Effects of Anthocyanins in Composite Meals on Cardiometabolic Outcomes—A Systematic Review of Randomized Controlled Feeding Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction
2.3. Quality Assessment Criteria
3. Results
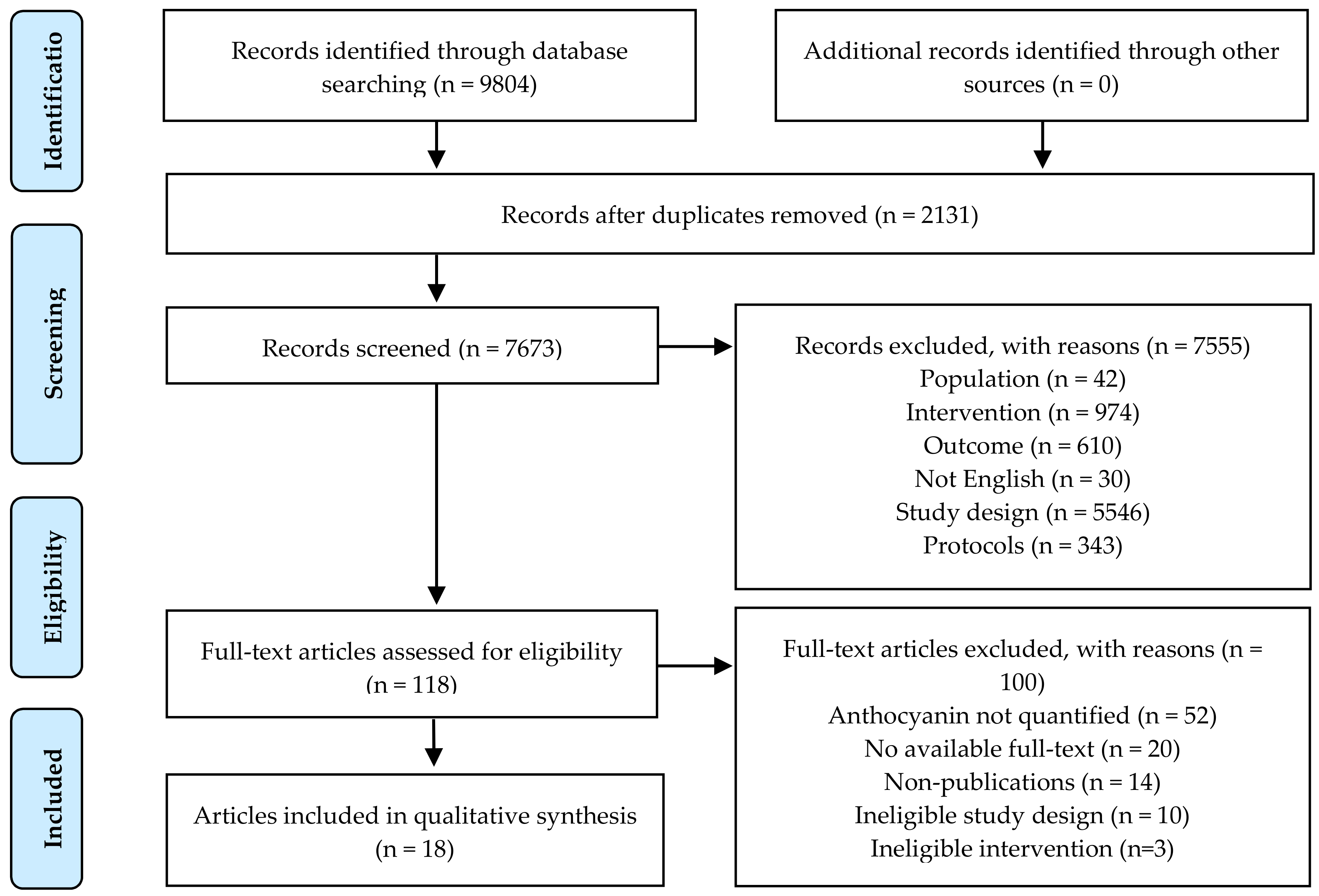
3.1. Literature Search and Study Selection
3.2. Study Characteristics
3.2.1. Population Characteristics
3.2.2. Study Design
3.2.3. Intervention Characteristics
3.2.4. Control Groups
3.2.5. Outcome Measurements
3.3. Quality Assessment
3.4. Energy Metabolism
3.5. Vascular Function
3.6. Incretins
3.7. Inflammation
3.8. Oxidative Stress and Antioxidant Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De la Iglesia, R.; Loria-Kohen, V.; Zulet, M.A.; Martinez, J.A.; Reglero, G.; Molina, A.R.D. Dietary Strategies Implicated in the Prevention and Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, X.; Zhang, Y.; Zhou, W. Bread fortified with anthocyanin-rich extract from black rice as nutraceutical sources: Its quality attributes and in vitro digestibility. Food Chem. 2016, 196, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Liu, C.; Sun, J.; Lu, Y.; Bo, Y. Effects of Anthocyanin on Serum Lipids in Dyslipidemia Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0162089. [Google Scholar] [CrossRef]
- Wallace, T.C.; Slavin, M.; Frankenfeld, C.L. Systematic Review of Anthocyanins and Markers of Cardiovascular Disease. Nutrients 2016, 8, 32. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Fatehi, P.; Jafari, T. Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food Chem. Toxicol. 2020, 135, 110922. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.0; Cochrane: London, UK, 2019. [Google Scholar]
- Richter, C.K.; Skulas-Ray, A.C.; Gaugler, T.L.; Lambert, J.D.; Proctor, D.N.; Kris-Etherton, P.M. Incorporating freeze-dried strawberry powder into a high-fat meal does not alter postprandial vascular function or blood markers of cardiovascular disease risk: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.; Edirisinghe, I.; Wei, H.; Vijayakumar, L.P.; Banaszewski, K.; Cappozzo, J.C.; Burton-Freeman, B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol. Nutr. Food Res. 2016, 60, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.; Betts, N.M.; Lyons, T.J.; Basu, A. Raspberries improve postprandial glucose and acute and chronic inflammation in adults with type 2 diabetes. Ann. Nutr. Metab. 2019, 74, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhu, L.; Edirisinghe, I.; Fareed, J.; Brailovsky, Y.; Burton-Freeman, B. Attenuation of Postmeal Metabolic Indices with Red Raspberries in Individuals at Risk for Diabetes: A Randomized Controlled Trial. Obesity 2019, 27, 542–550. [Google Scholar] [CrossRef]
- Blacker, B.C.; Snyder, S.M.; Eggett, D.L.; Parker, T.L. Consumption of blueberries with a high-carbohydrate, low-fat breakfast decreases postprandial serum markers of oxidation. Br. J. Nutr. 2013, 109, 1670–1677. [Google Scholar] [CrossRef]
