Abstract
Nitrate supplementation is an effective, evidence-based dietary strategy for enhancing sports performance. The effects of dietary nitrate seem to be mediated by the ability of oral bacteria to reduce nitrate to nitrite, thus increasing the levels of nitrite in circulation that may be further reduced to nitric oxide in the body. The gut microbiota has been recently implicated in sports performance by improving muscle function through the supply of certain metabolites. In this line, skeletal muscle can also serve as a reservoir of nitrate. Here we review the bacteria of the oral cavity involved in the reduction of nitrate to nitrite and the possible changes induced by nitrite and their effect on gastrointestinal balance and gut microbiota homeostasis. The potential role of gut bacteria in the reduction of nitrate to nitrite and as a supplier of the signaling molecule nitric oxide to the blood circulation and muscles has not been explored in any great detail.
1. Introduction
Recent studies describing the improvements in skeletal muscle function induced by nitrate supplementation have provided some evidence implicating the oral and gut microbiota as mediators in the underlying mechanisms. Yet, very little is known about how nitrate intake can enhance sports/exercise performance, and further investigation is needed to minimize the potential health risks associated with the dietary consumption of nitrate-containing foods. The efficacy of acute nitrate supplementation seems to be dependent on many factors such as age, health, diet, and fitness/training status, and it has the overall ability to rapidly influence vascular tone and peripheral tissue oxygenation [1,2,3]. The effect of longer-term nitrate supplementation for sports performance is, however, unclear. With regards to its impact on indices of exercise performance in healthy volunteers, the literature seems consistent in reporting that 2–6 days (or up to 15 days) of nitrate supplementation can increase sports performance [3].
Nitrate is naturally present in vegetables, particularly leafy vegetables, and both nitrate and nitrite are used as additives in processed meats. Historically, exogenous nitrate and nitrate have been considered as environmental pollutants and a potential source of health problems, as they can lead to the formation of probable carcinogenic N-nitrosamines [4,5], which are common in nitrite/nitrate-treated meat and poultry products. Accordingly, several restrictions have been placed upon the levels of acceptable nitrate, particularly in processed foods and cured meat [6]. In contrast to exogenous nitrate, many studies (reviewed in [7]) point to endogenous nitrate as an active component in vegetables that contributes to the beneficial health effects of this food group including protection against cardiovascular disease and type II diabetes. Regarding the intake of dietary nitrate/nitrate, both the former Scientific Committee on Food of the European Commission and the Joint FAO/WHO Expert Committee on Food Additives have established that the acceptable daily intake is 3.7 mg/kg bw/day [8]. The expert consensus is that nitrate supplementation with vegetable products such as beetroot juice is likely not harmful. Dietary nitrate is a precursor of the signaling molecule nitric oxide (NO), and has been recently identified both as a therapeutic agent [9] and an ergogenic aid [10]. Indeed, according to the Sports Supplement Framework developed by the Australian Sports Institute, which categorizes supplements into four categories based on scientific evidence (A, B, C, and D), nitrate has been considered as category A (a performance supplement) [11]. This is supported by other studies from the International Olympic Committee [10] and our own laboratory [12]. As mentioned earlier, the biological mechanisms by which dietary nitrate exerts its ergogenic effects is through direct vasodilatation, which promotes muscle blood flow, increasing oxygen consumption efficiency [13,14] and decreasing blood pressure [15,16,17,18]. It is known that dietary nitrate reduces the oxygen cost during physical activity and improves exercise tolerance [19,20,21]. A proposed mechanism to explain this effect is the improvement in mitochondrial efficiency. Indeed, nitrate affects basal mitochondrial function by enhancing oxidative phosphorylation efficiency (amount of oxygen consumed per ATP produced, P/O ratio), which correlates with the reduction of the oxygen cost during exercise [14].
A recent systematic review [19] suggested that nitrate supplementation (mainly tested in the form of beetroot juice) improves cardiorespiratory endurance in athletes by increasing skeletal muscle efficiency in oxygen uptake. Nitrate supplementation also enhances performance at various distances, increases the time to exhaustion at submaximal intensities, and may also improve cardiorespiratory performance at anaerobic threshold intensities and maximum oxygen uptake (VO2max). The ergogenic effects of nitrate have been demonstrated not only in endurance and submaximal exercises [13,22,23], but also in resistance-based exercises [12].
It has been extensively discussed that nitrate plays an important effect after exercise, being necessary to induce the vascular response in the first period of recovery by lowering blood pressure and stimulating greater skeletal muscle oxygenation [24]. Of note, the biological effects of dietary nitrate can be suppressed by the use of oral anti-bacterial mouthwash, which has been shown to reduce plasma nitrite levels [25,26,27,28] and to increase the risk of hypertension [29].
Several studies have hypothesized an important role for the oral microbiota in the health benefits of nitrogen-based dietary supplementation [30,31], but its specific role in the ergogenic effects of nitrate remains unclear. Nitrate is actively absorbed by the salivary glands and concentrated in saliva where it is secreted into the oral cavity and partially reduced to nitrite, a process that involves facultative anaerobic bacteria found in the deep clefts on the dorsal surface of the tongue [32]. Select oral bacteria contain specific nitrate reductase enzymes that use nitrate as an alternative electron acceptor to oxygen during respiration.
