Association between Serum Zinc Levels and Clinical Index or the Body Composition in Incident Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
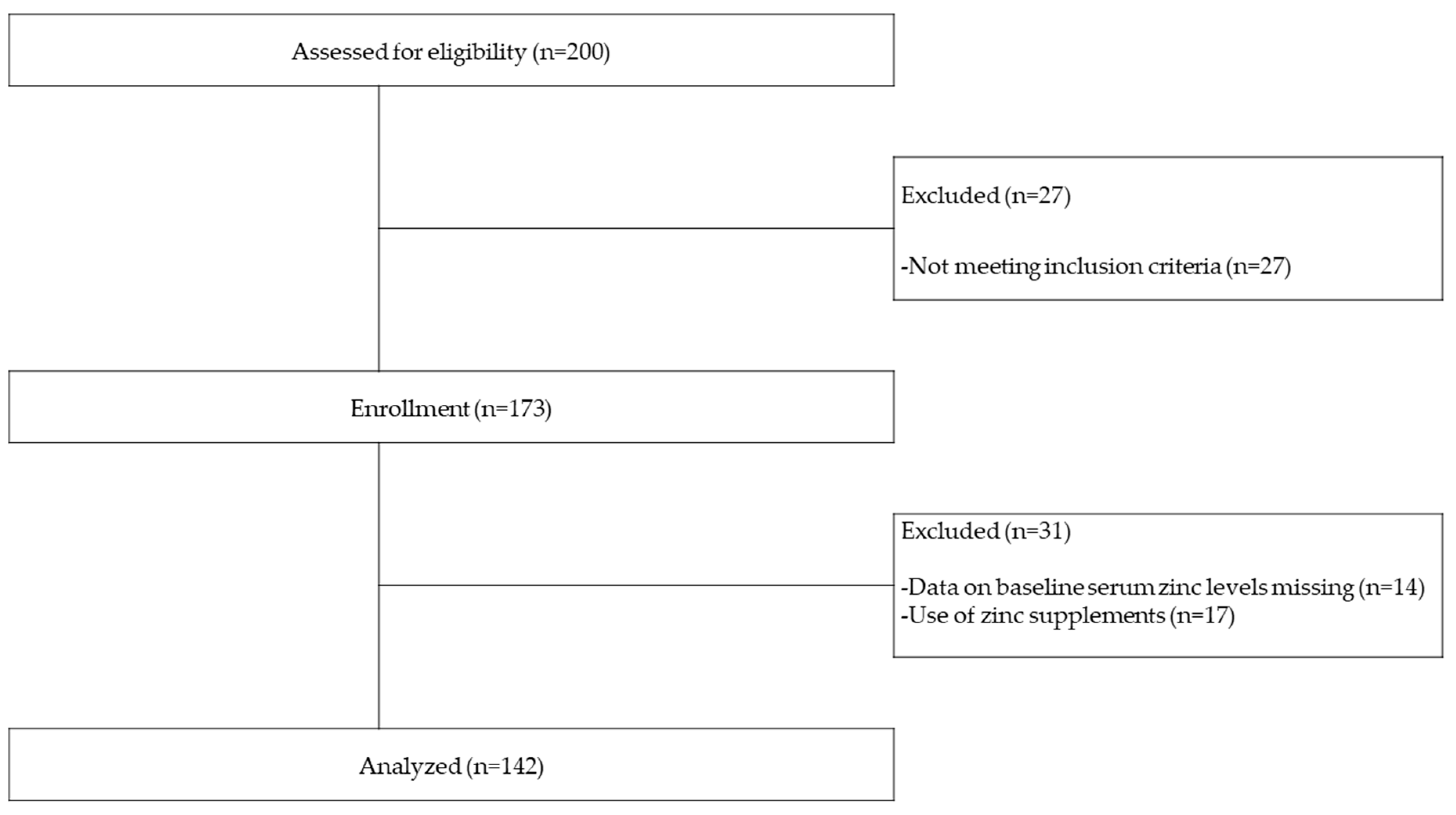
2.1. Study Design and Subjects
2.2. General Clinical Data, Measurement of Laboratory Indices, and Body Composition
2.3. Outcomes
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Study Participants and Baseline Characteristics
3.2. Correlation Analysis
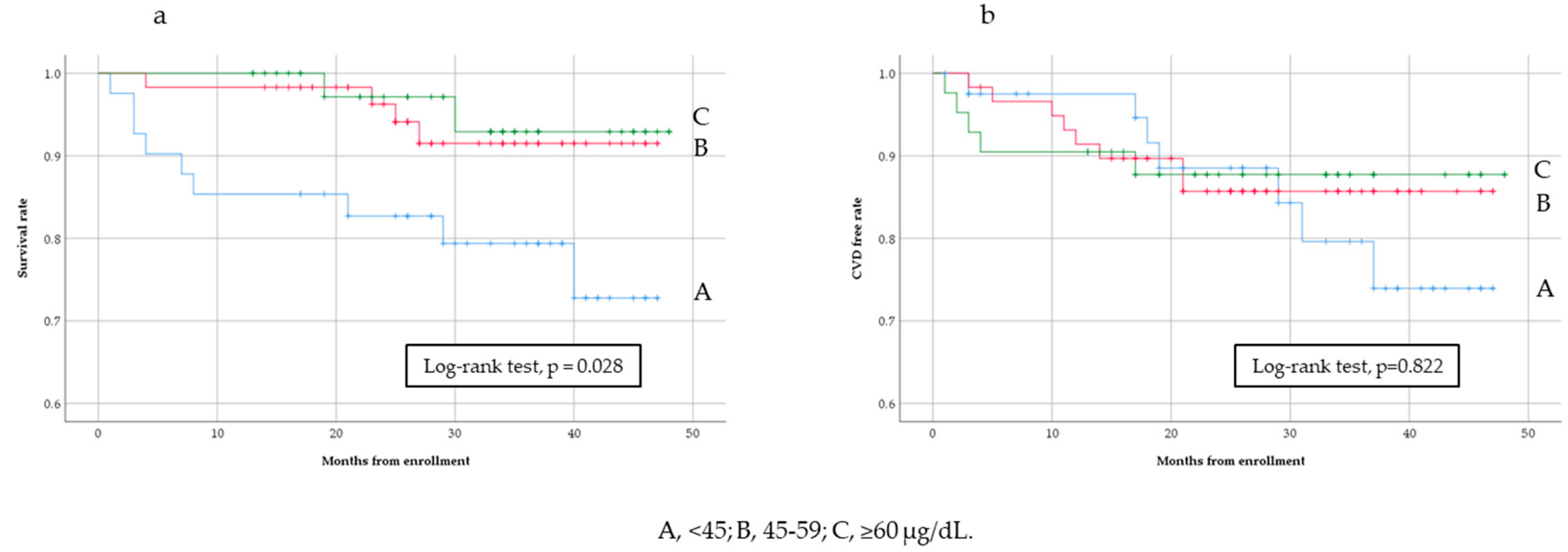
3.3. Analysis of All-Cause Mortality and Cardiovascular Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tonelli, M.; Network, T.A.K.D.; Wiebe, N.; Hemmelgarn, B.; Klarenbach, S.; Field, C.; Manns, B.; Thadhani, R.; Gill, J. The Alberta Kidney Disease Network. Trace elements in hemodialysis patients: A systematic review and meta-analysis. BMC Med. 2009, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alker, W.; Haase, H. Zinc and Sepsis. Nutrients 2018, 10, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiterer, G.; Macdonald, R.; Browning, J.D.; Morrow, J.; Matveev, S.V.; Daugherty, A.; Smart, E.; Toborek, M.; Hennig, B. Zinc Deficiency Increases Plasma Lipids and Atherosclerotic Markers in LDL-Receptor–Deficient Mice. J. Nutr. 2005, 135, 2114–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardozo, L.F.M.F.; Mafra, D. Don’t forget the zinc. Nephrol. Dial. Transplant. 2020, 35, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Zumkley, H.; Bertram, H.P.; Lison, A.; Knoll, O.; Losse, H. Aluminum, zinc and copper concentrations in plasma in chronic renal insufficiency. Clin. Nephrol. 1979, 12, 18–21. [Google Scholar]
- Dashti-Khavidaki, S.; Khalili, H.; Vahedi, S.-M.; Lessan-Pezeshki, M. Serum zinc concentrations in patients on maintenance hemodialysis and its relationship with anemia, parathyroid hormone concentrations and pruritus severity. Saudi J. Kidney Dis. Transplant. 2010, 21, 641–645. [Google Scholar]
- Muirhead, N.; Kertesz, A.; Flanagan, P.R.; Hodsman, A.B.; Hollomby, D.J.; Valberg, L.S. Zinc Metabolism in Patients on Maintenance Hemodialysis. Am. J. Nephrol. 1986, 6, 422–426. [Google Scholar] [CrossRef]