- Prior, R.L.; Gu, L.; Wu, X.; Jacob, R.A.; Sotoudeh, G.; Kader, A.A.; Cook, R.A. Plasma antioxidant capacity changes following a meal as a measure of the ability of a food to alter in vivo antioxidant status. J. Am. Coll. Nutr. 2007, 26, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Park, E.; Edirisinghe, I.; Burton-Freeman, B.M. Maximizing the health effects of strawberry anthocyanins: Understanding the influence of the consumption timing variable. Food Funct. 2016, 7, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Marsella, L.T.; Carraro, A.; Valente, R.; Gualtieri, P.; Gratteri, S.; Tomasi, D.; Gaiotti, F.; De Lorenzo, A. Changes in LDL Oxidative Status and Oxidative and Inflammatory Gene Expression after Red Wine Intake in Healthy People: A Randomized Trial. Mediat. Inflamm. 2015, 2015, 317348. [Google Scholar] [CrossRef]
- Miglio, C.; Peluso, I.; Raguzzini, A.; Villano, D.V.; Cesqui, E.; Catasta, G.; Toti, E.; Serafini, M. Fruit juice drinks prevent endogenous antioxidant response to high-fat meal ingestion. Br. J. Nutr. 2014, 111, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I.; Raguzzini, A.; Roberts, S.; Villano, D.; Cesqui, E.; Catasca, G.; Toti, E.; Miglio, C. High fat meal increase of il-17 is prevented by ingestion of fruit juice drink in healthy overweight subjects. Ann. Nutr. Metab. 2011, 58, 269. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Stone, S.G.; Mok, J.E.; Mhajan, R.K.; Fu, C.I.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Apple and blackcurrant polyphenol-rich drinks decrease postprandial glucose, insulin and incretin response to a high-carbohydrate meal in healthy men and women. J. Nutr. Biochem. 2017, 49, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, S.M.; Spencer, J.P.E.; Lovegrove, J.A. Acute impact of Hibiscus sabdariffa calyces on postprandial lipids, biomarkers of insulin resistance and inflammation in humans. Proc. Nutr. Soc. 2019, 75, E109. [Google Scholar] [CrossRef] [Green Version]
- Urquiaga, I.; Avila, F.; Echeverria, G.; Perez, D.; Trejo, S.; Leighton, F. A Chilean Berry Concentrate Protects against Postprandial Oxidative Stress and Increases Plasma Antioxidant Activity in Healthy Humans. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, J.; Flores, C.; Hidalgo, M.A.; Perez, M.; Yañez, A.; Quiñones, L.; Caceres, D.D.; Burgos, R.A. Delphinol® standardized maqui berry extract reduces postprandial blood glucose increase in individuals with impaired glucose regulation by novel mechanism of sodium glucose cotransporter inhibition. Panminerva Med. 2014, 56, 1–7. [Google Scholar]
- Stote, K.; Corkum, A.; Sweeney, M.; Shakerley, N.; Kean, T.; Gottschall-Pass, K. Postprandial Effects of Blueberry (Vaccinium angustifolium) Consumption on Glucose Metabolism, Gastrointestinal Hormone Response, and Perceived Appetite in Healthy Adults: A Randomized, Placebo-Controlled Crossover Trial. Nutrients 2019, 11, 202. [Google Scholar] [CrossRef] [Green Version]
- Murkovic, M.; Abuja, P.M.; Bergmann, A.R.; Zirngast, A.; Adam, U.; Winklhofer-Roob, B.M.; Toplak, H. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: A randomized, double-blind, placebo-controlled study. Eur. J. Clin. Nutr. 2004, 58, 244–249. [Google Scholar] [CrossRef]
- Salvatore, T.; Nevola, R.; Pafundi, P.C.; Monaco, L.; Ricozzi, C.; Imbriani, S.; Rinaldi, L.; Sasso, F.C. Incretin Hormones: The Link between Glycemic Index and Cardiometabolic Diseases. Nutrients 2019, 11, 1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Davy, K.P.; Davy, B.M. Advances in Nutrition Science and Integrative Physiology: Insights From Controlled Feeding Studies. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brnčić, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Mok, A.; Haldar, S.; Lee, J.C.-Y.; Leow, M.K.-S.; Henry, C.J. Postprandial changes in cardiometabolic disease risk in young Chinese men following isocaloric high or low protein diets, stratified by either high or low meal frequency a randomized controlled crossover trial. Nutr. J. 2016, 15, 27. [Google Scholar] [CrossRef] [Green Version]
