Abstract
The gluten-free diet (GFD) has gained increasing popularity in recent years, supported by marketing campaigns, media messages and social networks. Nevertheless, real knowledge of gluten and GF-related implications for health is still poor among the general population. The GFD has also been suggested for non-celiac gluten/wheat sensitivity (NCG/WS), a clinical entity characterized by intestinal and extraintestinal symptoms induced by gluten ingestion in the absence of celiac disease (CD) or wheat allergy (WA). NCG/WS should be regarded as an “umbrella term” including a variety of different conditions where gluten is likely not the only factor responsible for triggering symptoms. Other compounds aside from gluten may be involved in the pathogenesis of NCG/WS. These include fructans, which are part of fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), amylase trypsin inhibitors (ATIs), wheat germ agglutinin (WGA) and glyphosate. The GFD might be an appropriate dietary approach for patients with self-reported gluten/wheat-dependent symptoms. A low-FODMAP diet (LFD) should be the first dietary option for patients referring symptoms more related to FODMAPs than gluten/wheat and the second-line treatment for those with self-reported gluten/wheat-related symptoms not responding to the GFD. A personalized approach, regular follow-up and the help of a skilled dietician are mandatory.
1. Introduction
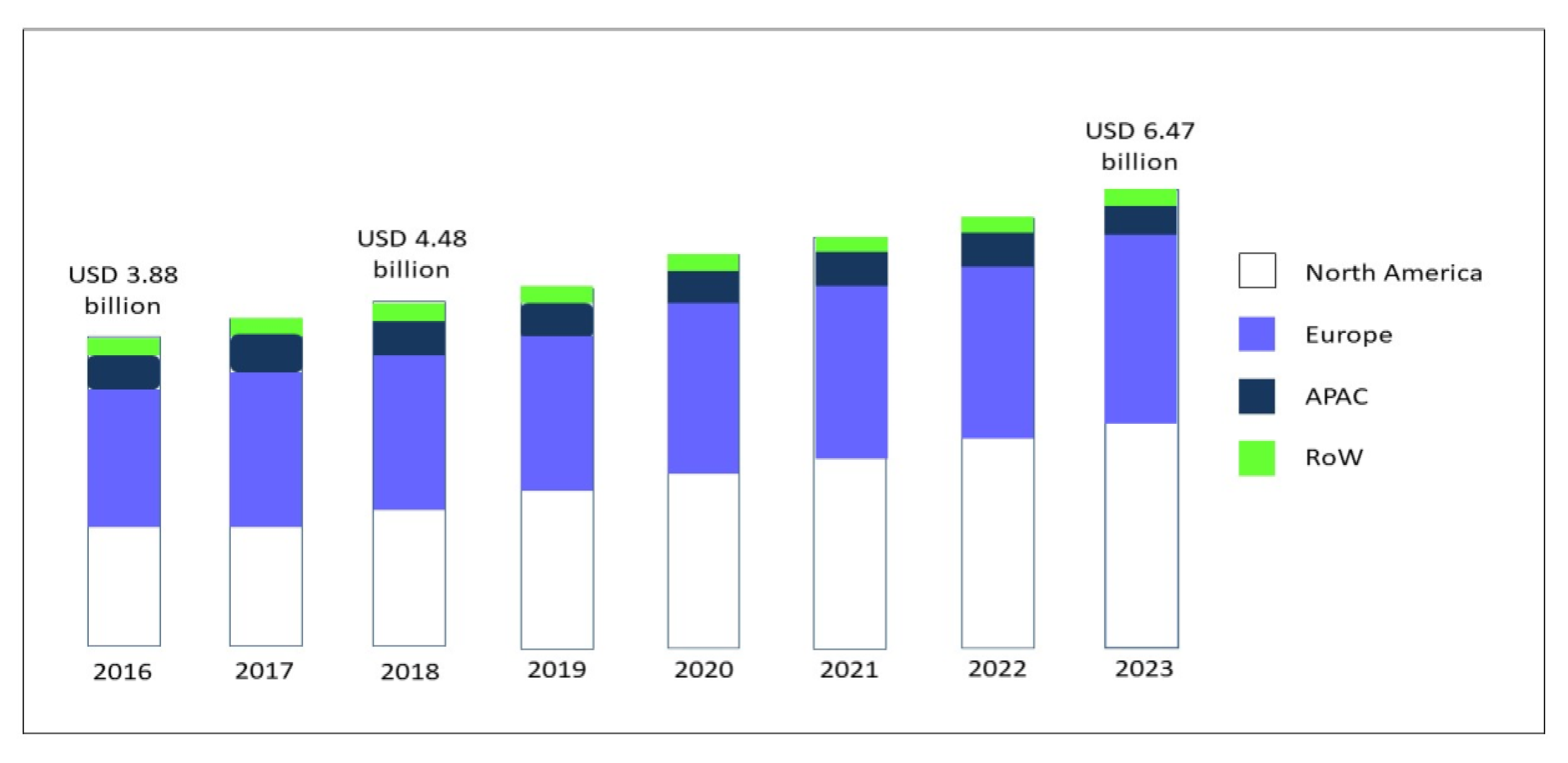
Over the last 30 years, the gluten-free diet (GFD) has gained increasing popularity associated with an exponential growth in the sales of gluten-free (GF) products [1]. The global market for GF food, driven by North America and Europe, but now spreading across the Asia-Pacific countries (APAC), was valued at USD 3.88 bn in 2016, and is foreseen to expand to USD 6.47 bn in 2023, at a compound annual growth rate (CAGR) of 7.60% [2] (Figure 1).
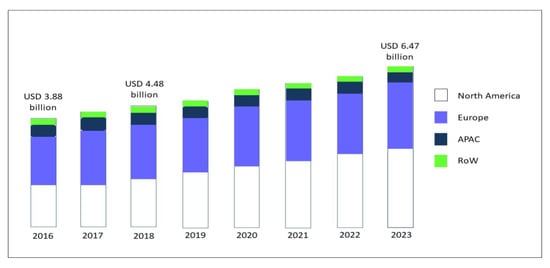
Figure 1.
GF product market, by region, during the forecast period of 2016 to 2023, modified from Research and Markets, Report May 2019 [2]. Abbreviations: GF: gluten-free; APAC: Asia-Pacific countries; RoW: rest of the world.
In the USA, a follow-up analysis of the National Health and Nutrition Examination Survey (NHANES) revealed that self-adoption of a GF diet without a diagnosis of celiac disease (CD) tripled from 2009–2010 (prevalence 0.52%) to 2013–2014 (prevalence 1.69%) [3] and NPD’s Dieting Monitor, which tracks nutrition-related issues of consumers, in 2013 reported that nearly 30% percent of adults claimed to cut down on or avoid gluten [4]. In Italy, where bread and pasta are the foundation of food culture, is in the vanguard of the European GF sector with the range of products jumping from 280 in 2001 to the current 6500 and a market amounting to EUR 320 million, of which only 215 are dispensed on prescription for celiac patients. The launch of innovative products containing no or less gluten and dominated by the bakery product segment is on the rise and contributes to boosting this industry. Suppliers continue to invest in innovation to improve taste, texture and overall quality in GF formulations.
The GFD is recommended as lifelong treatment for CD. However, neither government awareness campaigns and initiatives nor the improvement of diagnostic tools and increasing prevalence of CD [5] account for the overwhelming adoption of a GF lifestyle. Clinical application of GFDs continues to escalate as a therapeutic option for non-celiacs who seem to react negatively to gluten ingestion, are trying to lose weight [6] or simply want to reduce bloating after meals [7]. The reasons given for a self-imposed GFD include irritable bowel syndrome (IBS) and lactose intolerance [8]. Other persons spontaneously limit or eliminate gluten intake as a “healthy” dietary regimen without previous clinical tests due to the widespread consumer interest in free-from products and the growing adoption of specific eating patterns in pursuit of health and wellness [9]. In an Australian cross sectional population survey [10], symptomatic wheat avoidance was highly correlated with dairy avoidance, female gender and lesser and greater receptiveness to conventional and complementary medicine, respectively. While perception of the potential harm and expected benefits of gluten consumption/avoidance are high, real knowledge of gluten and GF-related implications for health is scarce. An American survey [11] found that over 30% of respondents had no specific reason for adopting a GF regimen, while less than 10% self-reported gluten sensitivity (GS). The other reasons were a healthy lifestyle, improvement of intestinal health or the presence of a gluten-sensitive family member. Different factors lie at the basis of the GF movement, mostly driven by non-scientific sources of information. While Google searches containing “low carb” and “low fat” have declined since 2004, worldwide searches for “gluten” showed a sharp upward trend, reaching the peak of food concerns from 2005 to 2014. From then on, they have remained generally steady. In Italy, an increase in the number of searches was observed until mid-2019, then there was a decline. This far exceeds lactose, genetically modified organisms (GMO) and palm oil, with ratios approaching 16:1, 6:1 and 2:1, respectively. Marketing campaigns aimed at extending the appeal of GF to every health-conscious consumer despite the high costs of products. Moreover, athletes and celebrities, together with mass media messages and social network platforms, all contribute to increasing awareness of gluten intolerance and fuel the interest in dietary treatments. Consumers commonly select GF products from aisles in major supermarkets and health food shops [12]; for many consumers, the front of package claims are more important determinants of GF product choices [13] than nutritional labeling [14]. Several studies have shown an excessive intake of fats and carbohydrates, with a lower consumption of dietary fibers, in CD patients on a GFD [15]. Moreover, clinically relevant deficiencies of iron, vitamin D, vitamin B6 and zinc have been reported in CD patients during treatment with a GFD, whereas data on deficiencies of vitamin B12, folic acid, calcium and magnesium are controversial [16]. The alarming discrepancy between media claims and scientific evidence [17] drives the motivation and reinforcement of people’s commitment to avoiding gluten, generating a great deal of confusion and misconceptions [18]. This article is aimed at discussing the actual role of gluten in non-celiac gluten/wheat sensitivity (NCG/WS).
2. What about Gluten?
2.1. Gluten Structure and Genetics
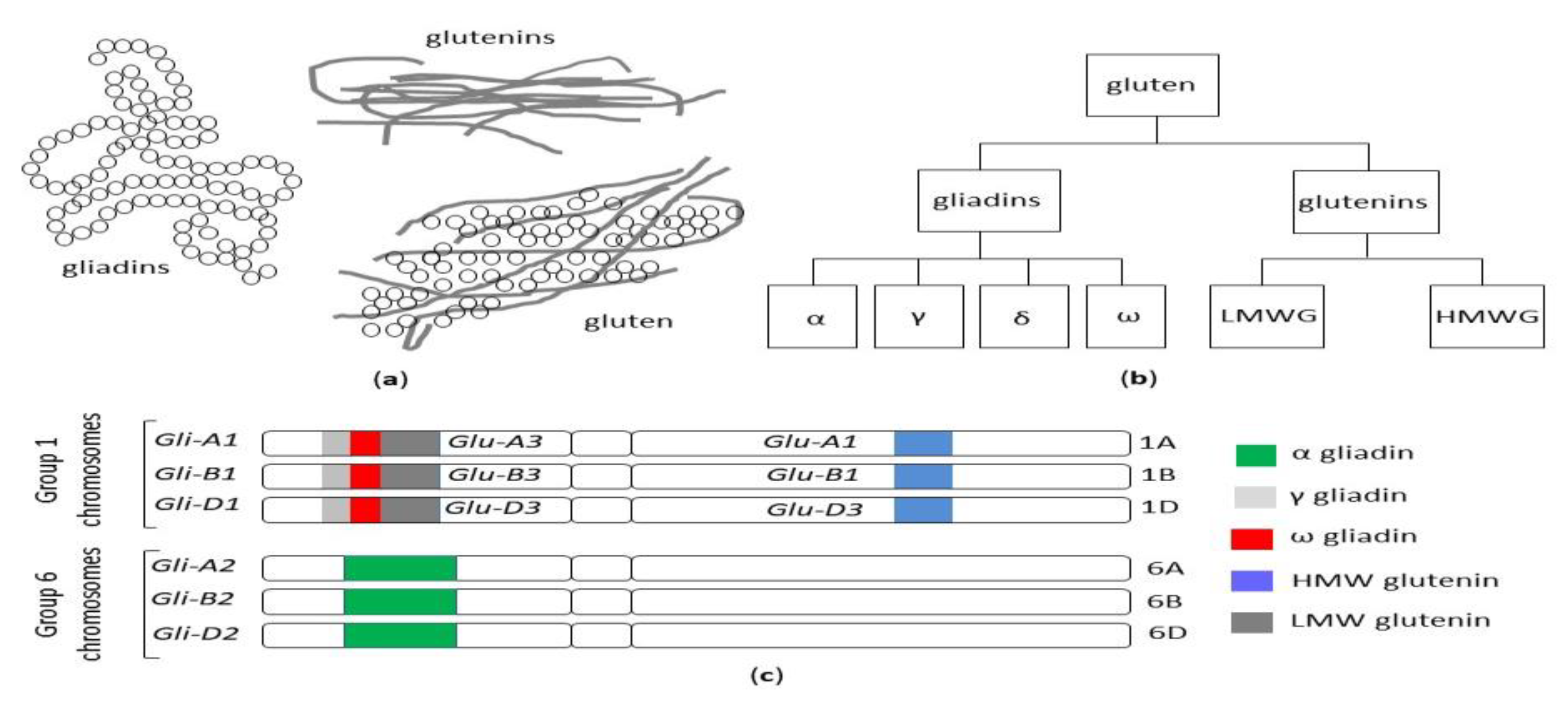
The term gluten collectively refers to a family of storage proteins, formally known as prolamins, naturally occurring in wheat, barley and rye, and their cross-bred grains [19]. Although oats also contain prolamins (avenins), they represent a small (10 to 15%) proportion of total protein content, in comparison with 80 to 85% of wheat gliadins. Moreover, avenins contain less proline than the other prolamins, are more easily digested and the peptides show less affinity for MHC II peptides encoded by HLA DQ2.5 haplotypes [20]. These properties could make oats generally safe for celiac patients, although individual hypersensitivity in some celiac patients can occur [21,22], and some varieties may display immunogenicity/toxicity [23,24]. The wheat prolamins are termed gliadins, monomeric proteins divided into four distinct subtypes, referred to as alpha, gamma, delta and omega gliadins and glutenins, and polymeric proteins composed of high (HMW-GS) and low molecular weight subunits (LMW-GS) linked by disulfide bonds [25] (Figure 2a,b).