The gut microbiota also seems to be involved in sports performance. For example, meta-omics analysis of the microbiota of elite athletes has identified a performance-enhancing bacterial genus that enhances sports performance via lactate metabolism [33]. In this regard, modulation of the gut microbiota by prebiotics, probiotics, and antibiotics has gained recent attention as being potentially effective for a variety of conditions and also as a sports performance enhancer [34,35]. If the oral and gut microbiota are involved in the nitrite-enhancing functions in sports performance, the possibility of modifying the microbiota to increase its nitrate-to-nitrite conversion, and therefore NO signaling, through diet or the use of supplements, opens a new avenue in the field of sports performance.
The objective of this narrative review is to assess the evidence for a role of the oral and gut microbiota in the enhanced sports performance mediated by dietary nitrate.
2. Nitrate Reduction in the Oral Cavity: The Role of Oral Microbiota
The nitrate—nitrite–NO pathway is an alternative system to the classical L-arginine–nitric oxide synthase (NOS) pathway for the generation of NO, supporting and complementing canonical NOS-dependent NO generation [36]. Dietary nitrate is derived mainly from green leafy vegetables such as beetroot, spinach, rocket, kale, celery, fennel and lettuce [37]. Oral bacteria use nitrate and nitrite as final electron acceptors in respiration, aiding the host in the conversion of nitrate to NO. Nitrate is first reduced to nitrite by commensal oral bacteria and, once absorbed into the blood circulation, nitrite is reduced to NO. Nitric oxide synthesis is important in the regulation of vascular tone and blood pressure, by promoting a reduction in vascular tone and blood pressure and improving the bioavailability of NO in different tissues and organs [36,37,38]. Nitric oxide production in the mouth is likely not relevant from a physiological point of view, because even if some oral bacteria do reduce nitrite to NO in saliva, the enzymatic process is slow and nitrite is rapidly extruded through continuous swallowing [39].
The oral microbiota is the second most diverse microbial community in the human body, and comprises 50–100 billion bacteria from more than 700 prokaryotic taxa, including a variety of facultative anaerobic bacteria with nitrate reductase activity [40]. Many of these bacteria also contain reductases that enable denitrification of nitrate to, ultimately, nitrogen gas (N2) through NO and nitrous oxide (N2O) formation [41]. Several bacterial species are strongly implicated in the reduction of nitrate to nitrite. The first identified nitrite-producing bacteria, Staphylococcus sciuri, Staphylococcus intermedius, Pasteurella spp., and Streptococcus spp., were isolated from the tongue of adult rats [42]. Recently, bacteria from the genus Streptococcus, including Streptococcus salivarius, S. mitis, S. bovis have been reported from human saliva [43]. Veillonella, Actinomyces, Rothia, Staphylococcus, Corynebacterium and Propionibacterium are also important nitrite-producing bacteria, with Veillonella being the most abundant group isolated from the tongue of adults, followed by Actinomyces [39]. Hyde et al. [44] confirmed Veillonella spp. as the most abundant nitrate-reducing genus in the tongue but also detected Prevotella, Neisseria, and Haemophilus at a higher abundance than Actinomyces spp. Veillonella spp. was also reported as the most abundant oral nitrate reducer in the elderly, indicating that its prevalence in the oral cavity does not change with age [45]. A recent study of oral nitrate-reducing bacteria in healthy individuals confirmed the occurrence of Prevotella, Veillonella and Haemophilus, but also detected Neisseria and Rothia, with Prevotella melaninogenica and Veillonella dispar as the most prevalent [46]. A study carried out in infants identified Prevotella and Veillonella but also Alloprevotella and Leptotrichia with oral nitrate-reducing activity [47] (Figure 1).
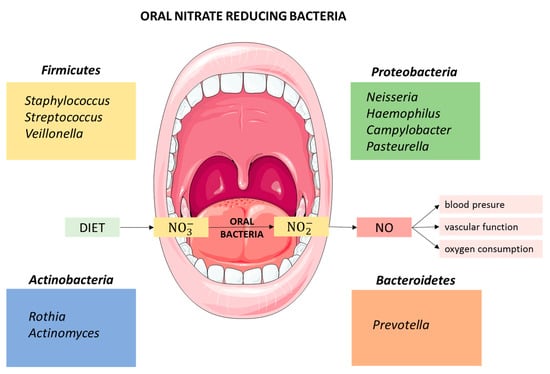
Figure 1.
Relevant genera of nitrate-reducing bacteria isolated from the oral cavity.