- Guo, C.-H.; Wang, C.-L. Effects of Zinc Supplementation on Plasma Copper/Zinc Ratios, Oxidative Stress, and Immunological Status in Hemodialysis Patients. Int. J. Med. Sci. 2013, 10, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Turk, S.; Bozfakioglu, S.; Ecder, S.; Kahraman, T.; Gürel, N.; Erkoc, R.; Aysuna, N.; Türkmen, A.; Bekiroglu, N.; Ark, E.; et al. Effects of Zinc Supplementation on the Immune System and on Antibody Response to Multivalent Influenza Vaccine in Hemodialysis Patients. Int. J. Artif. Organs 1998, 21, 274–278. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Society of Critical Care Medicine. American Society for Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, M.; Plauth, M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Kodama, H.; Tanaka, M.; Naito, Y.; Katayama, K.; Moriyama, M. Japan’s Practical Guidelines for Zinc Deficiency with a Particular Focus on Taste Disorders, Inflammatory Bowel Disease, and Liver Cirrhosis. Int. J. Mol. Sci. 2020, 21, 2941. [Google Scholar] [CrossRef] [PubMed]
- Nishime, K.; Kondo, M.; Saito, K.; Miyawaki, H.; Nakagawa, T. Zinc Burden Evokes Copper Deficiency in the Hypoalbuminemic Hemodialysis Patients. Nutrients 2020, 12, 577. [Google Scholar] [CrossRef] [Green Version]
- Fukasawa, H.; Niwa, H.; Ishibuchi, K.; Kaneko, M.; Iwakura, T.; Yasuda, H.; Furuya, R. The Impact of Serum Zinc Levels on Abdominal Fat Mass in Hemodialysis Patients. Nutrients 2020, 12, 656. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-J.; Wang, M.-Q.; Hu, R.; Yang, Y.; Huang, Y.-S.; Xian, S.-X.; Lu, L. Effect of Zinc Supplementation on Maintenance Hemodialysis Patients: A Systematic Review and Meta-Analysis of 15 Randomized Controlled Trials. Biomed. Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Qin, X.; Li, Y.; Yang, Y.; Yang, S.; Lu, Y.; Zhao, Y.; He, Y.; Li, Y.; Lei, Z.; et al. Comparison of three nutritional screening tools for predicting mortality in maintenance hemodialysis patients. Nutrients 2019, 110532. [Google Scholar] [CrossRef]