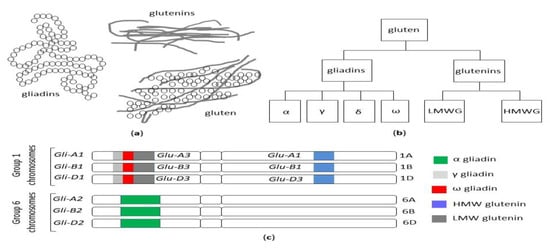
Figure 2.
(a) The structure of the gluten protein gluten network. (b) The classification of wheat gluten proteins. (c) Gliadin and glutenin loci in Triticum aestivum (AABBDD), modified from Sharma, 2020 [26]. HMW: high molecular weight, LMW: low molecular weight.
A significant proportion of prolamins are represented by repetitive sequences of glutamine and proline. The various wheat varieties differ in terms of prolamin molecular weight and microstructure (junction density, branching rate, lacunarity). These characteristics influence the strength of the network and the dough quality and, in turn, determine the tensile and cooking properties [19,25,27]. Due to its unique biochemical and functional features (water-binding and visco-elastic properties, gas retention), gluten is essential for baking but also widely used as an additive in processed food.
Besides its commercial value, the detrimental effects of gluten on human health have been described, mediated by immunological or toxic reactions [28]. Due to the high number of glutamine- and proline-rich periodic sequences, gluten peptides are highly resistant to gastric and intestinal proteolytic degradation, thus giving rise to potentially immunogenic fragments. In addition, gluten alters intestinal permeability, promotes oxidative stress, exerts cytotoxic and pro-inflammatory effects and negatively affects the microbiome; cell apoptosis is increased and cell differentiation is reduced [29]. In celiac patients carrying HLA DQ2/DQ8 haplotypes, gluten triggers an innate, as well as adaptative, Th1-driven immune response, amplified by transglutaminase-mediated synthesis of negatively charged glutamate residues from glutamine [26]. Since the Neolithic Age agricultural revolution, 10,000 years ago, ancient grasses have been domesticated and spread from the Fertile Crescent of the Middle East westward through Europe [30]. Agricultural techniques increased the abundance and availability of wheat, but it is only in the past 500 years that the gluten content of foods containing wheat has significantly increased. Modern hexaploid wheat cultivars have three different genomes (A, B and D) and evolved from the original diploid wheat, called einkorn (Triticum monococcum), through thousands of years of selective breeding and the development of tetraploid varieties [31] (Figure 2c). It has been posited that the genetic evolution, introducing new sequences into the wheat genome, could have potentially led to an increase in toxic and immunogenic epitopes responsible for the increased prevalence of CD [32] and, in general, of gluten-related disorders. A high-quality genome sequence was established from the reference wheat Chinese Spring, which made a complete set of gluten protein genes available from a single hexaploid cultivar [33,34]. Nevertheless, the large number of different wheat cultivars around the world, the high allelic variation in gluten genotypes among cultivars and the large number of immunogenic gluten epitopes make it difficult to draw firm conclusions, and the real contribution of modern wheat breeding practices to the increased prevalence of CD is still a matter of debate. Data on the reduced immunogenicity of old wheat genotypes because of the absence of the D-genome [32] have not been confirmed by more recent studies [35,36,37,38] and their health-promoting properties emerging from recent studies appear to rely on other features rather than their low immunogenicity. The macro- and micronutrient contents of ancient grains seem to decrease the risk of cardiovascular disease and metabolic syndrome, ameliorate the glycolipid profile and reduce oxidative stress and the level of pro-inflammatory cytokines. Furthermore, their consumption has been reported to curtail the extent and severity of IBS-related symptoms [39].
In the absence of convincing evidence for a role of wheat breeding in the increasing prevalence of gluten-related diseases, change in per capita consumption of wheat flour and the usage of vital gluten as a processed food additive have been postulated [40].
2.2. Is There a Role for Microbiota?
In recent years, the impact of gut microbiota on the loss of gluten tolerance has received increasing attention. The intestinal microbial communities represent a complex ecosystem, which plays a central role in modulating both innate and adaptive immune responses [41,42]. They are also involved in the maintenance of mucosal barrier function, which is a crucial mediator between our body and the external environment, and prevent the entry of toxic/immunogenic molecules across the intestinal wall [43,44].
In both stools and mucosal biopsies of celiac patients, a shift toward Bacteroides, Clostridium and Escherichia coli, with reduction in protective Bifidobacteria, Firmicutes and Lactobacilli, in comparison with non-celiac controls, has been described [45,46] as being partially restored by a GFD [47,48,49]. Both genetic makeup and environmental factors contribute to shaping the composition and diversity of the intestinal microbiota. Infants with a high genetic risk of developing CD harbor a higher proportion of Firmicutes and Proteobacteria and a lower proportion of Actinobacteria [50], resulting in an increased prevalence of pathogenic bacteria compared to those with a low risk [51]. According to the hygiene hypothesis, the decreased infectious pressure observed in industrialized countries over the last several decades should prevent the development of a functional immune system during early childhood, leading to an imbalance between pro-inflammatory and anti-inflammatory responses. Additional main drivers of microbial gut colonization, such as mode of delivery, infant feeding practice and antibiotic use, were not confirmed as risk factors for CD [52,53,54,55]. Although most studies report major differences in the composition of microbiota between celiac patients and healthy controls, a specific microbial profile cannot be identified in CD [56]. Evidence on the causal relationship between dysbiosis and disease occurrence is highly heterogeneous and controversial due to inter-individual variability, small sample sizes and different methodologies, which all hamper the interpretation of results [57]. Finally, it is still unclear whether an altered microbiota in CD patients is the cause or the consequence of mucosal inflammation [58]. The exact mechanisms by which a dysbiotic status could contribute to CD development are also still unknown and include the processing of gluten peptides, activation of innate immune response and modulation of intestinal permeability [59,60].
2.3. Gluten Consumption
The phenomenon of globalization is driving a revolution in food systems (supply, marketing and distribution) as well as in dietary patterns. Major changes in food culture are closely associated with urbanization, increasing incomes, capital flow and market liberalization, and are characterized by dietary convergence, a phenomenon occurring as a result of increased reliance on a narrow base of staple foods, among which the dominant staple grain is wheat [61]. Palatability, ease of large-scale cultivation, industrial food processing and low prices have all contributed to the global spread of wheat gluten consumption. Wheat production has increased sharply since 1955, showing an impressive tenfold increase in the annual rate of yield improvement, particularly in the 1960s, and gradually afterwards. This was thanks to a technology shift commonly labeled the “green revolution” [62,63]. The green revolution resulted in the development of rust-resistant semi-dwarf, high-yield wheat. Between 1980 and 2013, the world’s annual wheat yield increased by 1.41% [64]. Currently, North America maintains the leading position in the wheat gluten market, followed by Europe. The abundance of applications in the food industry and high demand for high-fiber and meat-free foods among an increasingly health-conscious and vegan/vegetarian population are considered key factors boosting the growth of the wheat gluten industry in Western countries [65]. The global wheat protein market was estimated to be valued at USD 2.04 billion in 2017 and it was foreseen to grow at a compound annual growth rate (CAGR) of 4.8% from 2017, to reach USD 2.58 billion by 2022 [66].
In highly populated, developing countries, particularly those in the Asian region, the growing middle class, adopting Western-style diets with a higher content of wheat products, have contributed to increasing its consumption [64]. Recently, global change in consumption patterns and consumer attitudes during coronavirus lockdowns, and in particular the boom of home baking, boosted a sharp increase in wheat consumption: the Spanish Minister of Agriculture, Luis Planas, revealed that sales of flour quadrupled during the third week of lockdown; Nielsen data showed that in March 2020, the retail flour sales in France, the US and Italy increased by 140, 154 and 185 percent, respectively, compared with the same period in 2019.
Vital wheat gluten (VWG) is obtained from wheat flour by removing soluble fibers and starch fractions and recovering gliadins and glutenins [67]. VWG is widely used as an additive in bakery products and pasta dough to increase yields and improve rheological, microstructure and quality characteristics [68,69]. Due to its visco-elasticity and the range of functional properties at a lower price than competitors, such as milk and soy proteins, have contributed to spreading its use in the food industry, leading to a tripled consumption since 1977, consistent with the epidemiology of CD [40].
2.4. Gluten Exorphins
Exogenous peptides with opioid-like activities, which include gluten exorphins (wheat), casomorphins (milk), rubiscolins (spinach) and soymorphins (soybean) [70], display regulatory functions both for the gastrointestinal and central nervous systems. In rodent behavioral models, food-derived, opioid-like peptides affect nociception, spontaneous behavior and memory. After oral, intracerebroventricular or intraperitoneal administration, some food-derived opioids also affect intestinal motility, hormone release, appetite, mucus production and local immunity [71,72]. In rats, the opioid antagonist naloxone drastically reduces the intake of preferred foods [73,74].
Enzymatic breakdown of gliadin from wheat by intestinal pepsin, leucine aminopeptidase and elastase generates morphine-like peptides, also known as gluten exorphins [75]. In healthy volunteers, early research showed that gluten exorphins induced a significant increase in gastrointestinal transit time, reversible after administration of the opioid antagonist naloxone [75,76].
In rodents, orally administered gluten exorphin A5 suppressed the endogenous pain-inhibitory system induced by socio-psychological stress and modified spontaneous behavior and learning/memory processes during several laboratory stressors, indicating that the peptides may cross the blood–brain barrier [77]. It has been suggested that the effects of food exorphins could be amplified if they are absorbed in excess through a disrupted mucosal barrier [78].
3. Not Only Gluten
Although general attention has focused on gluten as the only culprit of symptoms occurring in patients on a gluten-containing diet, a variety of substances, belonging to the non-gluten components of wheat, are potentially harmful, including wheat α-amylase/trypsin inhibitors (ATIs), wheat germ agglutinins (WGAs) and fructans. Moreover, glyphosate, a non-selective herbicide extensively used in farming against weeds, could play an important role due to its interference with agricultural crops.