Nitrate supplementation induces significant changes in the oral microbiome. Dietary sodium nitrate was found to increase the abundance of the nitrate-reducing genera Streptococcus and Haemophilus on the tongue of Wistar rats [48]. Similarly, in patients with hypercholesterolemia, nitrate supplementation (beetroot juice) significantly increased the abundance of Neisseria flavescens and Rothia mucilaginosa in the oral microbiome [18]. A similar result was reported by Vanthalo et al. [49], who also detected a decrease in the abundance of Prevotella and Veillonella after nitrate supplementation, which appears to contradict previous studies identifying key oral nitrate-reducing taxa. This could indicate that the increased nitrate availability in the oral cavity may not facilitate the growth of all nitrate-reducing bacteria. Thus, although the abundance of oral nitrate-producers contributes to the regulation of NO bioavailability [46], the total metabolic activity of the nitrate-reducing bacteria might be more important than the individual abundance of each bacterial species involved in this activity [44]. A summary of the studies identifying oral nitrate reducing bacteria is shown in Table 1.

Table 1.
Summary of studies identifying oral nitrate-reducing bacteria with or without nitrate supplementation.
3. Gastrointestinal Implications of Dietary Nitrate: A Potential Role of the Gut Microbiota
There is clinical evidence supporting the view that salivary nitrite is important for protection against gastrointestinal diseases. It has been known for many years that antibiotic treatments that inhibit nitrite-producing bacteria in the mouth increase the susceptibility to gastroenteritis [50], and that microbiota dysbiosis can be rescued by dietary nitrate following antibiotic therapy. It has also been reported that the conversion of nitrate to nitrite in the oral cavity “fuels” an important mammalian resistance mechanism against infectious diseases [30], which is controlled by the conversion of nitrite to antimicrobial NO in the acidic stomach. In fact, NO is generated in the human stomach at high concentrations, and its production is dependent on gastric acidity and involves the reduction of salivary-derived nitrite. High concentrations of NO are known to be bactericidal, and this could be a first-line defense against ingested pathogens. Another proposed role for gastric NO is in the regulation of mucosal blood flow and mucus production, two important protective mechanisms for gastric mucosal integrity [51,52]. This is consistent with the observation that the gastric levels of the tight junction proteins occludin and claudin are reduced following microbiota dysbiosis, and are recovered by increasing nitrate intake [53]. There is also evidence that the conversion of nitrate into oxides of nitrogen can prevent the formation of carcinogenic nitrosamines [50] and exert preventive and therapeutic effects in the colonic epithelium in addition to the stomach. This may involve the preservation of an intact adherent mucus layer, which is important to shield the colonic mucosa from bacterial infiltration, and the regulation of epithelial cell restitution [54]. Protective mechanisms could also involve reducing gastrointestinal inflammation. While the mechanisms responsible for the resolution of inflammation in the gut by nitrate metabolism are not well understood, they might involve the balance between the production of reactive nitrogen species (RNS) and the preservation of intestinal integrity [55]. These data, overall, highlight a potential role for gut microbiota in the resolution of inflammation by dietary nitrate. Indeed, a role for RNS derived from nitrate metabolism in maintaining gut microbiota homeostasis has been proposed in mice, with a unique role suggested for the ileum, which prevents bacterial overgrowth. The RNS, possibly peroxynitrite (ONOO-), are highly produced downstream of the reactions of iNOS and the enzyme NADPH oxidase 1 in the ileum of normal healthy mice. The composition and translocation of intestinal bacteria also appear to be regulated by RNS production [53]. iNOS has been described to be upregulated in the colon of patients with inflammatory bowel disease (IBD) [56], and dysbiosis and changes in NO metabolism have been linked to ulcerative colitis, a major form of IBD [57]. However, it is not clear whether dietary nitrate can stimulate the transformation of the bacterial community, for example, through an antimicrobial effect, or whether it is the microbiota and its nitrogen-based metabolic interactions that are responsible for the resolution of dysbiosis. Studies dealing with the effect of dietary nitrate on gut microbiota are summarized in Table 2.

Table 2.
Evidence on the effect of dietary nitrate-based products on the gut microbiota.
Whether an increase in the concentration of nitrate in the intestinal tract (e.g., after consumption of a diet rich in nitrate) produce changes in the microbiota is not clearly understood. While dietary nitrate has a beneficial impact on the gastric and gut mucosa during antibiotherapy [62], this can coincide with an increase in methane production [63], and further studies are needed to clarify these issues and to examine whether gut microbiota dysbiosis occurs after long-term nitrate supplementation as result of enhanced gut fermentation. In mice, long-term dietary nitrite/nitrate deficiency leads to the metabolic syndrome, endothelial dysfunction and cardiovascular death [60], indicating a novel pathogenetic role of the exogenous NO production system in the metabolic syndrome and its vascular complications. This latter study also suggested a causal role of gut microbiota dysbiosis in the development of the metabolic syndrome. By contrast, long-term nitrate supplementation in rodents seems to be effective in preventing the metabolic syndrome [64]. In this line, it has been suggested that the overall observed benefits of nitrate supplementation, and the greater NO production, are found between day 3 and 14 in healthy adults, after which time the supplementation needs to be reviewed [59], both in terms of microbiota and also physiological parameters such as NO. The potential role of nitrogen in the maintenance of the gastrointestinal function is summarized in Figure 2.