- Jacome-Sosa, M.; Parks, E.J.; Bruno, R.S.; Tasali, E.; Lewis, G.F.; Schneeman, B.O.; Rains, T.M. Postprandial Metabolism of Macronutrients and Cardiometabolic Risk: Recent Developments, Emerging Concepts, and Future Directions. Adv. Nutr. 2016, 7, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A. The post-prandial state and cardiovascular disease: Relevance to diabetes mellitus. Diabetes/Metab. Res. Rev. 2000, 16, 125–132. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bell, D.S.H. Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) Is a Cardiovascular Risk Factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef]
- Astley, C.M.; Todd, J.N.; Salem, R.M.; Vedantam, S.; Ebbeling, C.B.; Huang, P.L.; Ludwig, D.S.; Hirschhorn, J.N.; Florez, J.C. Genetic Evidence That Carbohydrate-Stimulated Insulin Secretion Leads to Obesity. Clin. Chem. 2018, 64, 192–200. [Google Scholar] [CrossRef]
- Blaak, E.E.; Antoine, J.M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2012, 13, 923–984. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istas, G.; Feliciano, R.P.; Weber, T.; Garcia-Villalba, R.; Tomas-Barberan, F.; Heiss, C.; Rodriguez-Mateos, A. Plasma urolithin metabolites correlate with improvements in endothelial function after red raspberry consumption: A double-blind randomized controlled trial. Arch. BioChem. Biophys. 2018, 651, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chusak, C.; Thilavech, T.; Henry, C.J.; Adisakwattana, S. Acute effect of Clitoria ternatea flower beverage on glycemic response and antioxidant capacity in healthy subjects: A randomized crossover trial. BMC Complement. Altern Med. 2018, 18, 6. [Google Scholar] [CrossRef]
- Moser, S.; Aragon, I.; Furrer, A.; Van Klinken, J.W.; Kaczmarczyk, M.; Lee, B.H.; George, J.; Hamaker, B.R.; Mattes, R.; Ferruzzi, M.G. Potato phenolics impact starch digestion and glucose transport in model systems but translation to phenolic rich potato chips results in only modest modification of glycemic response in humans. Nutr. Res. 2018, 52, 57–70. [Google Scholar] [CrossRef]
- Shah, K.; Shah, P. Effect of Anthocyanin Supplementations on Lipid Profile and Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cholesterol 2018, 2018, 8450793. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, J.; Lu, W.; Wang, X.; Wang, X.; Han, Z.; Qiu, C. Effects of blueberry supplementation on blood pressure: A systematic review and meta-analysis of randomized clinical trials. J. Hum. Hypertens. 2017, 31, 165–171. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar] [CrossRef] [Green Version]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef]
- Kan, L.; Oliviero, T.; Verkerk, R.; Fogliano, V.; Capuano, E. Interaction of bread and berry polyphenols affects starch digestibility and polyphenols bio-accessibility. J. Funct. Foods 2020, 68, 103924. [Google Scholar] [CrossRef]