3.1. Wheat α-Amylase/Trypsin Inhibitors (ATIs)
ATIs are a family of at least 11 proteins belonging to the non-gluten protein fraction. They are classified in monomeric, dimeric and tetrameric forms and represent 2–4% of total wheat protein content [79]. ATIs are contained in the endosperm of wheat seeds, where they play the multifunctional role of a natural defense against insects and parasites, inhibiting enzymes with amylase and trypsin-like activities and the regulation of starch metabolism during seed development and germination [80,81]. Identified as major allergens in baker’s asthma, as well as stimulators of innate immunity, ATIs promoted a strong innate immune response by engaging the TLR4–MD2–CD14 complex both in human and murine macrophages, monocytes and dendritic cells (DCs) and in vivo after oral or systemic challenge in mice. Furthermore, in duodenal biopsies from celiac patients in remission, ATIs induced an increase in IL-8 mRNA expression as well as a further increase in 33mer-induced IL-8 expression [82]. In line with these results, in gluten-sensitized mice expressing HLA-DQ8, ATI ingestion was recently shown to increase the inflammatory response to dietary gluten. Conversely, in ATI-fed control mice, a TLR4-mediated intestinal barrier dysfunction without mucosal damage was observed. In both cases, ATI-degrading lactobacilli decreased the inflammatory effects, suggesting new therapeutic strategies for wheat-related disorders [83]. ATIs are present and retain bioactivity in processed or baked foods. Wheat breeding practices aimed at developing high-yield, highly pest-resistant crops has led to an increased amount of ATIs in modern hexaploid wheat varieties; modern gluten-containing staples have been found to have higher levels of TLR4-activating ATIs than most gluten-free food; in mice, oral ingestion was shown to increase intestinal inflammation by activating gut and mesenteric lymph node myeloid cells [84]. Recently, a central role for ATIs has been proposed in the pathogenesis of NCG/WS within the context of a new theory which suggests a decrease in butyrate-producing intestinal bacteria as an initial trigger of the pathogenic cascade [85].
3.2. Wheat Germ Agglutinin (WGA)
A role of WGA as potentially responsible for many of wheat’s related, and difficult to diagnose, ill effects has been postulated. WGA belongs to the lectin group, a superfamily of carbohydrate-binding proteins present in a variety of plants with a protective role against external pathogens. It is a homodimer composed of subunits and each protomeric unit consists of four structurally homologous domains with a high degree of amino acid homology. Four interlocking disulfide bonds result in a compact, stable protein highly resistant to degradation [86]. It is present in its highest concentrations in the germ tissue of wheat kernels (up to 0.5 g/kg) [87], especially in whole wheat. Through thousands of years of selective wheat breeding to obtain increasingly higher protein content, the concentration of WGA lectin in wheat has increased proportionately, offering additional pest resistance and contributing to wheat’s global dominance as one of the world’s favored monocultures. WGA may adversely affect gastrointestinal function in various ways: it binds specifically to carbohydrates expressed by human enterocytes and immune cells and to the glycocalyx, the sialic acid coatings of the epithelial layer. In human basophils, WGA induced interleukin 4 (IL-4) and interleukin 13 (IL-13) release [88]. In an experimental model of human intestinal immune/epithelial cell interaction, it exhibited toxic and inflammatory effects by disrupting epithelial integrity and inducing the synthesis of pro-inflammatory cytokines, including interleukin 1, interleukin 6 and interleukin 8 by peripheral blood mononuclear cells (PBMCs) [89]. In murine spleen cells, WGA induced a T and B cell-independent production of interleukin 12 (IL12) and, in turn, the production of interferon gamma (IFN gamma) by T/natural killer lymphocytes [90]. In WGA-treated murine peritoneal macrophages, the production of pro-inflammatory cytokines anti-TNF alfa, interleukin 1 beta (IL-1 beta), IL-12 and IFN gamma was reported [91]. Human data on the in vivo immune-stimulatory activity of WGA are lacking [92], both in healthy subjects [93] and CD patients. However, the presence of IgG and IgA antibodies to WGA has been described, not cross reacting with gluten antigens, at higher levels in CD in comparison with patients with other intestinal diseases and healthy subjects. For this reason, a correlation with the pathogenesis of CD has been suggested [94]. Nevertheless, antibodies to wheat albumin and globulin [95], as well as to other dietary antigens such as casein, beta-lactoglobulin and ovalbumin, have also been reported in celiac patients [96] and the role of WGA in the pathogenesis of CD remains elusive.
In rodents, WGA displayed anti–nutrient effects reducing digestibility and utilization of dietary proteins: it mimicked the effects of epidermal growth factor (EGF), inducing cellular hyperplastic and hypertrophic growth [97]. In rats, it also caused damage to the intestinal brush border membrane, reduction in surface area, acceleration of cell losses, shortening of villi via binding to the villous surface [98] and cytoskeleton degradation, contributing to cell death and increased turnover.
3.3. Fructans
Wheat contains fructans, naturally occurring polymers of fructose molecules belonging to the fermentable oligosaccharides (fructo-oligosaccharides or fructans (FOSs), galacto-oligosaccharides (GOSs; stachyose, raffinose)), disaccharides (lactose), monosaccharides (fructose) and polyols (sorbitol, mannitol, xylitol and maltytol) (FODMAP) group [99]. FODMAP was named in 2005 by the Monash group in a paper on the link between a FODMAP-rich diet and lifestyle in Crohn’s disease patients [100]. FODMAPs can be found in a wide range of staple foods such as fruits, vegetables, legumes and cereals, honey, milk and dairy products and sweeteners [100,101].
Owing to their small size, they are osmotically active and rapidly fermented by gut bacteria in the large intestine [102]. The combination of osmotic activity with fluid retention within the intestinal lumen and gas production by fermentation of oligosaccharides and polyols induces a variety of symptoms. These include bloating, abdominal pain and diarrhea [103].
Fructans increase the tolerance of wheat to drought and cold [104]. Their content in wheat is highly variable and depends on the final product; no significant difference was found between wheat breads and the gluten-free counterparts (approximately 1% in both) [105,106]. Furthermore, gluten-free products, like corn, can have quite a large amount of FODMAPs, mainly fructans, galactans and fructose [105].
3.4. Wheat Glyphosate
Glyphosate is a non-selective herbicide and, since the late 1970s, one of the most extensively used in farming against weeds that interfere with agricultural crops like soy, corn and wheat [107]. A role as a causal factor for the worldwide increase of CD incidence was initially proposed, based on the glyphosate effects on intestinal microbiota, micronutrient absorption, enzymatic detoxification and serotonin signaling, as well as on the increased risk of non-Hodgkin’s lymphoma in celiac patients [108]. Nevertheless, the paper received criticism for being merely speculative. Other studies based on in vivo and in vitro animal models [109,110] and on cultured human and rat intestinal cell lines [111] have postulated a negative impact of glyphosate on intestinal microbiota, barrier properties and motility.
A strong limitation of these studies is that most of them, due to obvious ethical reasons, have been conducted on experimental models. In the absence of robust evidence, the causative link between glyphosate and gluten-related intestinal disorders remains hypothetical.
4. Gluten-Related Disorders
Wheat proteins are recognized as environmental triggers of two well-established immune-mediated disorders, CD and wheat allergy (WA). Furthermore, a gluten-related condition much debated in these last years is non-celiac gluten sensitivity (NCGS).
4.1. Celiac Disease (CD)
CD is a chronic small bowel enteropathy occurring in genetically susceptible individuals where dietary gluten peptides elicit both innate and adaptive Th1-driven immune responses, amplified by transglutaminase-mediated synthesis of negatively charged glutamate residues from glutamine [26]. Access of immunogenic gluten peptides to the small intestine lamina propria is fostered by gluten-induced up-regulation of zonulin, a modulator of intestinal tight junctions [112]. Besides the CD-predisposing HLA DQ2/DQ8 haplotypes, genome-wide association studies have identified 39 non-HLA loci affecting CD [113]. Environmental factors may also be of importance for CD development. A correlation with some viral infections, especially during early childhood, has been suggested, including rotavirus, reovirus, enterovirus A and B and acute respiratory infections [114,115,116]. Based on the evidence that the microbiota affects the immune response [117] and indications of microbiota alterations in celiac patients, a correlation between dysbiosis and the risk of developing CD has been postulated [118,119,120]. The role of other environmental factors, such as infant feeding practices, mode of delivery, age of gluten introduction and amount of gluten in early life or exposure to antibiotics, has not been confirmed or has given contradictory results [54,121,122,123,124,125,126].
CD is diagnosed more frequently in females with a F:M ratio of 2:1 on average and an onset following a bimodal age distribution, with an initial peak in the first 2 years of life and a second peak in the second or third decade, although about 25% of all diagnoses occur at the age of 60 years or more [127]. Clinical presentation ranges from virtually asymptomatic cases, despite typical mucosal damage (silent CD), to severe malabsorption and includes a variety of mild to severe intestinal and/or extraintestinal symptoms [128], especially involving iron and bone metabolism, central and peripheral nervous systems and reproductive system. Multiple autoimmune diseases have been described in association with CD, most commonly autoimmune thyroiditis, type 1 diabetes and liver and rheumatologic disorders [129]. Diagnosis of CD relies on the assessment of specific circulating antibodies and on the demonstration of duodenal mucosal damage (ranging from lymphocytic enteritis to severe villous atrophy); in selected cases, HLA DQ typing is recommended. According to the European guidelines, duodenal biopsies can be avoided in symptomatic children with high titer serology [130]. Owing to a misleading clinical presentation and/or lack of clear-cut diagnostic tests in many patients, diagnosis of CD requires time and expertise to properly combine clinical, serologic, histologic and genetic data. About 1% of patients, especially those with late diagnosis, low adherence to diet and HLA DQ2 homozygosis, develop pre-malignant or malignant complications (refractory CD, ulcerative jejunoileitis, enteropathy-associated T cell lymphoma (EATL), small bowel adenocarcinoma) [131,132] or hyposplenism [133].
4.2. Wheat Allergy (WA)
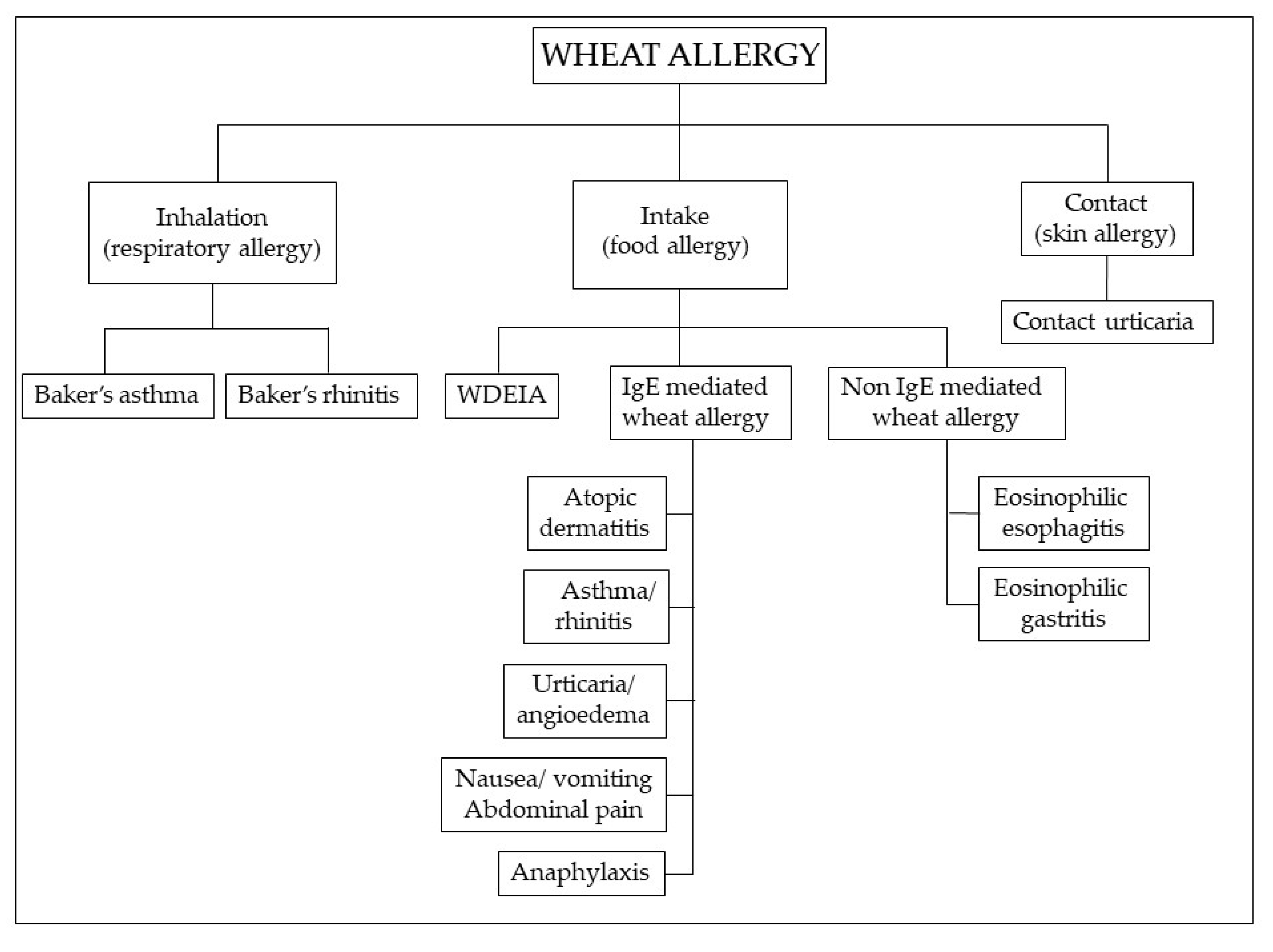
WA has a prevalence in the range of 0.2% to 1% [134]. Although more common in children [135], most of whom outgrow it by the age of 16 years [136], symptoms may occur at any stage of life, including later adulthood. The prevalent immune mechanism is IgE mediated, but non-IgE-mediated reactions are also described [137,138], characterized by chronic infiltration of eosinophils and lymphocytes in the gastrointestinal mucosa (Figure 3).