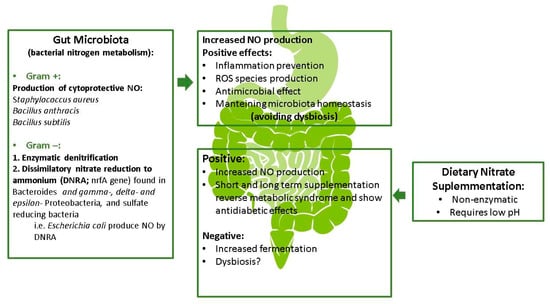
Figure 2.
Potential role of nitrogen metabolism in gastrointestinal functions (modified).
The metabolism of nitrate by some Gram-positive bacteria including Staphylococcus aureus, Bacillus anthracis and Bacillus subtilis has been shown to generate NO as a cytoprotective agent against oxidative stress and antibiotics [53,65]. Additionally, a broad range of denitrifying bacteria (mainly Gram-negative) are involved in enzymatic denitrification [66,67], although their role in the gastrointestinal tract is suggested to be minor [68]. The nrfA gene, which encodes a nitrite reductase involved in dissimilatory nitrate reduction to ammonium (DNRA), has been identified in Bacteroides species [69] and in gamma-, delta- and epsilon-subclasses of the Proteobacteria, and even in sulfate-reducing bacteria [56]. Escherichia coli, which is capable of DNRA but not denitrification, has been shown to generate substantial amounts of NO [70]. Whether or not the NO exerts a role in the complex ecosystem of the gut microbiota is not completely known and needs to be explored further.
There is sufficient evidence to indicate a role for the gut microbiota in the metabolism of dietary nitrogen to NO, although it is likely not substantial [56]. The main conversion of dietary nitrate seems to be non-enzymatic and requires an acidic pH, but the acidic conditions could depend on the production of lactic acid by the gut microbiota. While more evidence is needed, it appears that the microbial community creates a specific environment required for the non-enzymatic chemical conversion of nitrate [58].
4. Nitrate and the Oral and Gut-Muscle Axis: Potential Ergogenic Effects
Diet-induced changes in the composition of the gut microbiota markedly influence systemic metabolism, fuel availability and exercise capacity. The intake of dietary nitrate might modulate gut microbiota metabolism and contribute to local oxidation-reduction interactions, with a consequent beneficial effect on gut microbiota and health status [66].
Following its reduction by the oral bacteria, the metabolic fate of nitrate once it reaches the blood circulation is relatively unknown. The skeletal muscle has been proposed as a probable site of nitrate buffering, and both animal and human studies have revealed that skeletal muscle can act as a nitrate reservoir that can be drawn on following high intensity exercise [67]. The skeletal muscle appears to have the required molecular machinery for nitrate metabolism, transport, and storage [68]. The presence of a nitrate reservoir should be considered for nitrate supplementation, as it may not be necessary to administer nitrate chronically, but rather on an intermittent basis [28]. Although further studies are necessary to understand the mechanisms involved, it seems that dietary nitrate intake impacts the nitrate stores in skeletal muscle. Accordingly, skeletal muscle could play a key role in the metabolism, transport and storage of nitrate in humans.
Knowledge of the effect that nitrate has on the gut microbiota is very limited. This is in part because it is difficult to isolate its role from that of other compounds provided by dietary vegetable intake. No noteworthy changes in gut microbiota richness have been observed in rodent models of nitrate supplementation [61,62]. As discussed earlier, it is more clear that nitrate is capable of reaching the intestine, both through the digestive system and the bloodstream, where it plays an important protective and anti-inflammatory role in intestinal integrity [54], preserving the habitat of gut microbiota. Some anaerobic bacteria can use nitrate as a nutrient, and also as a final electron acceptor during respiration [71]. Although NOS-like enzymes have been identified in certain enteric bacteria, the vast majority of luminal NO is produced by anaerobic bacteria via the reductive metabolism of nitrate and nitrite [72]. However, the NO produced by this group of bacteria could act as an extra supply for NO bioavailability in skeletal muscle, which might be linked to the improvement of sports performance.
The practice of exercise is a well-known and important modulator of gut microbiota composition, and a very recent report has linked, for the first time, sports performance to the presence of a bacterial species in the gut microbiota [33]. These findings might be reconciled by the existence of a gut-muscle axis, in which the gut microbiota helps to maintain skeletal muscle mass and physical function [34,73]. The notion of crosstalk between the gut microbiota and skeletal muscle actually emerged from several early studies in animals that reported increases in specific bacterial species that produce short-chain fatty acids (SCFAs). It seems that the promotion of bacteria producing SCFAs could be beneficial for skeletal muscle mass and physical function in humans. Physical activity itself is able to stimulate the production of SCFAs, as can a high fiber diet [74]. The practice of physical exercise, in an appropriate manner, has been associated with higher microbiota diversity, an increased presence of health-promoting bacteria and an augmented production of SCFAs [75,76,77]. SCFAs are produced by colon fermentation of undigested carbohydrates and exert their effects both locally and systemically. They protect the integrity of the intestinal barrier and might also be involved in exercise adaptations by regulating intestinal hormones and metabolism, inducing gluconeogenesis and inhibiting lipogenesis [73]. SCFAs and muscle have a direct relationship through the activation of muscular AMP-activated protein kinase (AMPK) by SCFAs. AMPK is involved in the regulation of mitochondrial biogenesis, cholesterol levels and glucose and lipid muscle metabolism [34]. Moreover, SCFAs are also involved in aerobic energy metabolism due to the ability of butyrate, one of the most important SCFAs, to participate in the Krebs cycle [73].