- Hassimotto, N.M.; Pinto Mda, S.; Lajolo, F.M. Antioxidant status in humans after consumption of blackberry (Rubus fruticosus L.) juices with and without defatted milk. J. Agric. Food Chem. 2008, 56, 11727–11733. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Testa, M.F.; Villaño, D.; Pecorari, M.; van Wieren, K.; Azzini, E.; Brambilla, A.; Maiani, G. Antioxidant activity of blueberry fruit is impaired by association with milk. Free. Radic. Biol. Med. 2009, 46, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef] [Green Version]
- Bonta, D.V.; Brandon, D.C.; Hernandez, J.; Patel, M.; Grant, S.; Alazraki, N. Clinical intervention for quality improvement of gastric-emptying studies. J. Nucl. Med. Technol. 2014, 42, 274–277. [Google Scholar] [CrossRef] [Green Version]
- Erk, T.; Williamson, G.; Renouf, M.; Marmet, C.; Steiling, H.; Dionisi, F.; Barron, D.; Melcher, R.; Richling, E. Dose-dependent absorption of chlorogenic acids in the small intestine assessed by coffee consumption in ileostomists. Mol. Nutr. Food Res. 2012, 56, 1488–1500. [Google Scholar] [CrossRef]
- Cassidy, A.; Brown, J.E.; Hawdon, A.; Faughnan, M.S.; King, L.J.; Millward, J.; Zimmer-Nechemias, L.; Wolfe, B.; Setchell, K.D. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J. Nutr. 2006, 136, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.A.; Ashburn, L.A.; Kakazu, K.; Suzuki, S.; Wilkens, L.R.; Halm, B.M. Apparent bioavailability of isoflavones after intake of liquid and solid soya foods. Br. J. Nutr. 2009, 102, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Barik, S.K.; Russell, W.R.; Moar, K.M.; Cruickshank, M.; Scobbie, L.; Duncan, G.; Hoggard, N. The anthocyanins in black currants regulate postprandial hyperglycaemia primarily by inhibiting alpha-glucosidase while other phenolics modulate salivary alpha-amylase, glucose uptake and sugar transporters. J. Nutr. Biochem. 2020, 78, 108325. [Google Scholar] [CrossRef]
- Bitsch, R.; Netzel, M.; Frank, T.; Strass, G.; Bitsch, I. Bioavailability and Biokinetics of Anthocyanins From Red Grape Juice and Red Wine. J. Biomed. Biotechnol. 2004, 2004, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells—Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.L.; Dragsted, L.O.; Ravn-Haren, G.; Freese, R.; Rasmussen, S.E. Absorption and excretion of black currant anthocyanins in humans and watanabe heritable hyperlipidemic rabbits. J. Agric. Food Chem. 2003, 51, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, K.; Courtet-Compondu, M.C.; Williamson, G.; Rezzi, S.; Kussmann, M.; Rytz, A. Non-covalent binding of proteins to polyphenols correlates with their amino acid sequence. Food Chem. 2012, 132, 1333–1339. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Qi, B.; Sui, X.; Jiang, L. Complexation of thermally-denatured soybean protein isolate with anthocyanins and its effect on the protein structure and in vitro digestibility. Food Res. Int. 2018, 106, 619–625. [Google Scholar] [CrossRef]
- Xiao, D.; Sandhu, A.; Huang, Y.; Park, E.; Edirisinghe, I.; Burton-Freeman, B.M. The effect of dietary factors on strawberry anthocyanins oral bioavailability. Food Funct. 2017, 8, 3970–3979. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Roopchand, D.E.; Oren, A.; Grace, M.; Poulev, A.; Lila, M.A.; Havenaar, R.; Raskin, I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem. 2014, 142, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Sengul, H. Investigating the effects of food matrix and food components on bioaccessibility of pomegranate (Punica granatum) phenolics and anthocyanins using an in-vitro gastrointestinal digestion model. Food Res. Int. 2014, 62, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Mullen, W.; Edwards, C.A.; Serafini, M.; Crozier, A. Bioavailability of Pelargonidin-3-O-glucoside and Its Metabolites in Humans Following the Ingestion of Strawberries with and without Cream. J. Agric. Food Chem. 2008, 56, 713–719. [Google Scholar] [CrossRef]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milenkovic, D.; Morand, C.; Cassidy, A.; Konic-Ristic, A.; Tomás-Barberán, F.; Ordovas, J.M.; Kroon, P.; De Caterina, R.; Rodriguez-Mateos, A. Interindividual Variability in Biomarkers of Cardiometabolic Health after Consumption of Major Plant-Food Bioactive Compounds and the Determinants Involved. Adv. Nutr. 2017, 8, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.; Geleijnse, J.M.; de Roos, B. Inter-Individual Variation in Cancer and Cardiometabolic Health Outcomes in Response to Coffee Consumption: A Critical Review. Mol. Nutr. Food Res. 2020, 64, e1900479. [Google Scholar] [CrossRef] [PubMed]
| Reference | Country | Study Design | Subjects; M/F | Mean Age (SD) | Health Status | Intervention Length | %C/P/F; Meal Type; Meal Components | Anthocyanin Source | Dose | Control | Markers |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Murkovic et al., 2004 [32] | Austria | R/C/DB/P | 34; 20/14 | Elderberry: 30 (6) Control: 28 (4) | Healthy | 2 weeks | Breakfast: -; HC; coffee, bread rolls, butter, jam Lunch & dinner: 45/20/35; HC/MF; - (meals prepared at a local restaurant, standardized for nutrient distribution) | Gel capsules of spray-dried elderberry juice | 300 mg | Placebo gel capsules | TG, TC, HDL, LDL |
| R/C/DB/X | 6; - | 29 (5.1) | Healthy | 6 h | 18/11/71; HC; - | Gel capsules of spray-dried elderberry juice | 400 mg | Placebo gel capsules | TG, TC, Apo-B | ||
| Prior et al., 2007 [16] (Study #5) | USA | R/C/X | 6; 0/6 | 46.3 (5.6) | Healthy | 4 h | 80/7/13; HC; coconut milk, coffee creamer, ProMod protein powder, sugar, water, Polycose powder | Freeze-dried grape powder | 53 mg | No freeze-dried grape powder | ORAC |
| Edirisinghe et al., 2011 [17] | USA | R/C/SB/X | 24; 10/14 | 50.9 (15) | Overweight | 6 h | 56/15/29; HC; bagel, cream cheese, margarine, hard-boiled egg, cantaloupe, whole milk | Beverage containing freeze-dried strawberry powder | 39 mg | Calorie-matched strawberry-flavored beverage with no extract | GLU, INS, IL-6, IL1-b, TNF-⍺, CRP, PAI-1 |
| Serafini et al., 2011 [24] | Italy | R/C/DB/X | 14; 12/2 | 45.1 (8.6) | Overweight | 8 h | 30/15/55; HF; fried potatoes, fried eggs, cheese, white bread | Mixture of pineapple, plum, and blackcurrant juices | 32 mg | Calorie-matched placebo beverage | GLU, INS, TG, TC, APN, IL-6, IL-17, TNF-⍺, RARR2 |
| Blacker et al., 2013 [18] | USA | R/C/X | 15; - | 22.2 | Healthy | 3 h | -; HC; corn flakes, milk | Freeze-dried blueberry powder dissolved in water | 75, 161 mg | Calorie-matched control powder dissolved in water | GLU, ox-LDL, UA, ORAC |
| Hidalgo et al., 2014 [29] | Chile | R/C/DB/X | 10; - | - | - | 3 h | -; HC; boiled rice | Delphinol® tablet dissolved in water | 70 mg | Commercial instant powdered sugar-free berry juice with artificial coloring | GLU, INS |