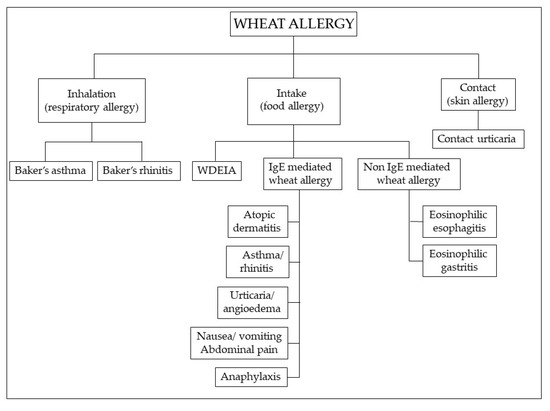
Figure 3.
Classification of wheat allergy depending on the route of exposure and the underlying immunologic mechanism. Abbreviations: WDEIA: wheat-dependent, exercise-induced anaphylaxis.
WA is a classic food allergy characterized by cutaneous, gastrointestinal or respiratory manifestations. These include wheat-dependent, exercise-induced anaphylaxis (WDEIA), which results from the combination of wheat ingestion and physical exercise, baker’s asthma and rhinitis, occurring after inhalation of wheat and cereal flours, which is one of the most common occupational allergies and contact urticaria [139]. Children with WA mainly display moderate-to-severe atopic dermatitis; wheat ingestion may also elicit IgE-mediated urticaria, angioedema, bronchial obstruction, nausea and abdominal pain, or even severe systemic anaphylaxis [140]. In adults, the most common variant is WDEIA, where symptoms range from urticaria to systemic reactions, including anaphylaxis [141]. Multiple allergens are involved in WA [142]: sera from patients with baker’s asthma and rhinitis react with amylase inhibitors, germ agglutinin, peroxidase and non-specific lipid transfer proteins (LTPs) [143]; WDEIA is induced by ω5-gliadins [144]; IgE from patients with atopic dermatitis, urticaria and anaphylaxis are reactive with α, β, γ, ω-gliadins, and low and high molecular weight subunits. Over 50% of patients with urticaria have IgE to ω5-gliadin [145]. The first-level diagnostic tests for WA are in vitro specific immunoglobulin E (sIgE) assays and skin prick tests (SPTs), which, however, have a low predictive value. Functional tests (bronchial challenge test in baker’s asthma and food challenge in food allergy) are considered the diagnostic gold standard for WA [146], but they are impractical and potentially dangerous. Molecular-based allergy (MA) diagnostics and a flow cytometry-assisted basophil activation test (BAT), an in vitro functional test for the diagnosis of immediate type allergy for patients at risk of severe anaphylactic reactions, are a novel diagnostic approach to allergic disorders that in some cases may represent an effective alternative to the in vivo functional tests [141,146].
4.3. Non-Celiac Gluten/Wheat Sensitivity (NCG/WS)
For the sake of simplicity, the wide range of intestinal and extra-intestinal symptoms occurring after the ingestion of gluten-containing food in subjects who do not have either CD or WA has been collectively defined as NCGS, more recently renamed non-celiac wheat sensitivity (NCWS) [147,148]. The occurrence of gluten-related disturbances beyond CD was initially reported in 1980 [149] and later in 2000 [150], but it was only in 2011 that NCG/WS took center stage as part of the spectrum of gluten-related disorders [151]. Since then, rising public interest and a growing body of research have fueled constant debate regarding this issue, with an overwhelming discrepancy between media messages and scientific citations [17]. The internet, the popular press, marketing claims and celebrities endorsing their gluten-free choices represent common sources of information with no reliable scientific evidence. The clinical picture is heterogeneous and non-specific, ranging from “IBS-like” symptoms (diarrhea, constipation, bloating, nausea and epigastric pain) to extra-intestinal manifestations (malaise, anxiety, fibromyalgia, skin rash, tiredness and chronic fatigue, “foggy mind” and headache) [152]. Owing to the lack of specific biomarkers, prevalence data in the general population are highly variable, ranging between 0.6% and 10.6% [92]. Recently, in NCGS, a significant increase in anti-gliadin IgG2 antibodies was described in comparison with healthy controls and an increase in anti-gliadin IgG4 antibodies was reported in comparison with CD and healthy controls, suggesting their potential role as diagnostic biomarkers [153]. Furthermore, evidence was provided for an overexpression of selected miRNA in the intestinal mucosa and peripheral blood leukocytes (PBLs) of NCWS patients if compared to symptomatic controls with functional dyspepsia or CD. Hsa-miR-30e-5p proved to be the best predictor of NCWS vs. CD in biopsies and vs. controls in PBLs [154]. The absence of validated diagnostic criteria also explains the high rate of self-diagnosis [155,156] as well as the impact of patients’ perception of symptoms and of the nocebo effect on the interpretation of study results [157]. In an attempt to establish the actual role of gluten, double-blind placebo-controlled (DBPC) gluten challenge trials have been suggested. Molina-Infante and Carroccio, in order to evaluate the accuracy of this approach, analyzed 10 of these trials including 1312 adults. The studies were different regarding the duration of the gluten challenge (1 day–6 weeks) and the wash out period (3 days–2 weeks), gluten daily dose (2–52 g) and the kind of placebo administered (gluten-free products, xylose, whey protein, rice or corn starch containing fermentable carbohydrates). Most of the trials reported that gluten was able to significantly aggravate symptoms when compared to placebo, but only 38 out of 231 patients (16%) specifically reacted to gluten. Moreover, a nocebo effect (similar or increased symptoms after placebo administration) was observed in 40% of the patients [158]. The heterogeneity of these studies should also be highlighted because this potentially affected the results, and these data prompt some doubts as to the role of gluten as a “trigger” food of symptoms because more than 80% of NCGS diagnosed on the basis of a positive response to a GFD cannot be formally diagnosed after a DBPC trial (not performed in all the studies according to the protocol recommended by the Salerno Experts [147]) and this sheds light on the possible importance of the so-called “nocebo” effect which cannot be excluded in studies involving a dietary approach.
Apart from gluten, potential dietary triggers include non-gluten wheat components such as ATIs, WGA and fructans. ATIs are highly resistant to intestinal proteolitic degradation and have been identified as strong activators of innate immune responses in human and murine macrophages, monocytes and DCs, eliciting the release of proinflammatory cytokines via the activation of TLR4 [82]. In mice, ATIs showed an additive effect on pre-existing low-level intestinal inflammation, with stimulatory activity increasing from the proximal intestine to the ileum and colon [84]. Involvement of an adaptive immune response by the migration of DCs to mesenteric lymph nodes and interaction with primed T cells could exacerbate the ongoing inflammation [84]. In vitro studies and in vivo animal models showed that WGA induces the release of pro-inflammatory cytokines and epithelial barrier disruption [89]. In vivo human studies are needed to better support the role of ATIs and WGA as triggering factors of NCG/WS. Unlike ATIs and WGA, fructans induce a variety of IBS-like symptoms, including bloating, abdominal pain and diarrhea, due to a combination of osmotic activity with fluid retention within the intestinal lumen and gas production by fermentation [102,159]. In a DBPC study, Biesiekierski et al. reported that patients with NCGS did not exhibit statistically significant effects after gluten was added to the diet in the presence of a low content of FODMAPs, indicating that symptoms may be due to fructans rather than gluten [160]. Another DBPC crossover study in patients with self-reported NCG/WS showed that fructans (rather than gluten) were more likely to induce symptoms, with no effect of gluten challenge [161]. However, Volta et al. argued that the authors had enrolled self-diagnosed NCG/WS, some extra-GI symptoms typical of NCG/WS had not been included in the evaluation, anti-gliadin IgG antibodies had not be assessed and only the prevalence of Hashimoto’s thyroiditis had been reported as an autoimmunity marker [162,163]. Hence, numerous patients with NCG/WS could have a diagnosis of IBS.
Exposure to hidden ingredients such as chemical additives and preservatives, commonly added to processed food as antimicrobial agents or to improve appearance, flavor or texture, might contribute to generating intestinal symptoms by inducing pro-inflammatory cytokines, altering gut microbiota composition and disrupting the mucosal barrier [164].
In the absence of a definite mechanism of action, the pathogenesis of NCG/WS remains a matter of debate; IgE-independent WA involving mast cells, eosinophils and other immune cells [165] has been postulated on the basis of past or current history of food allergy [166], eosinophil infiltration of the intestinal mucosa and in vitro basophil activation induced by food antigens in patients with NCG/WS diagnosed by DBPC challenge [167]. Furthermore, an increase in mucosal lymphocytes has been detected in some NCG/WS patients [167,168]. In particular, infiltration with innate lymphocyte-1 cells producing IFN-γ and responsive to a wheat-free diet has been described in the rectal mucosa of patients [169]. Current evidence suggests a complex interplay among systemic immune response, impaired intestinal barrier function and dysbiosis [170]. The early findings of a reduced intestinal permeability [168] in NCG/WS patients have not been confirmed: further studies have definitely shown intestinal epithelial damage leading to compromised barrier function [171,172] and microbial translocation from the lumen to the intestinal mucosa, resulting in a systemic, mainly innate, immune response [173,174]. In wheat-sensitive patients, altered expression of markers of an innate immune response has been described [168,175]. Positive serology for native gliadin in a proportion of patients [176] suggested a concomitant role of an adaptive immune response [173]. In wheat-sensitive individuals, Uhde et al. demonstrated increased serum markers of systemic innate immune activation as well as B cell response to microbial antigens associated with markers of intestinal epithelial cell damage as indicators of the translocation of microbial products across the intestinal mucosa, reversible on a GFD [177]. Based on the recognized involvement of an impaired intestinal permeability in the pathogenesis of NCG/WS [112,178] and on the studies regarding the immune-stimulating activity of ATIs [82,84], a new hypothesis has been formulated implicating the Western diet and lifestyle which, inducing dysbiosis with low levels of intestinal butyrate-producing bacteria, could lead to a vicious circle involving a disrupted intestinal barrier function, microbial lipopolysaccharide (LPS), decreased intestinal alkaline phosphatase (IAP) and intact ATI translocation [85].
In recent years, the overlap between NCG/WS and IBS has drawn increasing attention [92,179]; an Italian multicenter study found IBS in about 50% of these patients [152]. With an estimated 11.2% worldwide prevalence [180], IBS is the most prevalent functional gastrointestinal disorder (FGID) [181], causing a significant impairment of patients’ quality of life and productivity with a high social and economic impact [182]. Shared symptoms between IBS and NCG/WS are abdominal pain, altered bowel habits, bloating and/or extra-intestinal symptoms [183,184,185]. Food is regarded as a precipitating factor of symptoms by many IBS patients [186,187]. In the absence of specific tests, the diagnosis of IBS [188] essentially relies on symptom assessment, standardized in the Rome IV Criteria [189]. In recent years, a low-FODMAP diet (LFD) involving a global restriction of FODMAP intake followed by gradual re-introduction, according to individual tolerance and under the supervision of an expert dietician [190], has been widely employed for IBS treatment [191] and it is currently regarded as effective in reducing IBS symptoms, according to several studies [192,193,194,195].