SCFAs produced by the gut microbiota have recently been directly linked to improved exercise performance. Scheiman et al. showed that the abundance of members of the Veillonella genus of the gut microbiota was increased in marathon runners after a marathon [33]. Fascinatingly, the administration of V. atypica to mice resulted in an increase in their running time as compared with control mice. The authors hypothesized that systemic lactate produced from muscle during physical activity is able to enter the intestinal lumen and is metabolized to propionate by Veillonella. Propionate can be used by skeletal muscle to improve exercise performance and has also been shown to increase the heart rate and the maximum rate of oxygen consumption, and to affect blood pressure. When the authors introduced propionate intrarectally to mice, they observed a similar enhancement in exercise performance to that seen after V. atypica administration. Thus, the Veillonella genus is involved in performance through the metabolism and production of SCFAs (propionate) [33]. By contrast, the administration of antibiotics to mice has been shown to reduce their endurance capacity, as measured by treadmill testing, concomitant with a marked reduction of SCFAs in the cecum and plasma and dysbiosis of the gut microbiota [78]. The administration of acetate, but not butyrate (using an osmotic pump inserted subcutaneously), restored the endurance capacity and, consequentially, acetate could be an energy substrate for endurance exercise [78].
Of note, the same genus of Veillonella sp., found in the gut microbiota marathon runners [33] is present in the oral microbiota and is one of the main, if not the main, bacteria involved in the conversion of nitrate to nitrite. This genus thus seems to be strongly linked to sport performance, for its participation in the conversion of nitrate in the oral cavity and for its activity in the intestine as a producer of propionate from lactate generated during physical activity [21].
5. Conclusions
In this review, we have discussed the oral and gut bacteria involved in the reduction of dietary nitrate to nitrite and its subsequent conversion to NO, as well as the influence of nitrate on oral and gut microbial populations. The composition of the oral microbiota is modified by the intake of nitrate, favoring (although not all) those bacteria that reduce nitrate. However, the overall effect of the total nitrate-reductase activity in the mouth is unknown. The metabolism of nitrate and its derived compounds plays an important role in the homeostasis of the gastrointestinal system, including the microbiota, but there is a paucity of information on the effect of nitrate intake on the composition of the gut microbiota. Recent studies indicate that certain gut bacteria can enhance exercise performance through their metabolism, underscoring the existence of a microbiota-muscle axis with repercussions for sports performance. The fact that bacterial populations are modifiable with mouthwashes, in the case of oral microbiota, or through the use of probiotics or prebiotics, opens a door to enhance exercise performance through supplementation with nitrate-rich foods. Nevertheless, more research is needed to understand the relationships between nitrate intake and the oral and intestinal microbiota, and the possible storage of nitrate in muscle, which will ultimately lead to a better understanding of the global impact of these factors on sports performance.
Author Contributions
Conceptualization and supervision, R.G.-S. and M.L.; writing—original draft preparation, R.G.-S, M.B. and M.L.; writing—review and editing, R.G.-S., M.L., M.B. and B.d.L.; paper planification, ideas and final review examination, M.I.R.-G. and H.P.-G.; funding acquisition, M.L. and B.d.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Economy and Competitiveness, Spain, project AGL2016-77288-R and the project 2020/UEM03 from Universidad Europea de Madrid.
Acknowledgments
The authors acknowledge the study participants. We thank Kenneth McCreath for critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Engemann, A.; Focke, C.; Humpf, H.-U. Intestinal Formation ofN-Nitroso Compounds in the Pig Cecum Model. J. Agric. Food Chem. 2013, 61, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- De Lima Bezerra, Á.D.; Costa, E.C.; Pacheco, D.A.; Souza, D.C.; Farias-Junior, L.F.; Ritti-Dia, R.M.; Grigolo, G.B.; de Bittencourt Júnior, P.I.H.; Krause, M.; Fayh, A.P.T. Effect of Acute Dietary Nitrate Supplementation on the Post-Exercise Ambulatory Blood Pressure in Obese Males: A Randomized, Controlled, Crossover Trial. J. Sports Sci. Med. 2019, 18, 118–127. [Google Scholar] [PubMed]
- Jones, A.M. Dietary Nitrate Supplementation and Exercise Performance. Sports Med. 2014, 44, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Trivers, K.F.; Abrahamson, P.E.; Engel, L.S.; He, K.; Chow, W.-H.; Mayne, S.T.; Risch, H.A.; et al. Dietary intake of flavonoids and oesophageal and gastric cancer: Incidence and survival in the United States of America (USA). Br. J. Cancer 2015, 112, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wu, L.; Guan, W. Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef] [PubMed]
- EFSA. 2018. EFSA Explains Risk Assessment: Nitrites and Nitrates Added to Food. Available online: https://www.efsa.europa.eu/sites/default/files/corporate_publications/files/nitrates-nitrites-170614.pdf (accessed on 30 October 2020).