- Wong, C.P.; Ho, E. Zinc and its role in age-related inflammation and immune dysfunction. Mol. Nutr. Food Res. 2011, 56, 77–87. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Sezer, S.; Karakan, S.; Sasak, G.; Tutal, E.; Acar, F.N. Özdemir Body Fat Percentage as a Risk Factor for Atherosclerosis but not for Inflammation for Hemodialysis Patients: Differences between Genders. J. Ren. Nutr. 2012, 22, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Kanazawa, Y.; Nagaoka, Y.; Iwasawa, H.; Uchinaga, A.; Matsumoto, H.; Okada, T.; Yoshino, M. Body Protein Index Based on Bioelectrical Impedance Analysis Is a Useful New Marker Assessing Nutritional Status: Applications to Patients with Chronic Renal Failure on Maintenance Dialysis. Contrib. Nephrol. 2007, 155, 18–28. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Kalantar-Zadeh, K. Kidney cachexia or protein-energy wasting in chronic kidney disease: Facts and numbers. J. Cachexia Sarcopenia Muscle 2019, 10, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Beberashvili, I.; Azar, A.; Sinuani, I.; Shapiro, G.; Feldman, L.; Sandbank, J.; Stav, K.; Efrati, S. Geriatric nutritional risk index, muscle function, quality of life and clinical outcome in hemodialysis patients. Clin. Nutr. 2016, 35, 1522–1529. [Google Scholar] [CrossRef]
- Hao, X.; Li, D.; Zhang, N. Geriatric Nutritional Risk Index as a predictor for mortality: A meta-analysis of observational studies. Nutr. Res. 2019, 71, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Kubrusly, M.; Mota, R.S.; Silva, C.A.; Choukroun, G.; Oliveira, V.N. The Phase Angle and Mass Body Cell as Markers of Nutritional Status in Hemodialysis Patients. J. Ren. Nutr. 2010, 20, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Beberashvili, I.; Azar, A.; Sinuani, I.; Shapiro, G.; Feldman, L.; Stav, K.; Sandbank, J.; Averbukh, Z. Bioimpedance phase angle predicts muscle function, quality of life and clinical outcome in maintenance hemodialysis patients. Eur. J. Clin. Nutr. 2014, 68, 683–689. [Google Scholar] [CrossRef]
- Van Der Sande, F.M.; Van De Wal-Visscher, E.R.; Stuard, S.; Moissl, U.; Kooman, J.P. Using Bioimpedance Spectroscopy to Assess Volume Status in Dialysis Patients. Blood Purif. 2020, 49, 178–184. [Google Scholar] [CrossRef]
- Mahajan, S.K.; Bowersox, E.M.; Rye, D.L.; Abu-Hamdan, D.K.; Prasad, A.S.; McDonald, F.D.; Biersack, K.L. Factors underlying abnormal zinc metabolism in uremia. Kidney Int. Suppl. 1989, 27, S269–S273. [Google Scholar]