Unfortunately, only a few studies dealing with LFDs have been based on randomized placebo-controlled double-blind trials [190]. A recent systematic review and meta-analysis of randomized controlled trials (RCTs) examining the efficacy of an LFD and GFD in IBS provided evidence that an LFD is more effective than a GFD in reducing IBS symptoms [194], although the evidence is of very low quality. The authors justified the very low quality of evidence regarding LFD efficacy because of the heterogeneity of the studies, i.e., the different types of comparators used in the different studies and the low number of patients reporting global symptom improvement (189 out of 397 patients, whereas the GRADE system would require at least 300 patients) [196]. The authors also underlined that the problems could be solved if further trials were be carried out using similar comparator groups in order to provide more data [194]. Unfortunately, there is a problem of economic resources because it is quite difficult to find subjects interested in financing such studies [190].
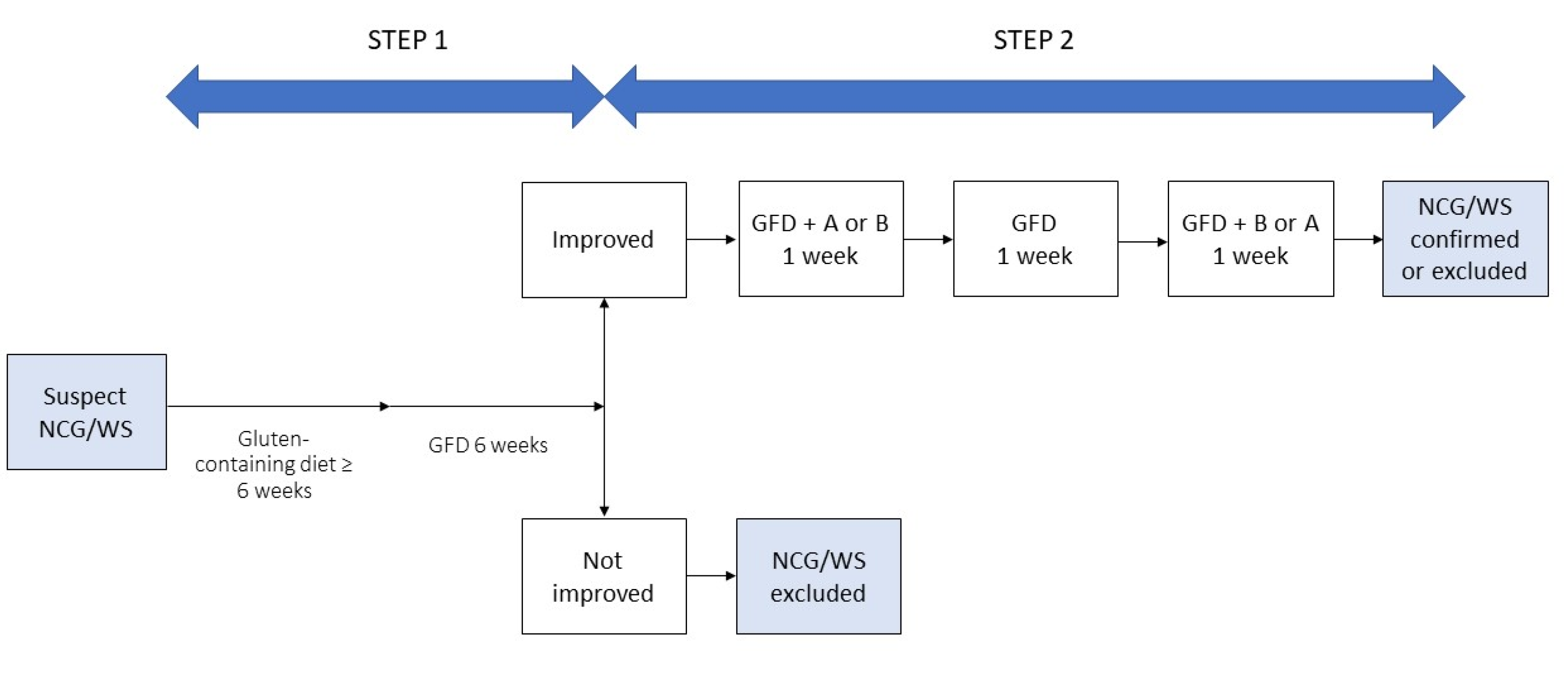
Differentiating IBS from NCG/WS can be cumbersome and needs to take into account the overlapping clinical picture, the lack of specific biomarkers, the putative role of the same dietary triggers and the influence of patients’ perceptions. Waiting for validated biomarkers able to obtain a differential diagnosis, some authors suggest that the patients’ opinions on the role of gluten in precipitating their digestive symptoms could be used as criteria to distinguish NCG/WS from IBS, although the gluten-related nocebo effect and the questionable reliability of patients in identifying the dietary culprit of their symptoms should be taken into account. With the aim of overcoming the limitation of a diagnosis merely based on exclusion criteria and to standardize the diagnostic procedure, an international group of experts elaborated the Salerno Experts’ criteria for the diagnosis of NCG/WS based on a double-step approach. In Step 1, after exclusion of CD and WA, patients start a six-week gluten-containing diet and report their symptoms according to a modified version of the Gastrointestinal Symptom Rating Scale (GSRS). Then they start a GFD for at least six weeks. A decrease of at least 30% of the baseline score is considered a positive response. Step 2 includes a one-week challenge (GFD and gluten or placebo) followed by a one-week washout of strict GFD and a crossover to the second one-week challenge. A variation of symptoms of at least 30% between gluten and placebo challenge discriminate a positive from a negative result [147] (Figure 4).
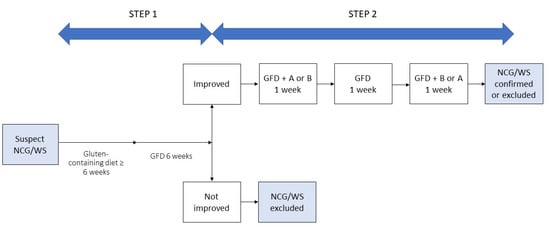
Figure 4.
Diagnosis of non-celiac gluten/wheat sensitivity (NCG/WS), modified from Catassi, 2015 [147]. GFD: gluten-free diet.
Although a DBPC approach represents the gold standard for a rigorous identification of NCG/WS [147], it is cumbersome and impractical for clinicians. Furthermore, wheat is commonly identified as a trigger when IBS patients are specifically interviewed [197,198,199]. The clinical effects of the aforementioned compounds could explain the overlapping symptoms of NCG/WS and IBS, as well as the causative role of wheat in a subgroup of IBS patients, and their symptomatic improvement after wheat elimination [92,183,200]. In this regard, the term “wheat-sensitive IBS” has been coined to describe patients who meet the Rome IV criteria for IBS and report gluten/wheat-related symptoms [92]. In everyday practice, it is not easy to clearly distinguish NCG/WS from “wheat-sensitive IBS”, which represents a “gray zone” where different concepts and symptoms can overlap. As a consequence, choosing the most appropriate dietary intervention within this overlap is challenging. However, the usefulness of such a distinction from a clinical point of view could be relatively unimportant. The GFD can be considered in patients with self-reported gluten/wheat-dependent symptoms, especially if associated with extra-intestinal manifestations, most likely not induced by fructans [6,92,201]. In non-responders or partial responders to a GFD, an LFD could be considered as a second-line treatment. Moreover, we think that in patients not reporting gluten/wheat as trigger of their symptoms and referring symptoms more related to FODMAPs other than fructans, an LFD could be the first dietary option. In any case, irrespective of the type of dietary approach, the patients’ preferences must be taken into account.
5. Conclusions
In the last 30 years, the GFD and related GF products have gained increasing popularity. These have been supported by marketing campaigns, athletes and celebrities, media messages and social networks. Nevertheless, real knowledge of gluten and GF-related implications for health is scarce in the population.
The role of potential causative factors in the increasing prevalence of CD is under debate. Modern wheat breeding practices have not been confirmed; per capita vital gluten consumption, variation in the amount and types of wheat intake and agronomic practices affecting wheat protein content have been proposed as contributors to the toxicity of wheat in genetically susceptible individuals [40]. Although general attention has focused on gluten as the only culprit of symptom occurrence in non-celiac patients on a gluten-containing diet, the role of a variety of compounds (ATIs, WGA, fructans and glyphosate) belonging to the non-gluten components of wheat appears to be prevalent.
Despite the wide acceptance of the term by the scientific community, the existence of NCG/WS as distinct entity has been questioned, and more properly could be regarded as a collective term for a variety of different conditions where gluten is directly involved only in a small minority of patients [158]. Likewise, the pathogenesis seems to be multifactorial, including innate immune response, altered mucosal barrier function and dysbiosis [202]. In the absence of specific diagnostic markers, and under the influence of marketing and media claims, a high rate of self-diagnosis occurs [152,156].
A GFD might be an appropriate dietary approach for patients with self-reported gluten/wheat-dependent symptoms. An LFD should be the first dietary option for patients referring symptoms more related to FODMAPs than gluten/wheat, and the second-line treatment for those with self-reported gluten/wheat-related symptoms who do not respond to a GFD. In any cases, a personalized approach, regular follow-up and the intervention of an expert dietician are recommended.
Author Contributions
Conceptualization, M.G.M., M.B. and A.R.; Methodology, M.G.M., M.B., F.C., L.C. and N.d.B.; Writing—original draft preparation, M.G.M., M.B. and F.R.; Review and editing of final manuscript, F.R. and S.M. (Sara Melissari); Supervision, S.M. (Santino Marchi). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to Chris Powell for the language revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rostami, K.; Bold, J.; Parr, A.; Johnson, M.W. Gluten-Free Diet Indications, Safety, Quality, Labels, and Challenges. Nutrients 2017, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets. Global Gluten-Free Food Market (2018–2023) Report; ID: 4856374; Research and Markets: Dublin, Ireland, 2019. [Google Scholar]
- Kim, H.S.; Patel, K.G.; Orosz, E.; Kothari, N.; Demyen, M.F.; Pyrsopoulos, N.; Ahlawat, S.K. Time Trends in the Prevalence of Celiac Disease and Gluten-Free Diet in the US Population. Results From the National Health and Nutrition Examination Surveys 2009–2014. JAMA Intern. Med. 2016, 176, 1716–1717. [Google Scholar] [CrossRef] [PubMed]
- NPD Group. Percentage of U.S. Adults Trying to Cut Down or Avoid Gluten in Their Diets Reaches New High in 2013. Available online: http://www.npd.com/wps/portal/npd/us/news/press-releases/percentage-of-us-adults-trying-to-cut-down-or-avoid-gluten-in-their-diets-reaches-new-high-in-2013-reports-npd (accessed on 27 June 2020).
- Gatti, S.; Lionetti, E.; Balanzoni, L.; Verma, A.K.; Galeazzi, T.; Gesuita, R.; Scattolo, N.; Cinquetti, M.; Fasano, A.; Catassi, C. Celiac Screening Team. Increased Prevalence of Celiac Disease in School-age Children in Italy. Clin. Gastroenterol. Hepatol. 2020, 18, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac gluten sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Arendt, E.K. Low FODMAPs and gluten-free foods for irritable bowel syndrome treatment: Lights and shadows. Food Res. Int. 2018, 110, 33–41. [Google Scholar] [CrossRef]
- Tanpowpong, P.; Broder-Fingert, S.; Katz, A.J.; Camargo, C.A., Jr. Predictors of dietary gluten avoidance in adults without a prior diagnosis of celiac disease. Nutrition 2015, 31, 236–238. [Google Scholar] [CrossRef]
- Lis, D.M. Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes. Sports Med. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public Health Nutr. 2015, 18, 490–499. [Google Scholar] [CrossRef]
- The Hartman Group, Inc. The Hartman Group’s Health & Wellness 2015 and Organic & Natural 2014 Reports. Available online: http://www.hartman-group.com/acumenPdfs/gluten-free-2015-09-03.pdf (accessed on 22 December 2015).