- Lundberg, J.O.; Carlström, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef] [PubMed]
- EFSA Nitrate in vegetables. Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA J. 2008, 6, 689. [Google Scholar]
- McDonagh, S.T.J.; Wylie, L.J.; Thompson, C.; Vanhatalo, A.; Jones, A.M. Potential benefits of dietary nitrate ingestion in healthy and clinical populations: A brief review. Eur. J. Sport Sci. 2018, 19, 15–29. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Australian Institute of Sport-AIS. AIS Sports Supplement Framework an Initiative of AIS Sports Nutrition Sports Gels. Available online: https://www.ais.gov.au/__data/assets/pdf_file/0004/698557/AIS-Sports-Supplement-Framework-2019.pdf (accessed on 30 October 2020).
- Valenzuela, P.L.; Morales, J.S.; Emanuele, E.; Pareja-Galeano, H.; Lucia, A. Supplements with purported effects on muscle mass and strength. Eur. J. Nutr. 2019, 58, 2983–3008. [Google Scholar] [CrossRef]
- Calvo, J.L.; Alorda-Capo, F.; Pareja-Galeano, H.; Jiménez, S.L. Influence of Nitrate Supplementation on Endurance Cyclic Sports Performance: A Systematic Review. Nutrients 2020, 12, 1796. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary Inorganic Nitrate Improves Mitochondrial Efficiency in Humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Ekblom, B.; Sahlin, K.; Lundberg, J.O.; Weitzberg, E. Effects of Dietary Nitrate on Blood Pressure in Healthy Volunteers. N. Engl. J. Med. 2006, 355, 2792–2793. [Google Scholar] [CrossRef] [PubMed]
- Sobko, T.; Marcus, C.; Govoni, M.; Kamiya, S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide Biol. Chem. 2010, 22, 136–140. [Google Scholar] [CrossRef]
- Hughes, W.E.; Ueda, K.; Treichler, D.P.; Casey, D.P. Effects of acute dietary nitrate supplementation on aortic blood pressure and aortic augmentation index in young and older adults. Nitric Oxide Biol. Chem. 2016, 59, 21–27. [Google Scholar] [CrossRef]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.J.; et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010, 48, 342–347. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Moretti, C.; Benjamin, N.; Weitzberg, E. Symbiotic bacteria enhance exercise performance. Br. J. Sports Med. 2020. [Google Scholar] [CrossRef]
- McMahon, N.F.; Leveritt, M.D.; Pavey, T.G. The Effect of Dietary Nitrate Supplementation on Endurance Exercise Performance in Healthy Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 735–756. [Google Scholar] [CrossRef]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Lozano-Estevan, M.D.C.; Veiga-Herreros, P.; Garnacho-Castaño, M.V. Effects of Beetroot Juice Supplementation on Cardiorespiratory Endurance in Athletes. A Systematic Review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.; Kiernan, M.; Willis, J.; Gallardo-Alfaro, L.; Casas-Agustench, P.; White, D.; Hickson, M.; Gabaldon, T.; Bescos, R. Post-exercise hypotension and skeletal muscle oxygenation is regulated by nitrate-reducing activity of oral bacteria. Free Radic. Biol. Med. 2019, 143, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A.; Cutler, C.; Farnham, G.; Liddle, L.; Burleigh, M.; Rodiles, A.; Sillitti, C.; Kiernan, M.; Moore, M.; Hickson, M.; et al. Dietary intake of inorganic nitrate in vegetarians and omnivores and its impact on blood pressure, resting metabolic rate and the oral microbiome. Free Radic. Biol. Med. 2019, 138, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Haydar, S.M.; Pearl, V.; Lundberg, J.O.; Weitzberg, E.; Ahluwalia, A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013, 55, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Harasawa, R. The effects of essential oil, povidone-iodine, and chlorhexidine mouthwash on salivary nitrate/nitrite and nitrate-reducing bacteria. J. Oral Sci. 2017, 59, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Tribble, G.D.; Angelov, N.; Weltman, R.; Wang, B.-Y.; Eswaran, S.V.; Gay, I.C.; Parthasarathy, K.; Dao, D.-H.V.; Richardson, K.N.; Ismail, N.M.; et al. Frequency of Tongue Cleaning Impacts the Human Tongue Microbiome Composition and Enterosalivary Circulation of Nitrate. Front. Cell. Infect. Microbiol. 2019, 9, 39. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Muñoz-Torres, F.; Fernández-Santiago, J.; Patel, R.P.; Lopez-Candales, A. Over-the-counter mouthwash use, nitric oxide and hypertension risk. Blood Press. 2019, 29, 103–112. [Google Scholar] [CrossRef]
- Rocha, B.; Correia, M.; Barbosa, R.; Laranjinha, J. A dietary-driven redox modulation of gut microbiome-host interactions: The rescue of epithelial barrier and mucus production during dysbiosis by dietary nitrate. Free Radic. Biol. Med. 2014, 75, S36–S37. [Google Scholar] [CrossRef]