- Mafra, D.; Cuppari, L.; Favaro, D.I.T.; Cozzolino, S.M.F. Zinc levels after iron supplementation in patients with chronic kidney disease. J. Ren. Nutr. 2004, 14, 164–169. [Google Scholar] [CrossRef]
- Kogirima, M.; Kurasawa, R.; Kubori, S.; Sarukura, N.; Nakamori, M.; Okada, S.; Kamioka, H.; Yamamoto, S. Ratio of low serum zinc levels in elderly Japanese people living in the central part of Japan. Eur. J. Clin. Nutr. 2007, 61, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Di Stasio, E.; Viola, A.; Cenerelli, S.; Leo, A.; Santarelli, S.; Monteburini, T. Dietary Daily Sodium Intake Lower than 1500 mg Is Associated with Inadequately Low Intake of Calorie, Protein, Iron, Zinc and Vitamin B1 in Patients on Chronic Hemodialysis. Nutrients 2020, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Peeters, M.J.; Brand, J.A.V.D.; Van Zuilen, A.D.; Koster, Y.; Bots, M.L.; Vervloet, M.G.; Blankestijn, P.J.; Wetzels, J.F.; MASTERPLAN Study Group. Abdominal aortic calcification in patients with CKD. J. Nephrol. 2016, 30, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toussaint, N.D.; Pedagogos, E.; Lau, K.K.; Heinze, S.; Becker, G.J.; Beavis, J.; Polkinghorne, K.R.; Damasiewicz, M.J.; Kerr, P.G. Lateral lumbar X-ray assessment of abdominal aortic calcification in Australian haemodialysis patients. Nephrology 2011, 16, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, K.; Takeshita, K.; Suzuki, S.; Tatami, Y.; Morimoto, R.; Okumura, T.; Yasuda, Y.; Murohara, T. Assessment of abdominal aortic calcification by computed tomography for prediction of latent left ventricular stiffness and future cardiovascular risk in pre-dialysis patients with chronic kidney disease: A single center cross-sectional study. Int. J. Med. Sci. 2019, 16, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Eisenberg, R.; Mowrey, W.B.; Wylie-Rosett, J.; Abramowitz, M.K.; A Bushinsky, D.; Melamed, M.L. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol. Dial. Transplant. 2020, 35, 1171–1178. [Google Scholar] [CrossRef]