| Miglio et al., 2014 [25] | Italy | R/C/DB/X | 14; - | 45 (9) | Overweight | 24 h | 29/15/55; HF; fried potatoes, fried eggs, cheese, white bread | Mixture of pineapple, plum, and blackcurrant juices | 32 mg | Calorie-matched placebo beverage | F2-iso, UA, thiols, TRAP |
| Di Renzo et al., 2015 [26] | Italy | R/C/X | 24; - | 31 (5,9) | Healthy | 3 h | 28/18/54; HF; McDonald’s meal comprising the Big Tasty Bacon burger and French Fries | Red wine | 541 mg/kg berry | Negative control: red wine only Positive control: test meal only | ox-LDL |
| Castro-Acosta et al., 2016 [12] | UK | R/C/DB/X | 22; 13/9 | 45.4 (13.7) | Healthy | 2 h | 92/-/-; HC; Sliced white bread, apricot jam | Beverage containing blackcurrant extract | 150, 300, 600 mg | Calorie-matched beverage with no extract | BP, DVP-SI, DVP-RI, GLU, INS, TG, GIP, GLP-1, NEFA, F2-iso |
| Huang et al., 2016 [19] | USA | R/C/SB/P | 24; 16/8 | Strawberry: 25 (4) Control: 27 (4) | Healthy | 10 h | 46/10/44; HC/HF; croissant, apple jelly, butter, frosted flake cereal, milk, breakfast sausage | Beverage containing freeze-dried strawberry powder | 49 mg | Calorie-matched control beverage | GLU, INS, TG, ox-LDL, IL-6 |
| Park et al., 2016 [20] | USA | R/C/SB/X | 21, 5/16 | 39.8 (13.8) | Obese with insulin resistance | 6 h | 61/15/24, HC | Beverage containing freeze-dried strawberry powder | 42, 88, 155 mg | Calorie-matched beverage with no powder | Glu, Ins, TG, ox-LDL, IL-6 |
| Castro-Acosta et al., 2017 [27] | UK | R/C/DB/X | 25; 20/5 | 32.3 (14.4) | Healthy | 2 h | 82/-/-; HC; white bread, apricot jam | Beverage containing blackcurrant and apple extracts | 600 mg | Calorie-matched beverage with no extract | BP, DVP-SI, DVP-RI, GLU, INS, TG, GIP, NEFA, CRP |
| Richter et al., 2017 [21] | USA | R/C/SB/X | 30; 17/13 | 28 (2) | Healthy, overweight, and obese | 4 h | 42/13/45; HF; cheese blintzes, heavy whipped cream, strawberry-flavored syrup, hard-boiled egg, bacon | Freeze-dried strawberry powder | 163 mg | Calorie-matched placebo powder with strawberry flavoring | AI, AP, PWV, BP, GLU, INS, TG, MDA, ox-LDL |
| Urquiaga et al., 2017 [30] | Chile | R/C/X | 9; 9/0 | 20 | Healthy | 6 h | -; HF; ground turkey leg meat burger | Chilean berry concentrate | Beverage: 90 mg; burger: 84.3 mg | Water | GLU, TG, FRAP, DPPH, CO, MDA, |
| Abubakar et al., 2019 [28] | UK | R/C/SB/X | 25; 25/0 | 49 (2) | 1–10% CVD risk in 10 years, as determined by QRISK 2 | 4 h | 37/4/59; HF; buttered croissant, butter, honey, | Hibiscus beverage | 150 mg | Water | AI, BP, HR, PP, FMD, NO2, NO3, NOx, GLU, INS, TG, NEFA, CRP, TAC |
| Schell et al., 2019 [22] | USA | R/C/X | 25; 5/20 | 54 (4.2) | Obese and diabetic | 4 h | 23/13/64; HF; scrambled eggs, butter, hash brown potatoes, buttermilk biscuits, sausage patty | Frozen red raspberries | 225 mg | Calorie and carbohydrate-matched control | BP, LAEI, SAEI, GLU, INS, TG, TC, HDL, LDL, IL-6, IL-1b, TNF-⍺, CRP, PAI-1, |
| Stote et al., 2019 [31] | Canada | R/C/X | 17; 4/13 | 47 (15) | Healthy | 2 h | 76/5/19; HC; waffles, maple syrup | Whole blueberries | 401 mg | Calorie-matched placebo gel | GLU, INS, PPY, GIP, GLP-1, PYY |
| Xiao et al., 2019 [23] | USA | R/C/SB/X | 21; 12/9 | All: 34 (12) PreDM + IR: 38 (13) | PreDM + IR | 24 h | 57/9/34; HC/MF; bagel, cream cheese, butter, cereal, whole milk | Frozen red raspberries | 73, 146 mg | No raspberries | GLU, INS, TG, ox-LDL, IL-6, IL-10 |
