High-Fiber Diet during Pregnancy Characterized by More Fruit and Vegetable Consumption
Abstract
:1. Introduction
2. Materials and Methods
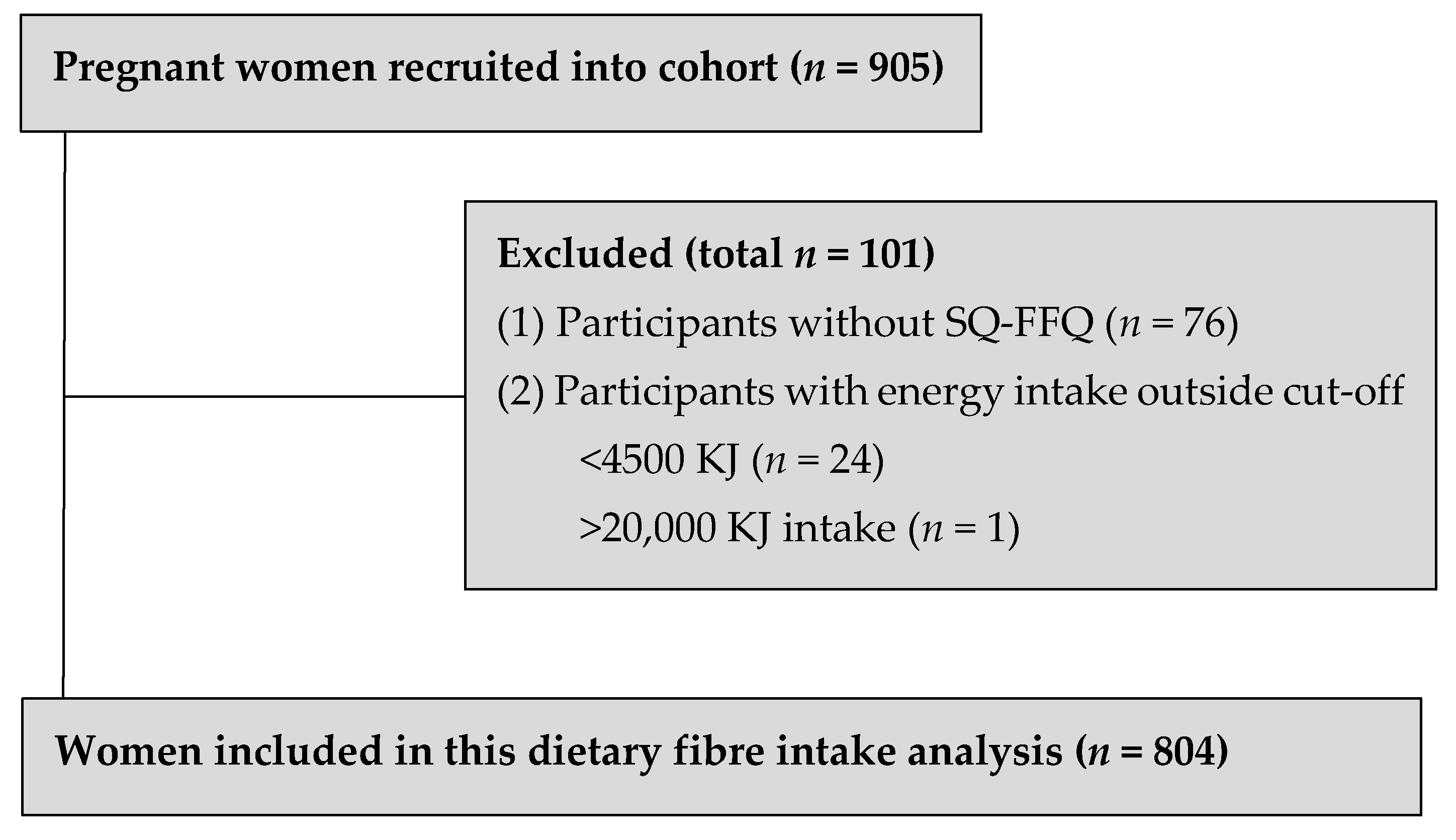
2.1. Study Population
2.2. Maternal Demographics and Characteristics
2.3. Maternal Dietary Assessment in Late Pregnancy
2.4. Infant Birth Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Participant Demographic Characteristics in Relation to Dietary Fiber Intakes
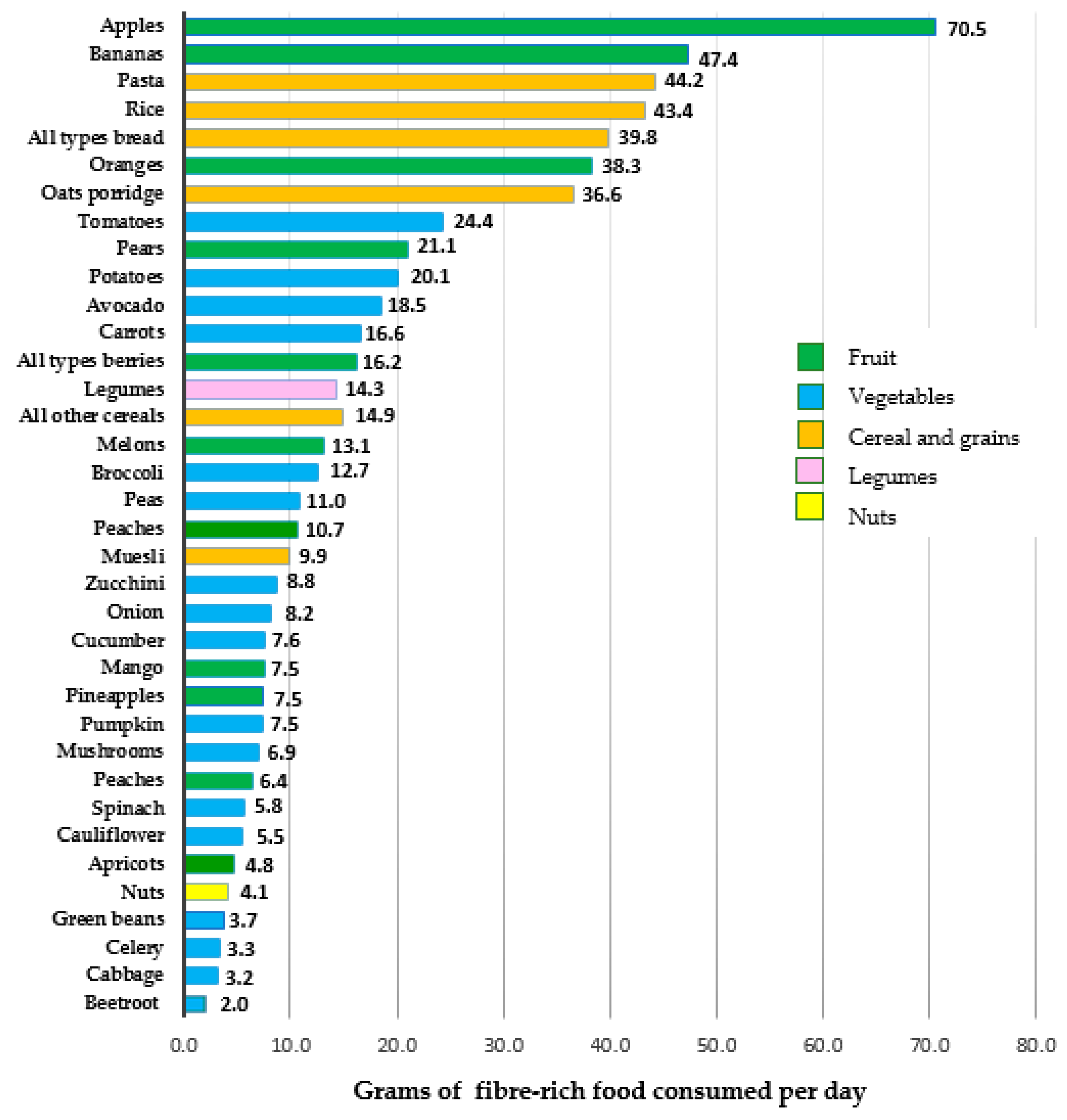
3.3. Fiber-Rich Foods Consumed during Late Pregnancy
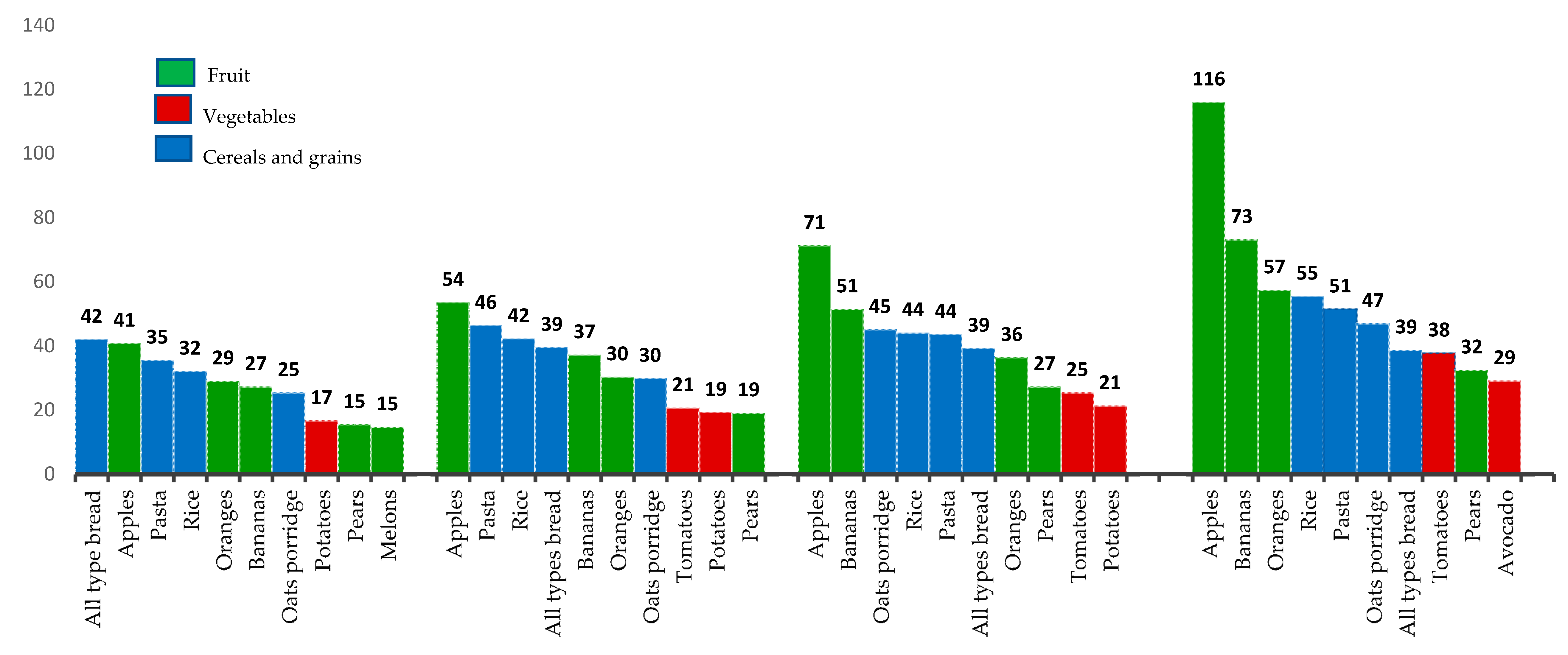
3.4. Top 10 Fiber-Rich Foods Consumed Based on Quartile Dietary Fiber-Intake Groupings
3.5. Associations between Dietary Fiber Intake during Late Pregnancy and Infant Birth Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Osmond, C.; Barker, D.J.P. Fetal, Infant, and Childhood Growth Are Predictors of Coronary Heart Disease, Diabetes, and Hypertension in Adult Men and Women. Environ. Health Perspect. 2000, 108, 545–553. [Google Scholar] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; H O’Keefe, J.; Brand-Miller, A.J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [Green Version]
- WHO. Diet, Nutrition, and the Prevention of Chronic Diseases; Report of a WHO Study Group Geneva; (WHO Technical Report Series, No.797-TRS 797); World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Fayet-Moore, F.; Cassettari, T.; Tuck, K.; McConnell, A.; Petocz, P. Dietary Fibre Intake in Australia. Paper I: Associations with Demographic, Socio-Economic, and Anthropometric Factors. Nutrients 2018, 10, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayet-Moore, F.; Cassettari, T.; Tuck, K.; McConnell, A.; Petocz, P. Dietary Fibre Intake in Australia. Paper II: Comparative Examination of Food Sources of Fibre among High and Low Fibre Consumers. Nutrients 2018, 10, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand including Recommended Dietary Intakes; NHMRC: Canberra, Australia, 2006.