- Shen, H.; Oesterling, E.; Stromberg, A.; Toborek, M.; Macdonald, R.; Hennig, B. Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-kappaB and PPAR signaling. J. Am. Coll. Nutr. 2008, 27, 577–587. [Google Scholar] [CrossRef]
- Summersgill, H.; England, H.; Lopez-Castejon, G.; Lawrence, C.B.; Luheshi, N.M.; Pahle, J.; Mendes, P.; Brough, D. Zinc depletion regulates the processing and secretion of IL-1beta. Cell Death Dis. 2014, 5, e1040. [Google Scholar] [CrossRef] [Green Version]
- Lobo, J.C.; Torres, J.P.M.; Fouque, D.; Mafra, D. Zinc deficiency in Chronic Kidney Disease: Is there a Relationship with Adipose Tissue and Atherosclerosis? Biol. Trace Element Res. 2009, 135, 16–21. [Google Scholar] [CrossRef]
- Lobo, J.C.; Stockler-Pinto, M.B.; Farage, N.E.; Faulin, T.D.E.S.; Abdalla, D.S.P.; Torres, J.P.M.; Velarde, L.G.C.; Mafra, D. Reduced Plasma Zinc Levels, Lipid Peroxidation, and Inflammation Biomarkers Levels in Hemodialysis Patients: Implications to Cardiovascular Mortality. Ren. Fail. 2013, 35, 680–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Serum Zinc Levels (µg/dL) | |||||
|---|---|---|---|---|---|
| All | <45 | 45–59 | ≥60 | p Value * | |
| n | 142 | 41 | 59 | 42 | |
| Age (years) | 70.0 ± 11.0 | 71.0 ± 11.2 | 69.8 ± 11.0 | 69.2 ± 11.0 | 0.073 |
| Male | 84, 59.2 | 32, 78.0 | 28, 47.5 | 24, 57.1 | 0.009 |
| Diabetes | 70, 49.3 | 23, 56.1 | 30, 50.8 | 17, 40.5 | 0.346 |
| BMI (kg/m2) | 24.1 ± 4.5 | 23.5 ± 3.7 | 24.4 ± 4.6 | 24.4 ± 5.0 | 0.548 |
| SBP (mmHg) | 157 ± 24 | 155 ± 21.4 | 159 ± 26 | 155 ± 26 | 0.549 |
| DBP (mmHg) | 78 ± 13 | 77 ± 15 | 77 ± 12 | 81 ± 14 | 0.318 |
| Previous history of CVD | 50, 35.2 | 20, 51.2 | 16, 27.1 | 14, 33.3 | 0.079 |
| <Lab data> | |||||
| Serum zinc (µg/dL) | 52.5 ± 12.5 | 37.4 ± 5.4 | 52.8 ± 4.4 | 66.8 ± 6.3 | <0.001 |
| Serum copper (µg/dL) | 106.5 ± 26.0 | 107.0 ± 30.4 | 106.1 ± 22.2 | 106.6 ± 27.0 | 0.985 |
| Serum iron (µg/dL) | 53 (33–77) | 44 (32–67) | 54 (31–79) | 56 (37–92) | 0.306 |
| TSAT (%) | 24.7 (16.8–35.9) | 21.4 (16.3–35.1) | 25.5 (15.5–34.8) | 25.3 (17.8–36.8) | 0.096 |
| Serum ferritin (ng/dL) | 149 (63–248) | 176 (124–266) | 114 (50–212) | 146 (69–264) | 0.069 |
| Hemoglobin (g/dL) | 8.8 ± 1.7 | 8.7 ± 2.5 | 8.6 ± 1.4 | 9.0 ± 1.3 | 0.510 |