- Nyakayiru, J.; Van Loon, L.J.; Verdijk, L. Could intramuscular storage of dietary nitrate contribute to its ergogenic effect? A mini-review. Free Radic. Biol. Med. 2020, 152, 295–300. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; Macdonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Ecerdá, B.; Epérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; Egonzález-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef]
- Sorrenti, V.; Fortinguerra, S.; Caudullo, G.; Buriani, A. Deciphering the Role of Polyphenols in Sports Performance: From Nutritional Genomics to the Gut Microbiota toward Phytonutritional Epigenomics. Nutrients 2020, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Hezel, M.P.; Weitzberg, E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2013, 21, 7–16. [Google Scholar] [CrossRef]
- Weitzberg, E.; Lundberg, J.O. Novel Aspects of Dietary Nitrate and Human Health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.Y.M.; Balligand, J.-L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef]
- Doel, J.J.; Benjamin, N.; Hector, M.P.; Rogers, M.; Allaker, R.P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005, 113, 14–19. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome–an update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Schreiber, F.; Stief, P.; Gieseke, A.; Heisterkamp, I.M.; Verstraete, W.; De Beer, D.; Stoodley, P. Denitrification in human dental plaque. BMC Biol. 2010, 8, 24. [Google Scholar] [CrossRef]
- Li, H.; Duncan, C.; Townend, J.; Killham, K.; Smith, L.M.; Johnston, P.; Dykhuizen, R.; Kelly, D.; Golden, M.; Benjamin, N.; et al. Nitrate-reducing bacteria on rat tongues. Appl. Environ. Microbiol. 1997, 63, 924–930. [Google Scholar] [CrossRef]
- Palmerini, C.A.; Palombari, R.; Perito, S.; Arienti, G. NO Synthesis in Human Saliva. Free Radic. Res. 2003, 37, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Hyde, E.R.; Andrade, F.; Vaksman, Z.; Parthasarathy, K.; Jiang, H.; Parthasarathy, D.K.; Torregrossa, A.C.; Tribble, G.; Kaplan, H.B.; Petrosino, J.F.; et al. Metagenomic Analysis of Nitrate-Reducing Bacteria in the Oral Cavity: Implications for Nitric Oxide Homeostasis. PLoS ONE 2014, 9, e88645. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Saito, M.; Harasawa, R. Salivary nitrate-nitrite conversion capacity after nitrate ingestion and incidence of Veillonella spp. in elderly individuals. J. Oral Sci. 2018, 60, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Burleigh, M.; Liddle, L.; Monaghan, C.; Muggeridge, D.J.; Sculthorpe, N.; Butcher, J.P.; Henriquez, F.L.; Allen, J.D.; Easton, C. Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic. Biol. Med. 2018, 120, 80–88. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, M.; Hernell, O.; Lönnerdal, B.; Nihlen, C.; Johanssson, I.; Weitzberg, E. Effects of age, sex and diet on salivary nitrate and nitrite in infants. Nitric Oxide Biol. Chem. 2019, 94, 73–78. [Google Scholar] [CrossRef]
- Hyde, E.R.; Luk, B.; Cron, S.; Kusic, L.; McCue, T.; Bauch, T.; Kaplan, H.; Tribble, G.; Petrosino, J.F.; Bryan, N.S. Characterization of the rat oral microbiome and the effects of dietary nitrate. Free Radic. Biol. Med. 2014, 77, 249–257. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Blackwell, J.R.; L’Heureux, J.E.; Williams, D.W.; Smith, A.; Van Der Giezen, M.; Winyard, P.G.; Kelly, J.; Jones, A.M. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 2018, 124, 21–30. [Google Scholar] [CrossRef]
- Duncan, C.; Li, H.; Dykhuizen, R.; Frazer, R.; Johnston, P.; Macknight, G.; Smith, L.; Lamza, K.; McKenzie, H.; Batt, L.; et al. Protection against oral and gastrointestinal diseases: Importance of dietary nitrate intake, oral nitrate reduction and enterosalivary nitrate circulation. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 939–948. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Biology of nitrogen oxides in the gastrointestinal tract. Gut 2013, 62, 616–629. [Google Scholar] [CrossRef]
- Björne, H.; Petersson, J.; Phillipson, M.; Weitzberg, E.; Holm, L.; Lundberg, J. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J. Clin. Investig. 2004, 113, 106–114. [Google Scholar] [CrossRef]
