Improvement of Executive Function after Short-Term Administration of an Antioxidants Mix Containing Bacopa, Lycopene, Astaxanthin and Vitamin B12: The BLAtwelve Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Study Outcomes
2.4. Cognitive Function Assessment
2.5. Blood Pressure Measurement
2.6. Laboratory Analysis
2.7. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Population
3.2. Primary Endpoints
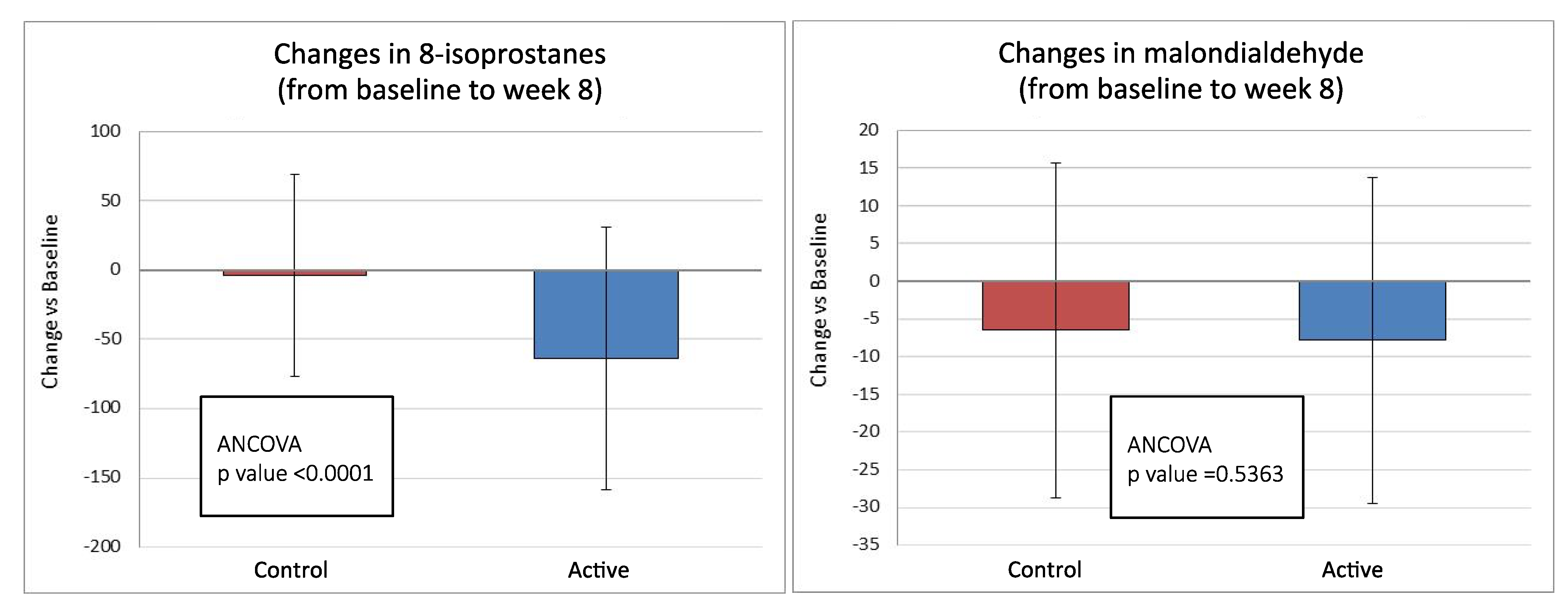
3.3. Secondary Endpoints
3.4. Safety Results
3.5. Adherence to the Study Protocol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1
| Average Amounts of Characterizing Ingredients Quantity | Quantity | %NRVs |
|---|---|---|
| (mg/dose) | ||
| Astaxanthin | 160,000 | 15,610 |
| Lycopene | 100,000 | 9756 |
| Bacopa (Bacopa monnieri (L.) Pennel) aerial part e.s. tit. 50% bacosides | 80,000 | 7805 |
| Cyanocobalamin (vitamin B12) | 6000 | 0.585 |
| Microcrystalline cellulose | 619,434 | 60,433 |
| Coating agent: hydroxypropylmethylcellulose | 15,819 | 1543 |
| Antiagglomerating agents: crosslinked sodium carboxymethylcellulose | 15,000 | 1463 |
| Antiagglomerating agents: polyethylene glycol | 8500 | 0.829 |
| Antiagglomerating agents: magnesium vegetable stearate | 8000 | 0.780 |
| Antiagglomerating agents: silicon dioxide | 6000 | 0.585 |
| Dye: titanium dioxide | 3651 | 0.356 |
| Antiagglomerating agent: vegetable stearic acid | 2434 | 0.237 |
| Dye: oxide of iron (red) | 0.150 | 0.015 |
| Dry: Patent blue V | 0.013 | 0.001 |
| Total | 1,025,000 | 100,000 |
| Average Amounts of Characterizing Ingredients Quantity | Quantity | % NRVs |
|---|---|---|
| (mg/dose) | ||
| Microcrystalline cellulose | 994,434 | 97,018 |
| Coating agent: hydroxypropylmethylcellulose | 15,819 | 1.543 |
| Antiagglomerating agents: magnesium vegetable stearate | 5.000 | 0.488 |
| Dye: titanium dioxide | 3.651 | 0.356 |
| Antiagglomerating agents: silicon dioxide | 3.000 | 0.293 |
| Antiagglomerating agent: vegetable stearic acid | 2.434 | 0.237 |
| Antiagglomerating agents: polyethylene glycol | 0.500 | 0.049 |
| Dye: oxide of iron (red) | 0.150 | 0.015 |
| Dry: Patent blue V | 0.013 | 0.001 |
| Total | 1,025,000 | 100,000 |
Appendix A.2