| Serum albumin (g/dL) | 3.1 ± 0.6 | 2.6 ± 0.6 | 3.1 ± 0.5 | 3.4 ± 0.5 | <0.001 |
| Serum BUN (mg/dL) | 84.3 ± 35.0 | 84.0 ± 30.8 | 80.3 ± 36.6 | 90.2 ± 36.6 | 0.376 |
| Serum Cre (mg/dL) | 7.9 ± 2.7 | 7.5 ± 3.5 | 8.0 ± 2.4 | 8.1 ± 2.2 | 0.619 |
| eGFR (ml/min/1.73 m2) | 5.7 (4.4–7.1) | 6.6 (5.2–8.3) | 5.3 (3.9–7.1) | 5.4 (4.3–6.6) | 0.024 |
| Serum CRP (mg/dL) | 0.4 (0.1–2.5) | 1.6 (0.2–8.2) | 0.3 (0.1–1.5) | 0.3 (0.1–1.3) | 0.003 |
| Serum aCa (mg/dL) | 8.9 (8.4–9.3) | 8.9 (8.6–9.3) | 8.8 (8.4–9.4) | 8.9 (8.4–9.2) | 0.652 |
| Serum P (mg/dL) | 5.7 (4.7–6.9) | 5.1 (4.3–6.0) | 5.9 (4.9–7.2) | 5.9 (4.7–6.9) | 0.069 |
| Serum iPTH (pg/dL) | 265 (154–437) | 231 (125–295) | 281 (158–476) | 288 (151–465) | 0.175 |
| NTproBNP (pg/mL) | 5084 (1740–17,508) | 6743 (2700–25,443) | 3296 (1629–14,078) | 5344 (1317–16,656) | 0.181 |
| GNRI | 85.1 (77.4–91.1) | 76.7 (70.6–84.6) | 85.0 (79.7–91.7) | 89.4 (86.4–96.5) | <0.001 |
| <Medication> | |||||
| Iron supplements | 13, 9.2 | 4, 9.8 | 6, 10.2 | 3, 7.1 | 0.863 |
| Phosphate binders | 38, 26.8 | 11, 26.8 | 16, 27.1 | 11, 26.2 | 0.995 |
| Anti-hypertensives | 115, 81.6 | 32, 80.0 | 49, 83.1 | 34, 81.0 | 0.922 |
| ESA | 133, 93.7 | 38, 92.7 | 57, 96.6 | 38, 90.5 | 0.439 |
| <BCM> | |||||
| PhA (degrees) | 3.7 ± 1.1 | 3.1 ± 1.0 | 3.9 ± 1.0 | 4.1 ± 1.1 | 0.001 |
| OH (liter) | 4.0 ± 3.5 | 5.2 ± 4.3 | 4.1 ± 3.2 | 2.6 ± 2.4 | 0.003 |
| OH/ECW | 0.2 (0.1–0.3) | 0.3 (0.2–0.4) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.001 |
| TBW (liter) | 33.7 (28.2–37.8) | 34.5 (31.0–41.0) | 32.6 (26.6–36.9) | 33.7 (27.9–37.5) | 0.158 |
| ECW (liter) | 17.0 (14.1–19.0) | 17.8 (16.2–21.2) | 16.5 (13.7–19.0) | 15.9 (13.8–18.5) | 0.061 |
| ICW (liter) | 16.1 (13.3–19.1) | 16.7 (14.5–19.0) | 15.2 (12.5–18.0) | 17.0 (12.9–20.0) | 0.158 |
| ECW/ICW | 1.1 (0.9–1.2) | 1.1 (1.0–1.2) | 1.1 (0.9–1.2) | 1.0 (0.9–1.1) | 0.005 |
| LTI (kg/m2) | 13.8 ± 3.4 | 13.8 ± 3.3 | 13.5 ± 3.3 | 14.2 ± 3.7 | 0.562 |
| FTI (kg/m2) | 8.6 ± 4.8 | 7.6 ± 3.7 | 9.3 ± 4.9 | 8.7 ± 5.6 | 0.248 |
| BCMI | 7.7 ± 2.4 | 7.7 ± 2.4 | 7.5 ± 2.3 | 8.0 ± 2.6 | 0.585 |
| All Patients (n = 142) | Male (n = 84) | Female (n = 58) | Diabetic (n = 70) | Non-Diabetic (n = 72) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| Age (years) | −0.150 | 0.862 | −0.130 | 0.238 | 0.217 | 0.102 | 0.064 | 0.597 | −0.127 | 0.289 |