- Hull, H.R.; Herman, A.; Gibbs, H.; Gajewski, B.; Krase, K.; Carlson, S.E.; Sullivan, D.K.; Goetz, J. The effect of high dietary fiber intake on gestational weight gain, fat accrual, and postpartum weight retention: A randomized clinical trial. BMC Pregnancy Childbirth 2020, 20, 319. [Google Scholar] [CrossRef]
- Zerfu, T.A.; Mekuria, A. Pregnant women have inadequate fiber intake while consuming fiber-rich diets in low-income rural setting: Evidences from Analysis of common “ready-to-eat” stable foods. Food Sci. Nutr. 2019, 7, 3286–3292. [Google Scholar] [CrossRef]
- Buss, C.; Nunes, M.A.; Camey, S.; Manzolli, P.; Soares, R.M.; Drehmer, M.; Giacomello, A.; Duncan, B.B.; Schmidt, M.I. Dietary fibre intake of pregnant women attending general practices in southern Brazil--the ECCAGE Study. Public Health Nutr. 2009, 12, 1392–1398. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Coughlin, K.B.; Frederick, I.O.; Sorensen, T.K.; Williams, M.A. Dietary fiber intake in early pregnancy and risk of subsequent preeclampsia. Am. J. Hypertens 2008, 21, 903–909. [Google Scholar] [CrossRef]
- Hodge, A.; Patterson, A.; Brown, W.; Ireland, P. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health 2000, 24, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Ireland, P.; Jolley, D.; Giles, G.; O’Dea, K.; Powles, P.; Rustishaese, I.; Whalqvist, M.; William, P. Development of Melbourne FFQ: A food frequency for use in a Australian prospective study involving and ethnically diverse cohort. Asian Pac. J. Allergy Immunol. 1994, 3, 19–31. [Google Scholar]
- Mahmood, M.W.; Abraham-Nordling, M.; Hakansson, N.; Wolk, A.; Hjern, F. High intake of dietary fibre from fruit and vegetables reduces the risk of hospitalisation for diverticular disease. Eur. J. Nutr. 2019, 58, 2393–2400. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P.; Bahadoran, Z.; Khalili Moghadam, S.; Zadeh Vakili, A.; Azizi, F. A Prospective Study of Different Types of Dietary Fiber and Risk of Cardiovascular Disease: Tehran Lipid and Glucose Study. Nutrients 2016, 8, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gresham, E.; Collins, C.E.; Mishra, G.D.; Byles, J.E.; Hure, A.J. Diet quality before or during pregnancy and the relationship with pregnancy and birth outcomes: The Australian Longitudinal Study on Women’s Health. Public Health Nutr. 2016, 19, 2975–2983. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, H.; Brantsæter, A.; Ydersbond, T.; Haugen, M.; Alexander, J. MoBa Dietary Support Group Methodological challenges when monitoring the diet of pregnant women in a large study: Experiences from the Norwegian Mother and Child Cohort Study (MoBa). Matern. Child Nutr. 2008, 4, 14–27. [Google Scholar] [CrossRef]
- Champ, M.; Hoebler, C. Functional food for pregnant, lactating women and in perinatal nutrition: A role for dietary fibres? Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 565–574. [Google Scholar] [CrossRef]
- Slater, K.; Rollo, M.E.; Szewczyk, Z.; Ashton, L.; Schumacher, T.; Collins, C. Do the Dietary Intakes of Pregnant Women Attending Public Hospital Antenatal Clinics Align with Australian Guide to Healthy Eating Recommendations? Nutrients 2020, 12, 2438. [Google Scholar] [CrossRef]
- Jang, W.; Kim, H.; Lee, B.E.; Chang, N. Maternal fruit and vegetable or vitamin C consumption during pregnancy is associated with fetal growth and infant growth up to 6 months: Results from the Korean Mothers and Children’s Environmental Health (MOCEH) cohort study. Nutr. J. 2018, 17, 105. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Yajnik, C.S.; Kanade, A.; Fall, C.H.D.; Margetts, B.M.; Jackson, A.A.; Shier, R.; Joshi, S.; Rege, S.; Lubree, H.; et al. Intake of Micronutrient-Rich Foods in Rural Indian Mothers Is Associated with the Size of Their Babies at Birth: Pune Maternal Nutrition Study. J. Nutr. 2001, 131, 1217–1224. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, T.B.; Osler, M.; Orozova-Bekkevold, I.; Knudsen, V.K.; Olsen, S.F. Association between fruit and vegetable consumption and birth weight: A prospective study among 43,585 Danish women. Scand. J. Public Health 2006, 34, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Tabassi, Z.; Samimi, M.; Fahiminejad, T.; Esmaillzadeh, A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: A randomised clinical trial. Br. J. Nutr. 2013, 109, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Review: Nutritional Requirements and Dietary Advice Targeted for Pregnant and Breastfeeding Women; National Health and Medical Research Council: Canberra, Australia, 2012.