- TMT is a frequently used neuropsychological test because of its sensitivity to brain damage. It explores visual–conceptual and visual–motor tracking. TMT is administered in two parts. Part A is a visual-scanning, timed task where participants are asked to connect with lines 25 circles numbered from 1 to 25, as quickly as possible. The test is terminated after 5 min even if not completed. In Part B participants are asked to connect circles containing numbers (from 1 to 13) or letters (from A to L) in an alternate numeric/alphabetical order. The test is terminated in every case after 10 min even if not completed. The TMT B minus TMT A score, calculated as the difference between TMT B and TMT A times, is considered a measure of cognitive flexibility, relatively independent of manual dexterity.
- VFT is a short test of verbal functioning. Participants are given 1 min to produce as many unique words as possible within a semantic category (category fluency) or starting with a given letter (letter fluency). The participant’s score in each task is the number of unique correct words.
- MoCA evaluates a broader array of cognitive domains (e.g., attention/executive functioning, visuospatial abilities, and language) and it has been demonstrated to be able to detect cognitive impairment with scores ranging from 0 to 30.
- MMSE is a widely used screening tool for cognitive impairment and covers five areas of cognitive function including orientation, attention, calculus, recall, and language with scores ranging from 0 to 30.
- AVLT is a neuropsychological assessment designed to evaluate the nature and severity of memory dysfunction, and to track changes in memory function. The examiner reads aloud a list of 15 words at the rate of one per second. The participant is then asked to repeat all words from the list that she/he can remember. This procedure is carried out a total of five times. After a 15-min delay, the participant is again asked to recall as many words as possible from the first list. The participant is then requested to read a list of words, and asked to indicate whether each word was from the first list. The score for each trial is the number of words correctly recollected.