- Gorgitano, M.T.; Sodano, V. Gluten-Free Products: From Dietary Necessity to Premium Price Extraction Tool. Nutrients 2019, 11, 1997. [Google Scholar] [CrossRef]
- Zysk, W.; Głąbska, D.; Guzek, D. Role of Front-of-Package Gluten-Free Product Labeling in a Pair-Matched Study in Women with and without Celiac Disease on a Gluten-Free Diet. Nutrients 2019, 11, 398. [Google Scholar] [CrossRef]
- Hartmann, C.; Hieke, S.; Taper, C.; Siegrist, M. European consumer healthiness evaluation of ‘Free-from’ labelled food products. Food Qual. Prefer. 2018, 68, 377–388. [Google Scholar] [CrossRef]
- Di Liberto, D.; Carlisi, D.; D’Anneo, A.; Emanuele, S.; Giuliano, M.; De Blasio, A.; Calvaruso, G.; Lauricella, M. Gluten Free Diet for the Management of Non Celiac Diseases: The Two Sides of the Coin. Healthcare 2020, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, J.M.; Adriaanse, M.P.M.; van der Ploeg, E.M.C.; Vreugdenhil, A.C.E. Narrative Review: Nutrient Deficiencies in Adults and Children with Treated and Untreated Celiac Disease. Nutrients 2020, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Corazza, G.R. Nonceliac gluten sensitivity: Sense or sensibility? Ann. Intern. Med. 2012, 156, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Gastroenterol. Hepatol. 2018, 14, 82–91. [Google Scholar]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef]
- Hoffmanová, I.; Sánchez, D.; Szczepanková, A.; Tlaskalová-Hogenová, H. The Pros and Cons of Using Oat in a Gluten-Free Diet for Celiac Patients. Nutrients 2019, 11, 2345. [Google Scholar] [CrossRef]
- Lundin, K.E.; Nilsen, E.M.; Scott, H.G.; Løberg, E.M.; Gjøen, A.; Bratlie, J.; Skar, V.; Mendez, E.; Løvik, A.; Kett, K. Oats induced villous atrophy in coeliac disease. Gut 2003, 52, 1649–1652. [Google Scholar] [CrossRef]
- Arentz-Hansen, H.; Fleckenstein, B.; Molberg, Ø.; Scott, H.; Koning, F.; Jung, G.; Roepstorff, P.; Lundin, K.E.; Sollid, L.M. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004, 1, e1. [Google Scholar] [CrossRef]
- Silano, M.; Pozo, E.P.; Uberti, F.; Manferdelli, S.; Del Pinto, T.; Felli, C.; Budelli, A.; Vincentini, O.; Restani, P. Diversity of oat varieties in eliciting the early inflammatory events in celiac disease. Eur. J. Nutr. 2014, 53, 1177–1186. [Google Scholar] [CrossRef]
- Poley, J.R. The Gluten-Free Diet: Can Oats and Wheat Starch Be Part of It? J. Am. Coll. Nutr. 2017, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P. What Is Gluten-Why Is It Special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Tong, J.; Yu, L.; Ding, M.; Zhang, Z.; Rehman, A.U.; Majzoobi, M.; Wang, Z.; Gao, X. The overexpression of high-molecular-weight glutenin subunit Bx7 improves the dough rheological properties by altering secondary and micro-structures of wheat gluten. Food Res. Int. 2020, 130, 108914. [Google Scholar] [CrossRef] [PubMed]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef]
- Lerner, A.; Shoenfeld, Y.; Matthias, T. Adverse effects of gluten ingestion and advantages of gluten withdrawal in nonceliac autoimmune disease. Nutr. Rev. 2017, 75, 1046–1058. [Google Scholar] [CrossRef]
- Balfourier, F.; Bouchet, S.; Robert, S.; De Oliveira, R.; Rimbert, H.; Kitt, J.; Choulet, F.; Paux, E. International Wheat Genome Sequencing Consortium; BreedWheat Consortium. Worldwide phylogeography and history of wheat genetic diversity. Sci. Adv. 2019, 5, eaav0536. [Google Scholar] [CrossRef]
- Venske, E.; Dos Santos, R.S.; Busanello, C.; Gustafson, P.; Costa de Oliveira, A. Bread wheat: A role model for plant domestication and breeding. Hereditas 2019, 156, 16. [Google Scholar] [CrossRef]
- Van den Broeck, H.C.; de Jong, H.C.; Salentijn, E.M.; Dekking, L.; Bosch, D.; Hamer, R.J.; Gilissen, L.J.; van der Meer, I.M.; Smulders, M.J. Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: Wheat breeding may have contributed to increased prevalence of celiac disease. Theor. Appl. Genet. 2010, 12, 1527–1539. [Google Scholar] [CrossRef]
- Huo, N.; Zhang, S.; Zhu, T.; Dong, L.; Wang, Y.; Mohr, T.; Hu, T.; Liu, Z.; Dvorak, J.; Luo, M.C.; et al. Gene Duplication and Evolution Dynamics in the Homeologous Regions Harboring Multiple Prolamin and Resistance Gene Families in Hexaploid Wheat. Front. Plant Sci. 2018, 9, 673. [Google Scholar] [CrossRef]
- Huo, N.; Zhu, T.; Altenbach, S.; Dong, L.; Wang, Y.; Mohr, T.; Liu, Z.; Dvorak, J.; Luo, M.C.; Gu, Y.Q. Dynamic Evolution of α-Gliadin Prolamin Gene Family in Homeologous Genomes of Hexaploid Wheat. Sci. Rep. 2018, 8, 5181. [Google Scholar] [CrossRef] [PubMed]
- Malalgoda, M.; Meinhardt, S.W.; Simsek, S. Detection and quantitation of immunogenic epitopes related to celiac disease in historical and modern hard red spring wheat cultivars. Food Chem. 2018, 264, 101–107. [Google Scholar] [CrossRef]
- Prandi, B.; Tedeschi, T.; Folloni, S.; Galaverna, G.; Sforza, S. Peptides from gluten digestion: A comparison between old and modern wheat varieties. Food Res. Int. 2017, 91, 92–102. [Google Scholar] [CrossRef]
- Ficco, D.B.; Prandi, B.; Amaretti, A.; Anfelli, I.; Leonardi, A.; Raimondi, S.; Pecchioni, N.; De Vita, P.; Faccini, A.; Sforza, S.; et al. Comparison of gluten peptides and potential prebiotic carbohydrates in old and modern Triticum turgidum ssp. genotypes. Food Res. Int. 2019, 120, 568–576. [Google Scholar] [CrossRef]
- Ribeiro, M.; Nunes, F.M. We might have got it wrong: Modern wheat is not more toxic for celiac patients. Food Chem. 2019, 278, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Whittaker, A.; Gori, A.M.; Cesari, F.; Surrenti, E.; Abbate, R.; Gensini, G.F.; Benedettelli, S.; Casini, A. Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: A double-blinded randomised dietary intervention trial. Br. J. Nutr. 2014, 111, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Kasarda, D.D. Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? J. Agric. Food Chem. 2013, 61, 1155–1159. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Spencer, S.P.; Fragiadakis, G.K.; Sonnenburg, J.L. Pursuing Human-Relevant Gut Microbiota-Immune Interactions. Immunity 2019, 51, 225–239. [Google Scholar] [CrossRef]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, F1000 Faculty Rev-69. [Google Scholar] [CrossRef]
- Alam, A.; Neish, A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers 2018, 6, 1539595. [Google Scholar] [CrossRef] [PubMed]
- Chibbar, R.; Dieleman, L.A. The Gut Microbiota in Celiac Disease and probiotics. Nutrients 2019, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific duodenal and faecal bacterial groups associated with paediatric coeliac disease. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Reddel, S.; Putignani, L.; Del Chierico, F. The Impact of Low-FODMAPs, Gluten-Free, and Ketogenic Diets on Gut Microbiota Modulation in Pathological Conditions. Nutrients 2019, 11, 373. [Google Scholar] [CrossRef]
- Olivares, M.; Neef, A.; Castillejo, G.; Palma, G.D.; Varea, V.; Capilla, A.; Palau, F.; Nova, E.; Marcos, A.; Polanco, I.; et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 2015, 64, 406–417. [Google Scholar] [CrossRef]
- Olivares, M.; Benítez-Páez, A.; de Palma, G.; Capilla, A.; Nova, E.; Castillejo, G.; Varea, V.; Marcos, A.; Garrote, J.A.; Polanco, I.; et al. Increased prevalence of pathogenic bacteria in the gut microbiota of infants at risk of developing celiac disease: The PROFICEL study. Gut Microbes 2018, 9, 551–558. [Google Scholar] [CrossRef]
- Lebwohl, B.; Murray, J.A.; Verdú, E.F.; Crowe, S.E.; Dennis, M.; Fasano, A.; Green, P.H.; Guandalini, S.; Khosla, C. Gluten Introduction, Breastfeeding, and Celiac Disease: Back to the Drawing Board. Am. J. Gastroenterol. 2016, 111, 12–14. [Google Scholar] [CrossRef]
- Koletzko, S.; Lee, H.S.; Beyerlein, A.; Aronsson, C.A.; Hummel, M.; Liu, E.; Simell, V.; Kurppa, K.; Lernmark, Å.; Hagopian, W.; et al. Cesarean Section on the Risk of Celiac Disease in the Offspring: The Teddy Study. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 417–424. [Google Scholar] [CrossRef]
- Kołodziej, M.; Patro-Gołąb, B.; Gieruszczak-Białek, D.; Skórka, A.; Pieścik-Lech, M.; Baron, R.; Szajewska, H. Association between early life (prenatal and postnatal) antibiotic administration and coeliac disease: A systematic review. Arch. Dis. Child. 2019, 104, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, K.M.; Vehik, K.; Lynch, K.F.; Larsson, H.E.; Canepa, R.J.; Simell, V.; Koletzko, S.; Liu, E.; Simell, O.G.; Toppari, J.; et al. Association between Early-Life Antibiotic Use and the Risk of Islet or Celiac Disease Autoimmunity. JAMA Pediatr. 2017, 171, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Verdu, E.F.; Galipeau, H.J.; Jabri, B. Novel players in coeliac disease pathogenesis: Role of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 497–506. [Google Scholar] [CrossRef]
- Valitutti, F.; Cucchiara, S.; Fasano, A. Celiac Disease and the Microbiome. Nutrients 2019, 11, 2403. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Olivares, M.; Codoñer-Franch, P.; Sanz, Y. Intestinal Microbiota and Celiac Disease: Cause, Consequence or Co-Evolution? Nutrients 2015, 7, 6900–6923. [Google Scholar] [CrossRef]
- Caminero, A.; Meisel, M.; Jabri, B.; Verdu, E.F. Mechanisms by which gut microorganisms influence food sensitivities. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; Verdu, E.F. Celiac disease: Should we care about microbes? Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G161–G170. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Globalization of Food Systems in Developing Countries: Impact on Food Security and Nutrition; FAO Food and Nutrition Paper 2004, n. 83; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; pp. 1–14, ISSN 0254-4725. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Crop Breeding: The Green Revolution and the Preceding Millennia. Available online: http://www.fao.org/english/newsroom/focus/2003/gmo2.htm (accessed on 28 June 2020).