- National Health and Medical Research Council. Australian Dietary Guidelines; NHMRC: Canberra, Australia, 2013.
- Malek, L.; Umberger, W.; Makrides, M.; Zhou, S.J. Adherence to the Australian dietary guidelines during pregnancy: Evidence from a national study. Public Health Nutr. 2016, 19, 1155–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Muggli, E.; Halliday, J.; Lewis, S.; Gasparini, E.; Forster, D. What do pregnant women eat, and are they meeting the recommended dietary requirements for pregnancy? Midwifery 2018, 67, 70–76. [Google Scholar] [CrossRef]
- Mishra, G.D.; Schoenaker, D.A.; Mihrshahi, S.; Dobson, A.J. How do women’s diets compare with the new Australian dietary guidelines? Public Health Nutr. 2015, 18, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Blumfield, M.L.; Hure, A.J.; MacDonald-Wicks, L.K.; Patterson, A.J.; Smith, R.; Collins, C.E. Disparities exist between National food group recommendations and the dietary intakes of women. BMC Womens Health 2011, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017, 69, 243–256. [Google Scholar] [CrossRef]
- Hyun, T.K.; Jang, K.I. Apple as a source of dietary phytonutrients: An update on the potential health benefits of apple. EXCLI J. 2016, 15, 565–569. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Prince, R.L.; Woodman, R.J.; Bondonno, C.P.; Ivey, K.L.; Bondonno, N.; Rimm, E.B.; Ward, N.C.; Croft, K.D.; Lewis, J.R. Apple intake is inversely associated with all-cause and disease-specific mortality in elderly women. Br. J. Nutr. 2016, 115, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Falcomer, A.L.; Riquette, R.F.R.; de Lima, B.R.; Ginani, V.C.; Zandonadi, R.P. Health Benefits of Green Banana Consumption: A Systematic Review. Nutrients 2019, 11, 1222. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Research advances on structural characterization of resistant starch and its structure-physiological function relationship: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1059–1083. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Tapsell, L.; Beck, E.L. Creation of a fibre categories database to quantify different dietary fibres. J. Food Compos. Anal. 2018, 71, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Lockyer, S.; Nugent, A.P. Health effects of resistant starch. Nutr. Bull. 2017, 42, 10–41. [Google Scholar] [CrossRef]
- Wang, J.; Tang, X.J.; Chen, P.S.; Huang, H.H. Changes in resistant starch from two banana cultivars during postharvest storage. Food Chem. 2014, 156, 319–325. [Google Scholar] [CrossRef]
- Campuzano, A.; Rosell, C.M.; Cornejo, F. Physicochemical and nutritional characteristics of banana flour during ripening. Food Chem. 2018, 256, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Koutsos, A.; Riccadonna, S.; Ulaszewska, M.; Franceschi, P.; Trošt, P.; Galvin, A.; Braune, T.; Fava, P.; Perenzoni, D.; Mattivi, F.; et al. Two apples a day lower serum cholesterol and improve cardiometabolic biomarkers in mildly hypercholesterolemic adults: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2020, 11, 307–318. [Google Scholar] [CrossRef]
- Phelan, S. Pregnancy: A “teachable moment” for weight control and obesity prevention. Am. J. Obstet. Gynecol. 2010, 202, 135.e1–135.e8. [Google Scholar] [CrossRef] [Green Version]
- Assari, S.; Lankarani, M.M. Educational Attainment Promotes Fruit and Vegetable Intake for Whites but Not Blacks. J Multidiscip. Sci. J. 2018, 1, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Prattala, R.; Hakala, S.; Roskam, A.J.; Roos, E.; Helmert, U.; Klumbiene, J.; Van Oyen, H.; Regidor, E.; Kunst, A.E. Association between educational level and vegetable use in nine European countries. Public Health Nutr. 2009, 12, 2174–2182. [Google Scholar] [CrossRef] [Green Version]
- Saunders, C.M.; Rehbinder, E.M.; Carlsen, K.C.L.; Gudbrandsgard, M.; Carlsen, K.H.; Haugen, G.; Hedlin, G.; Jonassen, C.M.; Sjoborg, K.D.; Landro, L.; et al. Food and nutrient intake and adherence to dietary recommendations during pregnancy: A Nordic mother-child population-based cohort. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.A.; Kim, K.; Kim, M.K. Educational attainment and differences in fruit and vegetable consumption among middle-aged adults in the Korean National Health and Nutrition Examination Survey IV. Nutr. Res. Pract. 2012, 6, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Stravik, M.; Jonsson, K.; Hartvigsson, O.; Sandin, A.; Wold, A.E.; Sandberg, A.S.; Barman, M. Food and Nutrient Intake during Pregnancy in Relation to Maternal Characteristics: Results from the NICE Birth Cohort in Northern Sweden. Nutrients 2019, 11, 1680. [Google Scholar] [CrossRef] [Green Version]
- Australian Institute of Health and Welfare. Australia’s Mothers and Babies 2015—in Brief. Perinatal Statistics Series no. 33. Cat no. PER 91; AIHW: Canberra, Australia, 2017.