| R/C/SB/X | 11; 5/6 | All: 34 (12) Healthy: 28 (6) | Healthy | 24 h | 57/9/34; HC/MF; bagel, cream cheese, butter, cereal, whole milk | Frozen red raspberries | 73, 146 mg | No raspberries | GLU, INS, TG |
| Reference | Random Sequence Generation | Allocation Concealment | Selective Reporting | Blinding (Participants and Personnel) | Blinding (Outcome Assessment) | Incomplete Outcome Data |
|---|---|---|---|---|---|---|
| Murkovic et al., 2004 [32] | U | U | L | L | U | L |
| Prior et al., 2007 [16] | U | U | L | L | U | L |
| Edirisinghe et al., 2011 [17] | U | U | L | L | L | L |
| Serafini et al., 2011 [24] | U | U | L | U | U | L |
| Blacker et al., 2013 [18] | U | U | L | U | L | L |
| Hidalgo et al., 2014 [29] | L | L | L | L | L | L |
| Miglio et al., 2014 [25] | U | U | L | U | L | L |
| Di Renzo et al., 2015 [26] | L | U | L | U | L | L |
| Castro-Acosta et al., 2016 [12] | L | L | L | L | L | L |
| Huang et al., 2016 [19] | L | U | L | L | U | L |
| Park et al., 2016 [20] | L | L | L | L | L | L |
| Castro-Acosta et al., 2017 [27] | L | L | L | L | L | L |
| Richter et al., 2017 [21] | L | L | L | L | L | L |
| Urquiaga et al., 2017 [30] | U | U | L | L | U | L |
| Abubakar et al., 2019 [28] | L | U | L | L | U | L |
| Schell et al., 2019 [22] | U | U | L | U | U | L |
| Stote et al., 2019 [31] | L | U | L | L | L | L |
| Xiao et al., 2019 [23] | L | U | L | L | L | L |
| Reference | Markers | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| GLU | INS | TG | TC | HDL | LDL | NEFA | APN | Apo-B | |
| Murkovic et al., 2004 [32] (2 weeks) | - | - | NS | NS | - | - | - | - | NS |
| Murkovic et al., 2004 [32] (6 h) | - | - | NS | NS | NS | NS | - | - | - |
| Edirisinghe et al., 2011 [17] | NS | ↓ | - | - | - | - | - | - | - |
| Serafini et al., 2011 [24] | NS | NS | NS | NS | - | - | - | NS | - |
| Blacker et al., 2013 [18] | NS | - | - | - | - | - | - | - | - |
| Hidalgo et al., 2014 [29] | ↓ conc. at 60 and 90 min | ↓ conc. at 60 min | - | - | - | - | - | - | - |
| Castro-Acosta et al., 2016 [12] | ↓ IAUC 0–30 min (600 mg) ↓ conc. 10–30 min and 75 min (600 mg) | ↓ IAUC 0–30 min (600 mg) ↓ conc. 10–30 min and 75 min (600 mg) | NS | - | - | - | NS | - | - |
| Huang et al., 2016 [19] | ↓ AUC 0–10 h (before- and after-meal groups only) | NS | NS | - | - | - | - | - | - |
| Park et al., 2016 [20] | NS | ↓ peak insulin (155 mg only ↓ conc. 0–6 h (155 mg) | NS | - | - | - | - | - | - |
| Castro-Acosta et al., 2017 [27] | ↓ IAUC 0–30 min only | NS | NS | - | - | - | ↓ conc. 60–90 min only | - | - |
| Richter et al., 2017 [21] | NS | NS | NS | - | - | - | - | - | - |
| Urquiaga et al., 2017 [30] | NS | - | NS | - | - | - | - | - | - |
| Abubakar et al., 2019 [28] | NS | NS | NS | - | - | - | NS | - | - |
| Schell et al., 2019 [22] | ↓ AUC ↓ conc at 4 h | NS | NS | NS | NS | NS | - | - | - |
| Stote et al., 2019 [31] | NS | NS | - | - | - | - | - | - | - |
| Xiao et al., 2019 [23] (healthy) | ↑ conc. at 2 h only | NS | NS | - | - | - | - | - | - |
| Xiao et al., 2019 [23] (PreDM) | ↓ peak glucose (146 mg) | ↓ | NS | - | - | - | - | - | - |
| Reference | Markers | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | DVP-SI | DVP-RI | AI | AP | PWV | HR | PP | FMD | LAEI | SAEI | NO2 | NO3 | NOx | |
| Castro-Acosta et al., 2016 [12] | NS | NS | NS | NS | - | - | - | - | - | - | - | - | - | - | - |
| Castro-Acosta et al., 2017 [27] | NS | NS | NS | NS | - | - | - | - | - | - | - | - | - | - | - |