| BMI (kg/m2) | 0.046 | 0.590 | 0.021 | 0.849 | 0.050 | 0.709 | 0.061 | 0.619 | 0.101 | 0.400 |
| SBP (mmHg) | 0.027 | 0.751 | −0.091 | 0.412 | 0.169 | 0.206 | 0.061 | 0.617 | −0.010 | 0.936 |
| DBP (mmHg) | 0.118 | 0.161 | 0.123 | 0.267 | 0.168 | 0.207 | 0.010 | 0.932 | 0.152 | 0.204 |
| <Lab data> | ||||||||||
| Serum copper (µg/dL) | −0.020 | 0.818 | −0.003 | 0.982 | 0.072 | 0.590 | −0.220 | 0.860 | 0.036 | 0.763 |
| Serum iron (µg/dL) | 0.152 | 0.082 | 0.215 | 0.061 | 0.076 | 0.587 | 0.153 | 0.219 | 0.111 | 0.379 |
| TSAT (%) | 0.092 | 0.301 | 0.128 | 0.269 | 0.051 | 0.715 | 0.068 | 0.588 | 0.080 | 0.531 |
| Serum ferritin (ng/dL) | −0.006 | 0.947 | −0.090 | 0.434 | 0.202 | 0.142 | −0.040 | 0.753 | 0.016 | 0.902 |
| Hemoglobin (g/dL) | 0.349 | 0.079 | 0.402 | 0.093 | 0.506 | 0.086 | 0.550 | 0.073 | 0.373 | 0.106 |
| Serum albumin (g/dL) * | 0.518 | <0.001 | 0.557 | <0.001 | 0.394 | 0.002 | 0.582 | <0.001 | 0.460 | <0.001 |
| eGFR (ml/min/1.73 m2) | −0.251 | 0.003 | −0.208 | 0.057 | −0.226 | 0.088 | −0.0178 | 0.140 | −0.287 | 0.014 |
| Serum CRP (mg/dL) | −0.353 | <0.001 | −0.380 | <0.001 | −0.157 | 0.238 | −0.530 | <0.001 | −0.241 | 0.042 |
| Serum aCa (mg/dL) | −0.440 | 0.607 | 0.013 | 0.909 | −0.146 | 0.274 | −0.092 | 0.450 | −0.012 | 0.920 |
| Serum P (mg/dL) | 0.158 | 0.060 | 0.188 | 0.087 | 0.052 | 0.698 | −0.099 | 0.416 | 0.320 | 0.006 |
| Serum iPTH (pg/dL) | 0.184 | 0.139 | 0.118 | 0.314 | 0.179 | 0.203 | 0.137 | 0.285 | 0.180 | 0.156 |
| NTproBNP (pg/mL) | 0.037 | 0.661 | 0.028 | 0.802 | 0.053 | 0.690 | −0.063 | 0.607 | 0.162 | 0.175 |
| GNRI * | 0.505 | <0.001 | 0.411 | 0.001 | 0.383 | 0.003 | 0.512 | <0.001 | 0.481 | <0.001 |
| <BCM> | ||||||||||
| PhA (degrees) * | 0.287 | 0.001 | 0.458 | 0.001 | 0.267 | 0.015 | 0.300 | 0.012 | 0.264 | 0.025 |
| OH (liter) * | −0.384 | <0.001 | −0.341 | 0.001 | −0.401 | 0.002 | −0.398 | 0.001 | −0.342 | 0.006 |
| OH/ECW * | −0.364 | <0.001 | −0.376 | 0.001 | −0.444 | <0.001 | −0.418 | <0.001 | −0.330 | 0.005 |
| TBW (liter) | −0.124 | 0.146 | −0.019 | 0.863 | −0.124 | 0.360 | −0.129 | 0.287 | −0.104 | 0.394 |