- Matziouridou, C.; Rocha, S.D.C.; Haabeth, O.A.; Rudi, K.; Carlsen, H.; Kielland, A. iNOS and NOX1-dependent ROS production maintains bacterial homeostasis in the ileum of mice. Mucosal Immunol. 2018, 11, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Jädert, C.; Phillipson, M.; Holm, L.; Lundberg, J.O.; Borniquel, S. Preventive and therapeutic effects of nitrite supplementation in experimental inflammatory bowel disease. Redox Biol. 2014, 2, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Geagea, A.G.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. 2016, 160, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, J.; Van De Wiele, T.; Van Nieuwenhuyse, G.; Boeckx, P.; Verstraete, W.; Boon, N. Sulfide- and nitrite-dependent nitric oxide production in the intestinal tract. Microb. Biotechnol. 2012, 5, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E.W. Review article: Nitric oxide from dysbiotic bacterial respiration of nitrate in the pathogenesis and as a target for therapy of ulcerative colitis. Aliment. Pharmacol. Ther. 2008, 27, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Tiso, M.; Schechter, A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar]
- Henning, S.M.; Yang, J.; Shao, P.; Lee, R.-P.; Huang, J.; Ly, A.; Hsu, M.; Lu, Q.-Y.; Thames, G.; Heber, D.; et al. Health benefit of vegetable/fruit juice-based diet: Role of microbiome. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Kina-Tanada, M.; Sakanashi, M.; Tanimoto, A.; Kaname, T.; Matsuzaki, T.; Noguchi, K.; Uchida, T.; Nakasone, J.; Kozuka, C.; Ishida, M.; et al. Long-term dietary nitrite and nitrate deficiency causes the metabolic syndrome, endothelial dysfunction and cardiovascular death in mice. Diabetologia 2017, 60, 1138–1151. [Google Scholar] [CrossRef]
- Conley, M.N.; Roberts, C.; Sharpton, T.J.; Iwaniec, U.T.; Hord, N.G. Increasing dietary nitrate has no effect on cancellous bone loss or fecal microbiome in ovariectomized rats. Mol. Nutr. Food Res. 2017, 61, 61. [Google Scholar] [CrossRef]
- Rocha, B.S.; Correia, M.G.; Pereira, A.; Henriques, I.; Da Silva, G.J.; Laranjinha, J. Inorganic nitrate prevents the loss of tight junction proteins and modulates inflammatory events induced by broad-spectrum antibiotics: A role for intestinal microbiota? Nitric Oxide Biol. Chem. 2019, 88, 27–34. [Google Scholar] [CrossRef]
- Natel, A.S.; Abdalla, A.L.; Araujo, R.; McManus, C.; Paim, T.D.P.; de Abdalla Filho, A.L.; Louvandini, H.; Nazato, C. Encapsulated nitrate replacing soybean meal changes in vitro ruminal fermentation and methane production in diets differing in concentrate to forage ratio. Anim. Sci. J. 2019, 90, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M.; Larsen, F.J.; Nyström, T.; Hezel, M.; Borniquel, S.; Weitzberg, E.; Lundberg, J.O. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc. Natl. Acad. Sci. USA 2010, 107, 17716–17720. [Google Scholar] [CrossRef] [PubMed]
- Gusarov, I.; Nudler, E. NO-mediated cytoprotection: Instant adaptation to oxidative stress in bacteria. Proc. Natl. Acad. Sci. USA 2005, 102, 13855–13860. [Google Scholar] [CrossRef] [PubMed]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 61. [Google Scholar] [CrossRef]
- Bu, C.; Wang, Y.; Ge, C.; Ahmad, H.A.; Gao, B.; Ni, S.-Q. Dissimilatory Nitrate Reduction to Ammonium in the Yellow River Estuary: Rates, Abundance, and Community Diversity. Sci. Rep. 2017, 7, 6830. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Allison, C.; Gibson, S.A.W.; Cummings, J.H. Contribution of the microflora to proteolysis in the human large intestine. J. Appl. Bacteriol. 1988, 64, 37–46. [Google Scholar] [CrossRef]
- Mohan, S.B.; Schmid, M.; Jetten, M.; Cole, J. Detection and widespread distribution of the nrfA gene encoding nitrite reduction to ammonia, a short circuit in the biological nitrogen cycle that competes with denitrification. FEMS Microbiol. Ecol. 2004, 49, 433–443. [Google Scholar] [CrossRef]
- Kaldorf, M.; Von Berg, K.-H.L.; Meier, U.; Servos, U.; Bothe, H. The reduction of nitrous oxide to dinitrogen by Escherichia coli. Arch. Microbiol. 1993, 160, 432–439. [Google Scholar] [CrossRef]
- Rocha, B.S.; Laranjinha, J. Nitrate from diet might fuel gut microbiota metabolism: Minding the gap between redox signaling and inter-kingdom communication. Free Radic. Biol. Med. 2020, 149, 37–43. [Google Scholar] [CrossRef]
- Espey, M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013, 55, 130–140. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-Muscle AxisExists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, M.S. The Role of the Gut Microbiome on Skeletal Muscle Mass and Physical Function: 2019 Update. Front. Physiol. 2019, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Van Treuren, W.; González, A.; Herrera, J.J.; Knight, R.; Fleshner, M. Exercise Is More Effective at Altering Gut Microbial Composition and Producing Stable Changes in Lean Mass in Juvenile versus Adult Male F344 Rats. PLoS ONE 2015, 10, e0125889. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2017, 67, 625–633. [Google Scholar] [CrossRef]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).