References
- Praticò, D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: Lights and shadows. Ann. N. Y. Acad. Sci. 2008, 1147, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Beal, M.F. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res. 2005, 49, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Pathways towards and a way from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, A.V.; Balachandran, B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr. Neurosci. 2002, 5, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, V.; Barberger-Gateau, P.; Peuchant, E.; Orgogozo, J.M. Nutritional factors in cerebral aging and dementia: Epidemiological arguments for a role of oxidative stress. Neuroepidemiology 2001, 20, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, M.; Geerlings, M.I.; Ruitenberg, A.; Van Swieten, J.C.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Diet and risk of dementia: Does fat matter?: The Rotterdam Study. Neurology 2002, 59, 1915–1921. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Alkerwi, A. Chocolate intake is associated with better cognitive function: The Maine-Syracuse Longitudinal Study. Appetite 2016, 100, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J. Nutr. 2009, 139, 120–127. [Google Scholar] [CrossRef]
- Socci, V.; Tempesta, D.; Desideri, G.; De Gennaro, L.; Ferrara, M. Enhancing human cognition with cocoa flavonoids. Front. Nutr. 2017, 19. [Google Scholar] [CrossRef] [Green Version]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [Green Version]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study-a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Spencer, J.P. Food for thought: The role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc. Nutr. Soc. 2008, 67, 238–252. [Google Scholar] [CrossRef] [Green Version]
- Feeney, J.; O’Leary, N.; Moran, R.; O’Halloran, A.M.; Nolan, J.M.; Beatty, S.; Young, I.S.; Kenny, R.A. Plasma lutein and zeaxanthin are associated with better cognitive function across multiple domains in a large population-based sample of older adults: Findings from the Irish longitudinal study on aging. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1431–1436. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the georgia centenarian study. J. Aging Res. 2013, 951786. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K.; Gleason, C.E.; Mares, J.A. Dietary carotenoids and cognitive function among US adults, NHANES 2011–2014. Nutr. Neurosci. 2020, 23, 554–562. [Google Scholar] [CrossRef]
- Cannavale, C.N.; Hassevoort, K.M.; Edwards, C.G.; Thompson, S.V.; Burd, N.A.; Holscher, H.D.; Erdman, J.W., Jr.; Cohen, N.J.; Khan, N.A. Serum lutein is related to relational memory performance. Nutrients 2019, 11, 768. [Google Scholar] [CrossRef] [Green Version]
- Zamroziewicz, M.K.; Paul, E.J.; Zwilling, C.E.; Johnson, E.J.; Kuchan, M.J.; Cohen, N.J.; Barbey, A.K. Parahippocampal cortex mediates the relationship between lutein and crystallized intelligence in healthy, older adults. Front. Aging Neurosci. 2016, 8, 297. [Google Scholar] [CrossRef] [Green Version]
- Power, R.; Coen, R.F.; Beatty, S.; Mulcahy, R.; Moran, R.; Stack, J.; Howard, A.N.; Nolan, J.M. Supplemental retinal carotenoids enhance memory in healthy individuals with low levels of macular pigment in a randomized, double-blind, placebo-controlled clinical trial. J. Alzheimers Dis. 2018, 61, 947–961. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R., Jr.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of lutein/zeaxanthin supplementation on the cognitive function of community dwelling older adults: A randomized, double-masked, placebo-controlled trial. Front. Aging Neurosci. 2017, 9, 254. [Google Scholar] [CrossRef]