| ECW (liter) | −0.237 | 0.005 | −0.192 | 0.080 | −0.152 | 0.254 | −0.257 | 0.032 | −0.195 | 0.101 |
| ICW (liter) | 0.063 | 0.457 | 0.121 | 0.272 | 0.228 | 0.850 | 0.060 | 0.612 | 0.047 | 0.693 |
| ECW/ICW * | −0.287 | 0.001 | −0.339 | 0.002 | −0.315 | 0.016 | −0.254 | 0.035 | −0.281 | 0.017 |
| LTI (kg/m2) | 0.048 | 0.573 | 0.140 | 0.208 | 0.049 | 0.716 | 0.122 | 0.313 | −0.024 | 0.844 |
| FTI (kg/m2) | 0.048 | 0.570 | 0.017 | 0.880 | 0.059 | 0.659 | 0.039 | 0.753 | 0.169 | 0.160 |
| BCMI | 0.053 | 0.532 | 0.136 | 0.221 | 0.046 | 0.731 | 0.129 | 0.285 | −0.027 | 0.823 |
| Serum Zinc Levels (µg/dL) | Number of Deaths, % | Unadjusted Model | Adjusted Model 1 * | Adjusted Model 2 ** |
|---|---|---|---|---|
| <45 | 9, 22.0 | 4.61 (0.99–21.33) | 4.23 (0.87–20.64) | 6.48 (0.25–166.94) |
| 45–59 | 4, 6.8 | 1.43 (0.26–7.79) | 1.44 (0.26–7.91) | 3.84 (0.21–50.94) |
| ≥60 | 2, 4.8 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Serum Zinc Levels (µg/dL) | Number of Deaths, % | Unadjusted Model | Adjusted Model 1 * | Adjusted Model 2 ** |
|---|---|---|---|---|
| <45 | 7, 17.1 | 1.40 (0.46–4.42) | 1.25 (0.41–3.84) | 2.47 (0.21–29.30) |
| 45–59 | 8, 13.6 | 1.10 (0.36–3.35) | 1.19 (0.37–3.84) | 1.58 (0.13–18.60) |
| ≥60 | 5, 11.9 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toida, T.; Toida, R.; Ebihara, S.; Takahashi, R.; Komatsu, H.; Uezono, S.; Sato, Y.; Fujimoto, S. Association between Serum Zinc Levels and Clinical Index or the Body Composition in Incident Hemodialysis Patients. Nutrients 2020, 12, 3187. https://doi.org/10.3390/nu12103187
Toida T, Toida R, Ebihara S, Takahashi R, Komatsu H, Uezono S, Sato Y, Fujimoto S. Association between Serum Zinc Levels and Clinical Index or the Body Composition in Incident Hemodialysis Patients. Nutrients. 2020; 12(10):3187. https://doi.org/10.3390/nu12103187
Chicago/Turabian StyleToida, Tatsunori, Reiko Toida, Shou Ebihara, Risa Takahashi, Hiroyuki Komatsu, Shigehiro Uezono, Yuji Sato, and Shouichi Fujimoto. 2020. "Association between Serum Zinc Levels and Clinical Index or the Body Composition in Incident Hemodialysis Patients" Nutrients 12, no. 10: 3187. https://doi.org/10.3390/nu12103187




