reactivity in healthy young adults. Nutr. J. 2014, 13, 115. [Google Scholar] [CrossRef] [Green Version]
- Van Bavel, D.; de Moraes, R.; Tibirica, E. Effects of dietary supplementation with creatine on homocysteinemia and systemic microvascular endothelial function in individuals adhering to vegan diets. Fundam. Clin. Pharmacol. 2018, 33, 428–440. [Google Scholar] [CrossRef]
- Lobo, D.M.; Tritto, A.C.; da Silva, L.R.; de Oliveira, P.B.; Benatti, F.B.; Roschel, H.; Nie, B.; Gualano, B.; Pereira, R.M.R. Effects of long-term low-dose dietary creatine supplementation in older women. Exp. Gerontol. 2015, 70, 97–104. [Google Scholar] [CrossRef]
- Domingues, W.J.R.; Ritti-Dias, R.M.; Cucato, G.G.; Wolosker, N.; Zerati, A.E.; Puech-Leão, P.; Nunhes, P.M.; Moliterno, A.A.; Avelar, A. Does Creatine Supplementation Affect Renal Function in Patients with Peripheral Artery Disease? A Randomized, Double Blind, Placebo-controlled, Clinical Trial. Ann. Vasc. Surg. 2020, 63, 45–52. [Google Scholar] [CrossRef]
- Van Lummel, R.C.; Walgaard, S.; Maier, A.B.; Ainsworth, E.; Beek, P.J.; Van Dieën, J.H. The instrumented Sit-To-Stand test (iSTS) has greater clinical relevance than the manually recorded sit-to-stand test in older adults. PLoS ONE 2016, 11, e0157968. [Google Scholar] [CrossRef] [Green Version]
- McDermott, M.M.; Guralnik, J.M.; Criqui, M.H.; Liu, K.; Kibbe, M.R.; Ferrucci, L. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014, 130, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Reeve, T.E.; Ur, R.; Craven, T.E.; Kaan, J.H.; Goldman, M.P.; Edwards, M.S.; Hurie, J.B.; Velazquez-Ramirez, G.; Corriere, M.A. Grip strength measurement for frailty assessment in patients with vascular disease and associations with comorbidity, cardiac risk, and sarcopenia. J. Vasc. Surg. 2018, 67, 1512–1520. [Google Scholar] [CrossRef] [Green Version]

| PLA (n = 15) | Cr (n = 14) | p-Value | |
|---|---|---|---|
| Women (%) a | 54 | 46 | 0.56 |
| Age (years) a | 64 ± 8 | 64 ± 10 | 0.54 |
| Weight (kg) a | 77 ± 10 | 68 ± 17 | 0.18 |
| Height (m) a | 1.64 ± 0.09 | 1.60 ± 0.06 | 0.21 |
| Body mass index (kg/m2) a | 28.7 ± 3.1 | 26.7 ± 6.5 | 0.43 |
| Ankle-brachial index (mmHg) a | 0.50 ± 0.13 | 0.51 ± 0.16 | 1.00 |
| Initial claudication distance (m) a | 143 ± 84 | 143 ± 65 | 0.88 |
| Total walking distance (m) a | 371 ± 81 | 344 ± 82 | 0.65 |
| Comorbidities (%) | |||
| Hypertension b | 86.7 | 78.6 | 0.67 |
| Diabetes b | 60.0 | 50.0 | 0.43 |
| Dyslipidemia b | 6.7 | 7.1 | 0.74 |
| Current smoking b | 78.6 | 78.6 | 0.68 |
| Coronary artery disease b | 46.7 | 28.6 | 0.26 |
| PLA (n = 15) | Cr (n = 14) | p-Value Interaction | |
|---|---|---|---|
| Baseline StO2 (%) | |||
| Pre | 68.8 ± 3.7 | 70.0 ± 4.6 | 0.082 |
| Post loading | 68.2 ± 2.8 | 70.5 ± 6.1 | |
| Post maintenance | 71.3 ± 5.7 | 71.1 ± 4.8 | |
| Minimum StO2 (%) | |||
| Pre | 54.2 ± 10.0 | 61.6 ± 13.0 | 0.191 |
| Post loading | 52.9 ± 9.3 | 61.7 ± 14.8 | |
| Post maintenance | 54.2 ± 7.7 | 62.8 ± 12.3 | |
| Time to minimum StO2 (s) | |||
| Pre | 52.5 ± 34.3 | 56.5 ± 46.1 | 0.833 |
| Post loading | 46.8 ± 58.6 | 44.4 ± 26.2 | |
| Post maintenance | 50.1 ± 88.3 | 58.4 ± 43.5 | |
| Completion of test (%) | |||
| Pre | 12.2 ± 8.5 | 7.2 ± 9.7 | 0.691 |
| Post loading | 12.1 ± 8.2 | 9.5 ± 7.1 | |
| Post maintenance | 9.2 ± 10.5 | 7.6 ± 10.4 | |
| Recovery time to maximum StO2 (s) | |||
| Pre | 158.9 ± 43.1 | 160.1 ± 175.1 | 0.723 |
| Post loading | 146.4 ± 75.1 | 177.1 ± 202.9 | |
| Post maintenance | 160.9 ± 119.1 | 181.9 ±125.8 | |
| Recovery phase time StO2 (s) | |||
| Pre | 126.7 ± 112.4 | 132.9 ± 194.2 | 0.827 |
| Post loading | 93.0 ± 77.7 | 121.1 ± 248.3 | |
| Post maintenance | 87.1 ± 137.5 | 130.3 ± 103.7 | |
| Full recovery maximum StO2 (%) | |||
| Pre | 69.5 ± 15.8 | 72.6 ± 12.4 | 0.312 |
| Post loading | 68.6 ± 9.7 | 72.3 ± 14.5 | |
| Post maintenance | 74.5 ± 15.8 | 73.1 ± 15.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues, W.J.R.; Ritti-Dias, R.M.; Cucato, G.G.; Wolosker, N.; Zerati, A.E.; Puech-Leão, P.; Coelho, D.B.; Nunhes, P.M.; Moliterno, A.A.; Avelar, A. Effect of Creatine Supplementation on Functional Capacity and Muscle Oxygen Saturation in Patients with Symptomatic Peripheral Arterial Disease: A Pilot Study of a Randomized, Double-Blind Placebo-Controlled Clinical Trial. Nutrients 2021, 13, 149. https://doi.org/10.3390/nu13010149
Domingues WJR, Ritti-Dias RM, Cucato GG, Wolosker N, Zerati AE, Puech-Leão P, Coelho DB, Nunhes PM, Moliterno AA, Avelar A. Effect of Creatine Supplementation on Functional Capacity and Muscle Oxygen Saturation in Patients with Symptomatic Peripheral Arterial Disease: A Pilot Study of a Randomized, Double-Blind Placebo-Controlled Clinical Trial. Nutrients. 2021; 13(1):149. https://doi.org/10.3390/nu13010149
Chicago/Turabian StyleDomingues, Wagner Jorge Ribeiro, Raphael Mendes Ritti-Dias, Gabriel Grizzo Cucato, Nelson Wolosker, Antônio Eduardo Zerati, Pedro Puech-Leão, Daniel Boari Coelho, Pollyana Mayara Nunhes, André Alberto Moliterno, and Ademar Avelar. 2021. "Effect of Creatine Supplementation on Functional Capacity and Muscle Oxygen Saturation in Patients with Symptomatic Peripheral Arterial Disease: A Pilot Study of a Randomized, Double-Blind Placebo-Controlled Clinical Trial" Nutrients 13, no. 1: 149. https://doi.org/10.3390/nu13010149