- Bovier, E.R.; Hammond, B.R. A randomized placebo-controlled study on the effects of lutein and zeaxanthin on visual processing speed in young healthy subjects. Arch. Biochem. Biophys. 2015, 572, 54–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: Meta-Analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beydoun, M.A.; Canas, J.A.; Fanelli-Kuczmarski, M.T.; Maldonado, A.I.; Shaked, D.; Kivimaki, M.; Evans, M.K.; Zonderman, A.B. Association of antioxidant vitamins A, C, E and carotenoids with cognitive performance over time: A cohort study of middle-aged adults. Nutrients 2020, 12, 3558. [Google Scholar] [CrossRef]
- Yasuno, F.; Tanimukai, S.; Sasaki, M.; Ikejima, C.; Yamashita, F.; Kodama, C.; Mizukami, K.; Asada, T. Combination of antioxidant supplements improved cognitive function in the elderly. J. Alzheimers Dis. 2012, 32, 895–903. [Google Scholar] [CrossRef]
- Crowe-White, K.M.; Phillips, T.A.; Ellis, A.C. Lycopene and cognitive function. J. Nutr. Sci. 2019, 8, e20. [Google Scholar] [CrossRef] [Green Version]
- Di Matteo, V.; Pierucci, M.; Di Giovanni, G.; Dragani, L.K.; Murzilli, S.; Poggi, A.; Esposito, E. Intake of tomato-enriched diet protects from 6-hydroxydopamine-induced degeneration of rat nigral dopaminergic neurons. J. Neural Transm. Suppl. 2009, 73, 333–341. [Google Scholar]
- Yi, F.; He, X.; Wang, D. Lycopene protects against MPP(+)-induced cytotoxicity by maintaining mitochondrial function in SH-SY5Y cells. Neurochem. Res. 2013, 38, 1747–1757. [Google Scholar] [CrossRef]
- Fujita, K.; Yoshimoto, N.; Kato, T.; Imada, H.; Matsumoto, G.; Inakuma, T.; Nagata, Y.; Miyachi, E. Lycopene inhibits ischemia/reperfusion-induced neuronal apoptosis in gerbil hippocampal tissue. Neurochem. Res. 2013, 38, 461–469. [Google Scholar] [CrossRef]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef]
- Karppi, J.; Laukkanen, J.A.; Sivenius, J.; Ronkainen, K.; Kurl, S. Serum lycopene decreases the risk of stroke in men: A population-based follow-up study. Neurology 2012, 79, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–356. [Google Scholar] [PubMed]
- Lim, S.Y.; Kim, E.J.; Kim, A.; Lee, H.J.; Choi, H.J.; Yang, S.J. Nutritional factors affecting mental health. Clin. Nutr. Res. 2016, 5, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterji, N.; Rastogi, R.P.; Dhar, M.L. Chemical examination of Bacopa munniera Wettst.: Part I—Isolation of chemical constituents. Indian J. Chem. 1963, 1, 212–215. [Google Scholar]
- Chatterji, N.; Rastogi, R.P.; Dhar, M.L. Chemical examination of Bacopa monniera Wettst: Part II—Isolation of chemical constituents. Indian J. Chem. 1965, 3, 24–29. [Google Scholar]
- Aguiar, S.; Borowski, T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013, 16, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.; Izzo, A.A.; Borrelli, F.; Renis, M.; Vanella, A. Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother. Res. 2003, 17, 870–875. [Google Scholar] [CrossRef]
- Stough, C.; Scholey, A.; Cropley, V.; Wesnes, K.; Zangara, A.; Pase, M.; Savage, K.; Nolidin, K.; Lomas, J.; Downey, L. Examining the cognitive effects of a special extract of Bacopa monniera (CDRI08: Keenmnd): A review of ten years of research at Swinburne University. J. Pharm. Pharm. Sci. 2013, 16, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Abichandani, L.G.; Thawani, V.; Gharpure, K.J.; Naidu, M.U.; Venkat Ramana, G. Efficacy of Standardized extract of Bacopa monnieri (Bacognize®) on cognitive functions of medical students: A six-week, randomized placebo-controlled trial. Evid. Based Complement. Altern. Med. 2016, 4103423. [Google Scholar] [CrossRef] [Green Version]
- Goswami, S.; Saoji, A.; Kumar, N.; Thawani, V.; Tiwari, M.; Thawani, M. Effect of Bacopa monnieri on cognitive functions in Alzheimer’s disease patients. Int. J. Collab. Res. Intern. Med. Public Health 2011, 3, 285–293. [Google Scholar]