| Richter et al., 2017 [21] | NS | - | - | - | NS | NS | NS | - | - | - | - | - | - | - | - |
| Abubakar et al., 2019 [28] | NS | NS | - | - | NS | - | - | NS | NS | ↑ %FMD 0–4 h | - | - | ↑ plasma conc. 0–4 h | ↑ urinary conc. at 4 h only | NS |
| Schell et al., 2019 [22] | NS | NS | - | - | - | - | - | - | - | - | NS | NS | - | - | - |
| Reference | Markers | |||
|---|---|---|---|---|
| PPY | GIP | GLP-1 | PYY | |
| Castro-Acosta et al., 2016 [12] | - | ↓ IAUC 0–2 h (600 mg) ↓ conc. 0–2 h (600 mg) ↓ peak GIP (600 mg) | ↓ conc. at 90 min only (600 mg) | - |
| Castro-Acosta et al., 2017 [27] | - | ↓ IAUC 0–30 min | - | - |
| Stote et al., 2019 [31] | ↑ conc. 30–120 min | NS | NS | NS |
| Reference | Markers | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ox-LDL | IL-6 | IL-17 | IL-1b | IL-10 | TNF-⍺ | CRP | PAI-1 | RARR2 | |
| Edirisinghe et al., 2011 [17] | - | ↓ conc. at 6 h only | - | NS | - | NS | ↓ mean 6 h-conc. | NS | - |
| Serafini et al., 2011 [24] | - | ↓ conc. 30–120 min | ↓ conc. at 4 h and 8 h | - | - | ↓ conc. 0.5–8 h | - | - | NS |
| Blacker et al., 2013 [18] | ↓ AUC 0–3 h | - | - | - | - | - | - | - | - |
| Di Renzo et al., 2015 [26] | NS | - | - | - | - | - | - | - | - |
| Huang et al., 2016 [19] | NS | ↓ AUC 0–10 h (before- and after-meal groups only) | - | - | - | - | - | - | - |
| Park et al., 2016 [20] | ↓ conc. 0–6 h (88mg) | NS | - | - | - | - | - | - | - |
| Castro-Acosta et al., 2017 [27] | - | - | - | - | - | - | ↓ IAUC 0–2 h | - | - |
| Richter et al., 2017 [21] | NS | - | - | - | - | - | - | - | - |
| Abubakar et al., 2019 [28] | - | - | - | - | - | - | NS | - | - |
| Schell et al., 2019 [22] | - | ↓ conc. at 4 h only | - | NS | - | ↓ conc. at 4 h only | NS | NS | - |
| Xiao et al., 2019 [23] (PreDM) | NS | NS | - | - | NS | - | - | - | - |
| Reference | Markers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORAC | FRAP | TRAP | DPPH | TAC | MDA | F2-iso | UA | SH | CO | |
| Prior et al., 2007 [16] | ↑ AUC 0–4 h | - | - | - | - | - | - | - | - | - |
| Blacker et al., 2013 [18] | ↑ AUC 0–1 h | - | - | - | - | - | - | NS | - | - |
| Miglio et al., 2014 [25] | - | - | NS | - | - | - | NS | ↓ conc. at 8 h only | ↓ conc. at 2 h, 4 h and 8 h only | - |
| Castro-Acosta et al., 2016 [12] | - | - | - | - | - | - | NS | - | - | - |
| Park et al., 2016 [20] | NS | - | - | - | - | - | - | - | - | - |
| Richter et al., 2017 [21] | - | - | - | - | - | NS | - | - | - | - |
| Urquiaga et al., 2017 [30] | - | NS | - | ↑ AUC 0–6 h | - | ↓ AUC 0–6 h | - | - | - | ↓ AUC 0–6 h |
| Abubakar et al., 2019 [28] | - | - | - | - | ↑ AUC 0–2 h | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, J.L.S.; Yang, D.; Liu, M.H. Effects of Anthocyanins in Composite Meals on Cardiometabolic Outcomes—A Systematic Review of Randomized Controlled Feeding Trials. Nutrients 2020, 12, 3781. https://doi.org/10.3390/nu12123781
Ou JLS, Yang D, Liu MH. Effects of Anthocyanins in Composite Meals on Cardiometabolic Outcomes—A Systematic Review of Randomized Controlled Feeding Trials. Nutrients. 2020; 12(12):3781. https://doi.org/10.3390/nu12123781
Chicago/Turabian StyleOu, Jun Leong Sean, Dimeng Yang, and Mei Hui Liu. 2020. "Effects of Anthocyanins in Composite Meals on Cardiometabolic Outcomes—A Systematic Review of Randomized Controlled Feeding Trials" Nutrients 12, no. 12: 3781. https://doi.org/10.3390/nu12123781