- Karim, M.B. The Green Revolution: An International Bibliography; Greenwood: Westport, CT, USA, 1986; pp. 1–28. [Google Scholar]
- Enghiad, A.; Ufer, D.; Countryman, A.M.; Thilmany, D.D. An Overview of Global Wheat Market Fundamentals in an Era of Climate Concerns. Int. J. Agron. 2017, 2017, 3931897. [Google Scholar] [CrossRef]
- Tiwari, H. Wheat Gluten Market: Global Industry Analysis 2014–2018 and Opportunity Assessment 2019–2029; Future Market Insights: London, UK, 2020. [Google Scholar]
- MarketsandMarkets. Wheat Protein Market; Report Code: FB 5949; MarketsandMarkets: Pune, India, 2018. [Google Scholar]
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-gluten uses and industry needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- Dhiraj, B.; Prabhasankar, P. Influence of Wheat-Milled Products and Their Additive Blends on Pasta Dough Rheological, Microstructure, and Product Quality Characteristics. Int. J. Food Sci. 2013, 2013, 538070. [Google Scholar] [CrossRef]
- Giannou, V.; Tzia, C. Addition of Vital Wheat Gluten to Enhance the Quality Characteristics of Frozen Dough Products. Foods 2016, 5, 6. [Google Scholar] [CrossRef]
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Curr. Pharm. Des. 2003, 9, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Udenigwe, C.C. Role of food-derived opioid peptides in the central nervous and gastrointestinal systems. J. Food Biochem. 2019, 43, e12629. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.; Fletcher, P.J.; Nobrega, J.N.; Remington, G. Behavioral effects of food-derived opioid-like peptides in rodents: Implications for schizophrenia? Pharmacol. Biochem. Behav. 2015, 134, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.J.; Grace, M.; Cleary, J.P.; Billington, C.J.; Levine, A.S. Potency of naloxone’s anorectic effect in rats is dependent on diet preference. Am. J. Physiol. 1996, 271, R217–R221. [Google Scholar] [CrossRef] [PubMed]
- Boggiano, M.M.; Chandler, P.C.; Viana, J.B.; Oswald, K.D.; Maldonado, C.R.; Wauford, P.K. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav. Neurosci. 2005, 119, 1207–1214. [Google Scholar] [CrossRef]
- Morley, J.E.; Levine, A.S.; Yamada, T.; Gebhard, R.L.; Prigge, W.F.; Shafer, R.B.; Goetz, F.C.; Silvis, S.E. Effect of exorphins on gastrointestinal function, hormonal release, and appetite. Gastroenterology 1983, 84, 1517–1523. [Google Scholar] [CrossRef]
- Corazza, G.R.; Frazzoni, M.; Strocchi, A.; Prati, C.; Sarchielli, P.; Capelli, M. Alimentary exorphin actions on motility and hormonal secretion of gastrointestinal tract. In Opioid Peptides in the Periphery; Fraioli, F., Isidori, A., Mazzetti, M., Eds.; Elsevier Sciences Publisher: Amsterdam, NL, USA, 1984; pp. 243–247. [Google Scholar]
- Takahashi, M.; Fukunaga, H.; Kaneto, H.; Fukudome, S.; Yoshikawa, M. Behavioral and pharmacological studies on gluten exorphin A5, a newly isolated bioactive food protein fragment, in mice. Jpn. J. Pharmacol. 2000, 84, 259–265. [Google Scholar] [CrossRef]
- Bressan, P.; Kramer, P. Bread and Other Edible Agents of Mental Disease. Front. Hum. Neurosci. 2016, 10, 130. [Google Scholar] [CrossRef]
- Dupont, F.M.; Vensel, W.H.; Tanaka, C.K.; Hurkman, W.J.; Altenbach, S.B. Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Sci. 2011, 9, 10. [Google Scholar] [CrossRef]
- Guo, G.; Lv, D.; Yan, X.; Subburaj, S.; Ge, P.; Li, X.; Hu, Y.; Yan, Y. Proteome characterization of developing grains in bread wheat cultivars (Triticum aestivum L.). BMC Plant Biol. 2012, 12, 147. [Google Scholar] [CrossRef]
- Finnie, C.; Melchior, S.; Roepstorff, P.; Svensson, B. Proteome analysis of grain filling and seed maturation in barley. Plant Physiol. 2002, 129, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; McCarville, J.L.; Zevallos, V.F.; Pigrau, M.; Xuechen, B.Y.; Jury, J.; Galipeau, H.J.; Clarizio, A.V.; Casqueiro, J.; Murray, J.A.; et al. Lactobacilli Degrade Wheat Amylase Trypsin Inhibitors to Reduce Intestinal Dysfunction Induced by Immunogenic Wheat Proteins. Gastroenterology 2019, 156, 2266–2280. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Russel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Leccioli, V.; Oliveri, M.; Romeo, M.; Berretta, M.; Rossi, P. A New Proposal for the Pathogenic Mechanism of Non-Coeliac/Non-Allergic Gluten/Wheat Sensitivity: Piecing Together the Puzzle of Recent Scientific Evidence. Nutrients 2017, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.J.; Winter, H.C.; Poretz, R.D. Glicoproteins II. Wheat germ agglutinin. New Comprehensive. Biochemistry 1997, 29 Pt B, 403–474. [Google Scholar]
- Peumans, W.J.; Van Damme, E.J.M. Prevalence, biological activity and genetic manipulation of lectins in foods. Trends Food Sci. Technol. 1996, 7, 132–138. [Google Scholar] [CrossRef]
- Haas, H.; Falcone, F.H.; Schramm, G.; Haisch, K.; Gibbs, B.F.; Klaucke, J.; Poppelmann, M.; Becker, W.M.; Gabius, H.J.; Schlaak, M. Dietary lectins can induce in vitro release of IL-4 and IL-13 from human basophils. Eur. J. Immunol. 1999, 29, 918–927. [Google Scholar] [CrossRef]
- Dalla Pellegrina, C.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef]
- Muraille, E.; Pajak, B.; Urbain, J.; Leo, O. Carbohydrate-bearing cell surface receptors involved in innate immunity: Interleukin-12 induction by mitogenic and nonmitogenic lectins. Cell Immunol. 1999, 191, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, A.; Kesherwani, V. Production of TNF-alpha, IL-1beta, IL-12 and IFN-gamma in murine peritoneal macrophages on treatment with wheat germ agglutinin in vitro: Involvement of tyrosine kinase pathways. Glycoconj. J. 2007, 24, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The overlapping area of non-celiac gluten sensitivity (NCGS) and wheat-sensitive irritable bowel syndrome (IBS): An update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef]
- Tchernychev, B.; Wilchek, M. Natural human antibodies to dietary lectins. FEBS Lett. 1996, 397, 139–142. [Google Scholar] [CrossRef]
- Sollid, L.M.; Kolberg, J.; Scott, H.; Ek, J.; Fausa, O.; Brandtzaeg, P. Antibodies to wheat germ agglutinin in coeliac disease. Clin. Exp. Immunol. 1986, 63, 95–100. [Google Scholar]
- Troncone, R.; Auricchio, S.; De Vincenzi, M.; Donatiello, A.; Farris, E.; Silano, V. An analysis of cereals that react with serum antibodies in patients with coeliac disease. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 346–350. [Google Scholar] [CrossRef]
- Volta, U.; Lazzari, R.; Bianchi, F.B.; Lenzi, M.; Baldoni, A.M.; Cassani, F.; Collina, A.; Pisi, E. Antibodies to dietary antigens in coeliac disease. Scand. J. Gastroenterol. 1986, 21, 935–940. [Google Scholar] [CrossRef]
- Pusztai, A.; Ewen, S.W.; Grant, G.; Brown, D.S.; Stewart, J.C.; Peumans, W.J.; Van Damme, E.J.; Bardocz, S. Antinutritive effects of wheat- germ agglutinin and other N-acetylglucosamine-specific lectins. Br. J. Nutr. 1993, 70, 313–321. [Google Scholar] [CrossRef]
- Lorenzsonn, V.; Olsen, W.A. In vivo responses of rat intestinal epithelium to intraluminal dietary lectins. Gastroenterology 1982, 82, 838–848. [Google Scholar] [CrossRef]
- Gibson, P.R.; Muir, J.G.; Newnham, E.D. Other Dietary Confounders: FODMAPS et al. Dig. Dis. 2015, 33, 269–276. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought—Western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Bazzichi, L.; Bassotti, G.; Mumolo, M.G.; Fani, B.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; et al. Bioelectrical impedance vector analysis in patients with irritable bowel syndrome on a low FODMAP diet: A pilot study. Tech. Coloproctol. 2017, 21, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; de Bari, O.; Lembo, A.; Ballou, S. Irritable bowel syndrome and diet. Gastroenterol. Rep. 2017, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; Ghanati, F.; Gavlighi, H.A.; Sharifi, M. Fructan dynamics and antioxidant capacity of 4-day-old seedlings of wheat (Triticum aestivum) cultivars during drought stress and recovery. Funct. Plant Biol. 2018, 45, 1000–1008. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of fructans, galacto-oligosaccharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Abrahmsohn, O.; David, G.J.; Staudacher, H.; Irving, P.; Lomer, M.C.; Ellis, P.R. Fructan content of commonly consumed wheat, rye and gluten-free breads. Int. J. Food Sci. Nutr. 2011, 62, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate, pathways to modern diseases II: Celiac sprue and gluten intolerance. Interdiscip. Toxicol. 2013, 6, 159–184. [Google Scholar] [CrossRef]
- Mesnage, R.; Teixeira, M.; Mandrioli, D.; Falcioni, L.; Ducarmon, Q.R.; Zwittink, R.D.; Amiel, C.; Panoff, J.M.; Belpoggi, F.; Antoniou, M.N. Shotgun metagenomics and metabolomics reveal glyphosate alters the gut microbiome of Sprague-Dawley rats by inhibiting the shikimate pathway. BioRxiv 2019. [Google Scholar] [CrossRef]
- Chłopecka, M.; Mendel, M.; Dziekan, N.; Karlik, W. Glyphosate affects the spontaneous motoric activity of intestine at very low doses—In vitro study. Pestic. Biochem. Physiol. 2014, 113, 25–30. [Google Scholar] [CrossRef]
- Vasiluk, L.; Pinto, L.J.; Moore, M.M. Oral bioavailability of glyphosate: Studies using two intestinal cell lines. Environ. Toxicol. Chem. 2005, 24, 153–160. [Google Scholar] [CrossRef]
- Drago, S.; El Asmar, R.; Di Pierro, M.; Grazia Clemente, M.; Tripathi, A.; Sapone, A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2006, 41, 408–419. [Google Scholar] [CrossRef]
- Dubois, P.C.; Trynka, G.; Franke, L.; Hunt, K.A.; Romanos, J.; Curtotti, A.; Zhernakova, A.; Heap, G.A.; Adány, R.; Aromaa, A.; et al. Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 2010, 42, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Stene, L.C.; Honeyman, M.C.; Hoffenberg, E.J.; Haas, J.E.; Sokol, R.J.; Emery, L.; Taki, I.; Norris, J.M.; Erlich, H.A.; Eisenbarth, G.S.; et al. Rotavirus Infection Frequency and Risk of Celiac Disease Autoimmunity in Early Childhood: A Longitudinal Study. Am. J. Gastroenterol. 2006, 101, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, R.; Cielo, D.; de Falco, R.; Galatola, M.; Bruno, V.; Malamisura, B.; Limongelli, M.G.; Troncone, R.; Greco, L. Respiratory Infections and the Risk of Celiac Disease. Pediatrics 2017, 140, e20164102. [Google Scholar] [CrossRef]
- Caminero, A.; McCarville, J.L.; Galipeau, H.J.; Deraison, C.; Bernier, S.P.; Constante, M.; Rolland, C.; Meisel, M.; Murray, J.A.; Yu, X.B.; et al. Duodenal bacterial proteolytic activity determines sensitivity to dietary antigen through protease-activated receptor-2. Nat. Commun. 2019, 10, 1198. [Google Scholar] [CrossRef]
- Pozo-Rubio, T.; Olivares, M.; Nova, E.; De Palma, G.; Mujico, J.R.; Ferrer, M.D.; Marcos, A.; Sanz, Y. Immune development and intestinal microbiota in celiac disease. Clin. Dev. Immunol. 2012, 2012, 654143. [Google Scholar] [CrossRef][Green Version]
- Olivares, M.; Walker, A.W.; Capilla, A.; Benítez-Páez, A.; Palau, F.; Parkhill, J.; Castillejo, G.; Sanz, Y. Gut Microbiota Trajectory in Early Life May Predict Development of Celiac Disease. Microbiome 2018, 6, 36. [Google Scholar] [CrossRef]
- Rintala, A.; Riikonen, I.; Toivonen, A.; Pietilä, S.; Munukka, E.; Pursiheimo, J.P.; Elo, L.L.; Arikoski, P.; Luopajärvi, K.; Schwab, U.; et al. Early fecal microbiota composition in children who later develop celiac disease and associated autoimmunity. Scand. J. Gastroenterol. 2018, 53, 403–409. [Google Scholar] [CrossRef]
- Lionetti, E.; Castellaneta, S.; Francavilla, R.; Pulvirenti, A.; Tonutti, E.; Amarri, S.; Barbato, M.; Barbera, C.; Barera, G.; Bellantoni, A.; et al. SIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD Risk. Introduction of gluten, HLA status, and the risk of celiac disease in children. N. Engl. J. Med. 2014, 371, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, C.A.; Lee, H.S.; Liu, E.; Uusitalo, U.; Hummel, S.; Yang, J.; Hummel, M.; Rewers, M.; She, J.X.; Simell, O.; et al. Age at gluten introduction and risk of celiac disease. Pediatrics 2015, 135, 239–245. [Google Scholar] [CrossRef]
- Leonard, M.M.; Fasano, A. Gluten and celiac disease risk: Is it just a matter of quantity? JAMA 2019, 322, 510–511. [Google Scholar] [CrossRef]