- Aisen, P.S.; Schneider, L.S.; Sano, M.; Diaz-Arrastia, R.; van Dyck, C.H.; Weiner, M.F.; Bottiglieri, T.; Jin, S.; Stokes, K.T.; Thomas, R.G.; et al. Alzheimer disease cooperative study. High-Dose B vitamin supplementation and cognitive decline in Alzheimer disease: A randomized controlled trial. JAMA 2008, 300, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Mander, A.; Ames, D.; Carne, R.; Sanders, K.; Watters, D. Cognitive impairment and vitamin B12: A review. Int. Psychogeriatr. 2012, 24, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.; Calvaresi, E.; Hughes, D. Short-Term folate, vitamin B-12 or vitamin B-6 supplementation slightly affects memory performance but not mood in women of various ages. J. Nutr. 2002, 132, 1345–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Tian, Y.X.; Yang, F.; Zhang, J.P.; Skibsted, L.H. Antioxidant synergism between carotenoids in membranes. Astaxanthin as a radical transfer bridge. Food Chem. 2009, 115, 1437–1442. [Google Scholar] [CrossRef]
- Shi, J.; Qu, Q.; Kakuda, Y.; Xue, S.J.; Jiang, Y.; Koide, S.; Shim, Y.Y. Investigation of the antioxidant and synergistic activity of lycopene and other natural antioxidants using LAME and AMVN model systems. J. Food Compos. Anal. 2007, 20, 603–608. [Google Scholar] [CrossRef]
- Zanotta, D.; Puricelli, S.; Bonoldi, G. Cognitive effects of a dietary supplement made from extract of Bacopa monnieri, astaxanthin, phosphatidylserine, and vitamin E in subjects with mild cognitive impairment: A noncomparative, exploratory clinical study. Neuropsychiatr. Dis. Treat. 2014, 10, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Castelli, V.; Melani, F.; Ferri, C.; d’Angelo, M.; Catanesi, M.; Grassi, D.; Benedetti, E.; Giordano, A.; Cimini, A.; Desideri, G. Neuroprotective activities of bacopa, lycopene, astaxanthin, and vitamin B12 combination on oxidative stress-dependent neuronal death. J. Cell. Biochem. 2020, 121, 4862–4869. [Google Scholar] [CrossRef]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Viña, J.; Lloret, A.; Ortí, R.; Alonso, D. Molecular bases of the treatment of Alzheimer’s disease with antioxidants: Prevention of oxidative stress. Mol. Asp. Med. 2004, 25, 117–123. [Google Scholar] [CrossRef]
- Tadokoro, K.; Ohta, Y.; Inufusa, H.; Loon, A.F.N.; Abe, K. Prevention of cognitive decline in Alzheimer’s disease by novel antioxidative supplements. Int. J. Mol. Sci. 2020, 21, 1974. [Google Scholar] [CrossRef] [Green Version]
- Grassi, D.; Ferri, C.; Desideri, G. Brain protection and cognitive function: Cocoa flavonoids as nutraceuticals. Curr. Pharm. Des. 2016, 22, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Socci, V.; Tempesta, D.; Ferri, C.; De Gennaro, L.; Desideri, G.; Ferrara, M. Flavanol-Rich chocolate acutely improves arterial function and working memory performance counteracting the effects of sleep deprivation in healthy individuals. J. Hypertens. 2016, 34, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Jama, J.W.; Launer, L.J.; Witteman, J.C.M.; den Breeijen, J.H.; Breteler, M.M.; Grobbee, D.E.; Hofman, A. Dietary antioxidants and cognitive function in a population-based sample of older persons. Am. J. Epidemiol. 1996, 144, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, K.H.; Losonczy, K.G.; Izmirlian, G.; Foley, D.J.; Ross, G.W.; Petrovitch, H.; Havlik, R.; White, L.R. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 2000, 54, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Feskens, E.J.M.; Launer, L.J.; Kromhout, D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am. J. Epidemiol. 1997, 145, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Wilson, R.S. Vitamin E and cognitive decline in older persons. Arch. Neurol. 2002, 59, 1125–1132. [Google Scholar] [CrossRef]
- Berr, C.; Balansard, B.; Arnaud, J.; Roussel, A.M.; Alperovitch, A. Cognitive decline is associated with systemic oxidative stress: The EVA study. J. Am. Geriatr. Soc. 2000, 48, 1285–1291. [Google Scholar] [CrossRef]
- Grodstein, F.; Chen, J.; Willett, W.C. High-Dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am. J. Clin. Nutr. 2003, 77, 975–984. [Google Scholar] [CrossRef]