- Dunneram, Y.; Jeewon, R. Healthy Diet and Nutrition Education Program among Women of Reproductive Age: A Necessity of Multilevel Strategies or Community Responsibility. Health Promot. Perspect. 2015, 5, 116–127. [Google Scholar] [CrossRef] [Green Version]
| Maternal Characteristics | All Participants (n = 804) | Q1 Fiber intake * 15.9 (14.4–17.5) n = 202 | Q2 Fiber Intake * 21.4 (20.3–22.6) n = 202 | Q3 Fiber Intake * 26.5 (25.2–27.6) n = 199 | Q4 Fiber Intake * 34.8 (32.1–39.5) n = 201 | p Value |
|---|---|---|---|---|---|---|
| Age (years) # | 32.9 (29.2–35.8) | 31.0 (27.8–34.7) | 33.0 (30.0–35.9) | 33.3 (30.4–36.0) | 32.9 (29.6–35.9) | <0.01 |
| Parity (0) ¥ | 374/754 (49.6%) | 102/187 (54.5%) | 80/187 (42.8%) | 96/188 (51.1%) | 97/192 (50.5%) | 0.14 |
| Ethnicity (Caucasian) ¥ | 665/734 (90.6%) | 161/177 (91.0%) | 160/184 (87.0%) | 172/184 (93.5%) | 173/189 (91.5%) | 0.18 |
| Further education post-secondary school ¥ | 601/784 (76.7%) | 138/197 (70.1%) | 149/197 (75.6%) | 15/192 (80.7%) | 159/198 (80.3%) | 0.04 |
| Food Group | Recommended Daily Serving | Consumed Less Than Recommended | Meeting Recommendation and Consuming More Than Recommended |
|---|---|---|---|
| Fruit | 2 | 604 (75.1%) | 200 (24.8%) |
| Vegetables (and legumes) | 5 | 776 (96.5%) | 28 (3.5%) |
| Infant Characteristics | All Participants n = 804 | Q1 15.9 (14.4–17.5) * n = 202 | Q2 21.4 (20.3–22.6) * n = 202 | Q3 26.5 (25.2–27.6) * n = 199 | Q4 34.8 (32.1–39.5) * n = 201 | p Value |
|---|---|---|---|---|---|---|
| Gender (male) ¥ | 394/768 (51.3%) § | 101/192 (47.4%) | 96/189 (51.0%) | 100/194 (51.5%) | 99/196 (50%) | 0.56 |
| Birth weight ** | 3499.1 (435.0) * | 3466.8 (485.0) | 3514.5 (418.3) | 3489.5 (411.5) | 3523.6 (422.0) | 0.55 |
| Gestational age # | 39.3 (38.6–40.1) ★ | 39.2 (38.4–40.3) | 39.0 (38.6–40.0) | 39.4 (38.6–40.1) | 39.4 (37.7–40.3) | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pretorius, R.A.; Palmer, D.J. High-Fiber Diet during Pregnancy Characterized by More Fruit and Vegetable Consumption. Nutrients 2021, 13, 35. https://doi.org/10.3390/nu13010035
Pretorius RA, Palmer DJ. High-Fiber Diet during Pregnancy Characterized by More Fruit and Vegetable Consumption. Nutrients. 2021; 13(1):35. https://doi.org/10.3390/nu13010035
Chicago/Turabian StylePretorius, Rachelle A., and Debra J. Palmer. 2021. "High-Fiber Diet during Pregnancy Characterized by More Fruit and Vegetable Consumption" Nutrients 13, no. 1: 35. https://doi.org/10.3390/nu13010035