- Lund-Blix, N.A.; Mårild, K.; Tapia, G.; Norris, J.M.; Stene, L.C.; Størdal, K. Gluten Intake in Early Childhood and Risk of Celiac Disease in Childhood: A Nationwide Cohort Study. Am. J. Gastroenterol. 2019, 114, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Dydensborg Sander, S.; Hansen, A.V.; Størdal, K.; Andersen, A.N.; Murray, J.A.; Husby, S. Mode of delivery is not associated with celiac disease. Clin. Epidemiol. 2018, 10, 323–332. [Google Scholar] [CrossRef]
- Sander, S.D.; Andersen, A.M.; Murray, J.A.; Karlstad, Ø.; Husby, S.; Størdal, K. Association Between Antibiotics in the First Year of Life and Celiac Disease. Gastroenterology 2019, 156, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Vilppula, A.; Luostarinen, L.; Holmes, G.K.T.; Kaukinen, K. Review article: Coeliac disease in later life must not be missed. Aliment Pharmacol. Ther. 2018, 47, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Denham, J.M.; Hill, I.D. Celiac disease and autoimmunity: Review and controversies. Curr. Allergy Asthma Rep. 2013, 13, 347–353. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Eigner, W.; Bashir, K.; Primas, C.; Kazemi-Shirazi, L.; Wrba, F.; Trauner, M.; Vogelsang, H. Dynamics of occurrence of refractory coeliac disease and associated complications over 25 years. Aliment Pharmacol. Ther. 2017, 45, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Rosado, M.M.; Cazzola, P.; Riboni, R.; Biagi, F.; Carsetti, R.; Corazza, G.R. Splenic Hypofunction and the Spectrum of Autoimmune and Malignant Complications in Celiac Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 179–186. [Google Scholar] [CrossRef]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008, 121, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Bockelbrink, A.; Beyer, K.; Keil, T.; Niggemann, B.; Grüber, C.; Wahn, U.; Lau, S. Primary versus secondary immunoglobulin E sensitization to soy and wheat in the Multi-Centre Allergy Study cohort. Clin. Exp. Allergy 2008, 38, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Keet, C.A.; Matsui, E.C.; Dhillon, G.; Lenehan, P.; Paterakis, M.; Wood, R.A. The natural history of wheat allergy. Ann. Allergy Asthma Immunol. 2009, 102, 410–415. [Google Scholar] [CrossRef]
- Devonshire, A.L.; Durrani, S.; Assa‘ad, A. Non-IgE mediated food allergy during infancy. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 292–298. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef]
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat Allergy in Children: A Comprehensive Update. Medicina 2019, 55, 400. [Google Scholar] [CrossRef]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K.A.; Brockow, K.; Biedermann, T.; Koehler, P.; Wieser, H. Wheat-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2016, 46, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.S.; Shewry, P.R. Allergens to wheat and related cereals. Clin. Exp. Allergy 2008, 38, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, G.; Quirce, S.; Diaz-Perales, A. Wheat allergens associated with Baker’s asthma. J. Investig. Allergol. Clin. Immunol. 2011, 21, 81–94. [Google Scholar]
- Le, T.A.; Al Kindi, M.; Tan, J.A.; Smith, A.; Heddle, R.J.; Kette, F.E.; Hissaria, P.; Smith, W.B. The clinical spectrum of omega-5-gliadin allergy. Intern. Med. J. 2016, 46, 710–716. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Jiang, N.N.; Wen, L.P.; Li, H.; Yin, J. Wheat allergy in patients with recurrent urticaria. World Allergy Organ. J. 2019, 12, 100013. [Google Scholar] [CrossRef]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Dolan, R.; Chey, W.D.; Eswaran, S. The role of diet in the management of irritable bowel syndrome: A focus on FODMAPs. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 607–615. [Google Scholar] [CrossRef]
- Cooper, B.T.; Holmes, G.K.; Ferguson, R.; Thompson, R.A.; Allan, R.N.; Cooke, W.T. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology 1980, 79, 801–806. [Google Scholar] [CrossRef]
- Kaukinen, K.; Turjanmaa, K.; Mäki, M.; Partanen, J.; Venäläinen, R.; Reunala, T.; Collin, P. Intolerance to cereals is not specific for coeliac disease. Scand. J. Gastroenterol. 2000, 35, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabro, A.; Troncone, R.; Corazza, G.R. Study Group for Non-Celiac Gluten Sensitivity. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Caio, G.; De Giorgio, R.; Green, P.H.; Volta, U.; Alaedini, A. Subclass Profile of IgG Antibody Response to Gluten Differentiates Nonceliac Gluten Sensitivity From Celiac Disease. Gastroenterology 2020, 159, 1965–1967. [Google Scholar] [CrossRef] [PubMed]
- Clemente, E.; Efthymakis, K.; Carletti, E.; Capone, V.; Sperduti, S.; Bologna, G.; Marchisio, M.; Di Nicola, M.; Neri, M.; Sallese, M. An explorative study identifies miRNA signatures for the diagnosis of non-celiac wheat sensitivity. PLoS ONE 2019, 14, e0226478. [Google Scholar] [CrossRef]
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfield, S.N.; Rugg, N.; Kelsall, A.; Newrick, L.; Sanders, D.S. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur. J. Gastroenterol. Hepatol. 2014, 26, 33–39. [Google Scholar] [CrossRef]
- Carroccio, A.; Giambalvo, O.; Blasca, F.; Iacobucci, R.; D’Alcamo, A.; Mansueto, P. Self-Reported Non-Celiac Wheat Sensitivity in High School Students: Demographic and Clinical Characteristics. Nutrients 2017, 9, 771. [Google Scholar] [CrossRef]
- Khan, A.; Suarez, M.G.; Murray, J.A. Nonceliac Gluten and Wheat Sensitivity. Clin. Gastroenterol. Hepatol. 2020, 18, 1913–1922. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients After Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef]
- Ong, D.K.; Mitchell, S.B.; Barrett, J.S.; Shepherd, S.J.; Irving, P.M.; Biesiekierski, J.R.; Smith, S.; Gibson, P.R.; Muir, J.G. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2010, 25, 1366–1373. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No Effects of Gluten in Patients with Self-Reported Non-Celiac Gluten Sensitivity After Dietary Reduction of Fermentable, Poorly Absorbed, Short-Chain Carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology 2018, 154, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Caio, G.; De Giorgio, R. More Than One Culprit for Nonceliac Gluten/Wheat Sensitivity. Gastroenterology 2018, 155, 227. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Henriksen, C.; Veierød, M.B.; Gibson, P.R.; Lundin, K.E.A. More Than One Culprit for Nonceliac Gluten/Wheat Sensitivity Reply. Gastroenterology 2018, 155, 228. [Google Scholar] [CrossRef] [PubMed]
- Laudisi, F.; Stolfi, C.; Monteleone, G. Impact of Food Additives on Gut Homeostasis. Nutrients 2019, 1, 2334. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.; Crowe, S.E. Gastrointestinal food allergy: New insights into pathophysiology and clinical perspectives. Gastroenterology 2005, 128, 1089–1113. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; D’Alcamo, A.; Iacono, G. Non-celiac wheat sensitivity as an allergic condition: Personal experience and narrative review. Am. J. Gastroenterol. 2013, 108, 1845–1853. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2012, 107, 1898–1906. [Google Scholar] [CrossRef]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.T.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC Med. 2011, 9, 23. [Google Scholar] [CrossRef]
- Di Liberto, D.; Mansueto, P.; D’Alcamo, A.; Lo Pizzo, M.; Lo Presti, E.; Geraci, G.; Fayer, F.; Guggino, G.; Iacono, G.; Dieli, F.; et al. Predominance of type 1 innate lymphoid cells in the rectal mucosa of patients with non-celiac wheat sensitivity: Reversal after a wheat-free diet. Clin. Transl. Gastroenterol. 2016, 7, e178. [Google Scholar] [CrossRef]
- Volta, U.; Caio, G.; Karunaratne, T.B.; Alaedini, A.; De Giorgio, R. Non-coeliac gluten/wheat sensitivity: Advances in knowledge and relevant questions. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Röcken, C.; Brasch, J.; Bethge, J.; Böttner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef]
- Volta, U.; De Giorgio, R.; Caio, G.; Uhde, M.; Manfredini, R.; Alaedini, A. Nonceliac Wheat Sensitivity: An Immune-Mediated Condition with Systemic Manifestations. Gastroenterol. Clin. N. Am. 2019, 48, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Rotondi Aufiero, V.; Fasano, A.; Mazzarella, G. Non-Celiac Gluten Sensitivity: How Its Gut Immune Activation and Potential Dietary Management Differ from Celiac Disease. Mol. Nutr. Food Res. 2018, 62, e1700854. [Google Scholar] [CrossRef] [PubMed]
- Vazquez–Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Tovoli, F.; Cicola, R.; Parisi, C.; Fabbri, A.; Piscaglia, M.; Fiorini, E.; Caio, G. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J. Clin. Gastroenterol. 2012, 46, 680–685. [Google Scholar] [CrossRef]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Hollon, J.; Puppa, E.L.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015, 7, 1565–1576. [Google Scholar] [CrossRef]
- Usai-Satta, P.; Bassotti, G.; Bellini, M.; Oppia, F.; Lai, M.; Cabras, F. Irritable Bowel Syndrome and Gluten-Related Disorders. Nutrients 2020, 12, 1117. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Patel, N.K. Rome criteria and a diagnostic approach to irritable bowel syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable bowel syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef]
- De Giorgio, R.; Volta, U.; Gibson, P.R. Sensitivity to wheat, gluten and FODMAPs in IBS: Facts or fiction? Gut 2016, 65, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Czaja-Bulsa, G. Non coeliac gluten sensitivity—A new disease with gluten intolerance. Clin. Nutr. 2015, 34, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Makharia, A.; Catassi, C.; Makharia, G.K. The overlap between irritable bowel syndrome and non-celiac gluten sensitivity: A clinical dilemma. Nutrients 2015, 7, 10417–10426. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.A.; Fraher, M.H.; Quigley, E.M. Irritable bowel syndrome: The role of food in pathogenesis and management. Gastroenterol. Hepatol. 2014, 10, 164–174. [Google Scholar]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-Related Gastrointestinal Symptoms in the Irritable Bowel Syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable bowel syndrome: A disease still searching for pathogenesis, diagnosis and therapy. World J. Gastroenterol. 2014, 20, 8807–8820. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R. History of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32, 5–7. [Google Scholar] [CrossRef]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Morganti, R.; Pancetti, A.; Bertani, L.; de Bortoli, N.; Costa, F.; Mosca, M.; Marchi, S.; et al. A Low-FODMAP Diet for Irritable Bowel Syndrome: Some Answers to the Doubts from a Long-Term Follow-Up. Nutrients 2020, 12, 2360. [Google Scholar] [CrossRef]
- AtkinsD, B.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association systematic review and evidence based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 549–575. [Google Scholar] [CrossRef]
- Morcos, A.; Dinan, T.; Quigley, E.M. Irritable bowel syndrome: Role of food in pathogenesis and management. J. Dig. Dis. 2009, 10, 237–246. [Google Scholar] [CrossRef]
- Bohn, L.; Storsrud, S.; Tornblom, H.; Bengtsson, U.; Simren, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.L.; Vazquez-Roque, M.I.; Carlson, P.; Burton, D.; Grover, M.; Camilleri, M.; Turner, J.R. Gluten-induced symptoms in diarrhea-predominant irritable bowel syndrome are associated with increased myosin light chain kinase activity and claudin-15 expression. Lab. Investig. 2017, 97, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Nardelli, A.; Borojevic, R.; De Palma, G.; Calo, N.C.; McCarville, J.; Caminero, A.; Basra, D.; Mordhorst, A.; Ignatova, E.; et al. Gluten-free Diet Reduces Symptoms, Particularly Diarrhea, in Patients With Irritable Bowel Syndrome and Anti-gliadin IgG. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Caio, G.; Tovoli, F.; De Giorgio, R. Non-celiac gluten sensitivity: Questions still to be answered despite increasing awareness. Cell Mol. Immunol. 2013, 10, 383–392. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).