- Basambombo, L.L.; Carmichael, P.H.; Côté, S.; Laurin, D. Use of vitamin E and C supplements for the prevention of cognitive decline. Ann. Pharmacother. 2017, 51, 118–124. [Google Scholar] [CrossRef]
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C. Cache county study group. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch. Neurol. 2004, 61, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Devore, E.E.; Grodstein, F.; van Rooij, F.J.; Hofman, A.; Stampfer, M.J.; Witteman, J.C.; Breteler, M.M. Dietary antioxidants and long-term risk of dementia. Arch. Neurol. 2010, 67, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelhart, M.J.; Geerlings, M.I.; Ruitenberg, A.; van Swieten, J.C.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Dietary Intake of antioxidants and risk of Alzheimer Disease. JAMA 2002, 287, 3223–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heart Protection Study Collaborative Group. RC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 23–33. [Google Scholar] [CrossRef]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A.; et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaffe, K.; Clemons, T.E.; McBee, W.L.; Lindblad, A.S.; Age-Related Eye Disease Study Research Group. Impact of antioxidants, zinc and copper on cognition in the elderly: A randomized controlled trial. Neurology 2004, 63, 1705–1707. [Google Scholar] [PubMed] [Green Version]
- Kang, J.H.; Cook, N.; Manson, J.E.; Buring, J.E.; Grodstein, F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch. Intern. Med. 2006, 166, 2462–2468. [Google Scholar] [CrossRef] [Green Version]
- Luchsinger, J.A.; Tang, M.X.; Shea, S.; Mayeux, R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch. Neurol. 2003, 60, 203–208. [Google Scholar] [CrossRef]
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of antioxidant supplement use and dementia in the prevention of Alzheimer’s disease by vitamin E and Selenium trial (PREADViSE). JAMA Neurol. 2017, 74, 567–573. [Google Scholar] [CrossRef]
- Grodstein, F.; Kang, J.H.; Glynn, R.J.; Cook, N.R.; Gaziano, J.M. A randomized trial of beta carotene supplementation and cognitive function in men: The Physicians’ Health Study II. Arch. Intern. Med. 2007, 167, 2184–2190. [Google Scholar] [CrossRef] [Green Version]
- Park, D.C.; Reuter-Lorenz, P. The adaptive brain: Aging and neurocognitive scaffolding. Annu. Rev. Psychol. 2009, 60, 173–196. [Google Scholar] [CrossRef] [Green Version]
- Moscovitch, M.; Winocur, G. The neuropsychology of memory and aging. In The Handbook of Aging and Cognition; Salthouse, T.A., Craik, F.I.M., Eds.; Erlbaum: Hillsdale, NJ, USA, 1992; pp. 315–372. [Google Scholar]
- Patrono, C.; Fitzgerald, G.A. Isoprostanes: Potential markers of oxidant stress in atherothrombotic disease. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Minuz, P.; Andrioli, G.; Degan, M.; Gaino, S.; Ortolani, R.; Tommasoli, R.; Zuliani, V.; Lechi, A.; Lechi, C. The F2-isoprostane 8-epiprostaglandin F2 increases platelet adhesion and reduces the antiadhesive and antiaggregatory effects of NO. Arterioscler. Thromb. Vasc. Biol. 1998, 8, 1248–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, L.; Giagulli, C.; Minuz, P.; Lechi, A.; Laudanna, C. 8-Iso-PGF2 induces 2-integrin-mediated rapid adhesion of human polymorphonuclear neutrophils: A link between oxidative stress and ischemia/reperfusion injury. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praticò, D. Increase of brain oxidative stress in mild cognitive impairment. A possible predictor of Alzheimer’s disease. Arch. Neurol. 2002, 59, 972–976. [Google Scholar] [CrossRef] [Green Version]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G.; et al. Antioxidants for Alzheimer disease: A randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012, 69, 836–841. [Google Scholar] [CrossRef] [Green Version]

| Placebo (n = 40) | Active (n = 40) | p Value | |
|---|---|---|---|
| Gender (m/f) | 13/27 | 12/28 | 0.809 |
| Age (years) | 62.05 (1.55) | 61.88 (1.36) | 0.593 |
| BMI (kg/m2) | 26.48 (2.03) | 27.23 (2.49) | 0.145 |
| SBP (mmHg) | 129.62 (10.0) | 131.55 (7.41) | 0.329 |
| DBP (mmHg) | 83.28 (7.12) | 83.45 (5.51) | 0.902 |
| TC (mg/dL) | 203.93 (42.15) | 202.35 (39.74) | 0.864 |
| LDL-C (mg/dL | 134.85 (38.40) | 130.33 (35.22) | 0.584 |
| HDL-C (mg/dL) | 54.40 (11.02) | 55.48 (14.13) | 0.705 |
| TG (mg/dL) | 115.18 (56.30) | 120.25 (70.31) | 0.722 |
| Glucose (mg/dL) | 85.08 (16.58) | 83.05 (13.46) | 0.550 |
| Insulin (mU/L) | 10.70 (8.23) | 8.60 (4.09) | 0.153 |
| HOMA-IR | 2.27 (1.90) | 1.80 (0.95) | 0.162 |
| Uricacid (mg/dL) | 4.93 (1.12) | 4.79 (1.48) | 0.616 |
| MDA (pmol/mL) | 91.30 (52.31) | 85.44 (38.59) | 0.570 |
| 8-isoprostanes (pg/mL) | 160.44 (162.18) | 147.60 (151.79) | 0.716 |
| MMSE | 28.67 (0.57) | 28.64 (0.51) | 0.831 |
| TMT-B (s) | 54.64 (21.30) | 56.97 (25.37) | 0.794 |
| TMT-A (s) | 24.20 (7.37) | 25.17 (10.53) | 0.814 |
| TMT-B minus TMT-A (s) | 30.44 (17.96) | 31.80 (22.32) | 0.799 |
| MOCA | 25.18 (1.82) | 25.50 (1.83) | 0.428 |
| GDS | 1.93 (1.19) | 1.83 (1.28) | 0.718 |
| Placebo (n = 40) Week 8 | Active (n = 38) Week 8 | p Value | |
|---|---|---|---|
| TMT B (s) | 3.46 (13.08) | −17.63 (18.93) | <0.0001 |
| TMT A (s) | −0.37 (5.31) | −6.86 (10.00) | <0.0001 |
| TMT B-A (s) | 3.84 (11.65) | −10.46 (16.62) | <0.0001 |
| VFT letter (n) | 1.07 (4.80) | 5.28 (6.25) | 0.0009 |
| VFT category (n) | 0.46 (1.79) | 0.74 (1.67) | 0.4915 |
| AVL (n) | −0.13 (49.02) | −4.87 (43.09) | 0.750 |
| Delayed AVL (n) | 4.60 (11.42) | 4.38 (12.84) | 0.591 |
| MOCA | 0.55 (2.59) | 1.13 (2.17) | 0.150 |
| MMSE | 0.03 (0.16) | 0.03 (0.16) | 0.907 |
| Placebo (n = 40) Week 4 | Active (n = 38) Week 4 | p Value | Placebo (n = 40) Week 8 | Active (n = 38) Week 8 | p Value | |
|---|---|---|---|---|---|---|
| SBP (mmHg) | 0.52 (2.46) | 0.13 (2.65) | 0.745 | 0.54 (2.08) | 1.18 (2.45) | 0.108 |
| DBP (mmHg) | 0.42 (2.14) | −0.49 (2.72) | 0.122 | 0.33 (2.32) | 0.03 (2.72) | 0.719 |
| TC (mg/dL) | 0.70 (29.50) | 1.77 (28.14) | 0.864 | −0.18 (31.51) | 3.95 (30.36) | 0.563 |
| LDL-C (mg/dL) | 0.15 (29.11) | 2.31 (23.13) | 0.965 | −1.38 (31.30) | 5.03 (26.07) | 0.432 |
| HDL-C (mg/dL) | −1.15 (8.13) | 0.10 (9.79) | 0.349 | 2.13 (9.67) | 2.08 (11.54) | 0.819 |
| TG (mg/dL) | 3.20 (47.41) | −6.26 (49.30) | 0.435 | −0.13 (49.02) | −4.87 (43.09) | 0.750 |
| Glucose (mg/dL) | 3.10 (10.81) | 7.03 (12.16) | 0.164 | 4.60 (11.42) | 4.38 (12.84) | 0.591 |
| Insulin (mU/L) | −1.57 (5.41) | 0.99 (5.42) | 0.116 | −2.31 (6.50) | 0.70 (3.51) | 0.036 |
| HOMA-IR | −0.27 (1.19) | 0.32 (1.23) | 0.095 | −0.41 (1.56) | 0.25 (0.90) | 0.080 |
| Uric acid (mg/dL) | 0.07 (0.89) | 0.22 (0.76) | 0.530 | −0.06 (1.13) | 0.01 (0.99) | 0.915 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crosta, F.; Stefani, A.; Melani, F.; Fabrizzi, P.; Nizzardo, A.; Grassi, D.; Bocale, R.; Necozione, S.; Lombardi, F.; Castelli, V.; et al. Improvement of Executive Function after Short-Term Administration of an Antioxidants Mix Containing Bacopa, Lycopene, Astaxanthin and Vitamin B12: The BLAtwelve Study. Nutrients 2021, 13, 56. https://doi.org/10.3390/nu13010056
Crosta F, Stefani A, Melani F, Fabrizzi P, Nizzardo A, Grassi D, Bocale R, Necozione S, Lombardi F, Castelli V, et al. Improvement of Executive Function after Short-Term Administration of an Antioxidants Mix Containing Bacopa, Lycopene, Astaxanthin and Vitamin B12: The BLAtwelve Study. Nutrients. 2021; 13(1):56. https://doi.org/10.3390/nu13010056
Chicago/Turabian StyleCrosta, Francesca, Amanda Stefani, Francesco Melani, Paolo Fabrizzi, Andrea Nizzardo, Davide Grassi, Raffaella Bocale, Stefano Necozione, Francesca Lombardi, Vanessa Castelli, and et al. 2021. "Improvement of Executive Function after Short-Term Administration of an Antioxidants Mix Containing Bacopa, Lycopene, Astaxanthin and Vitamin B12: The BLAtwelve Study" Nutrients 13, no. 1: 56. https://doi.org/10.3390/nu13010056







