Weight Reduction by the Low-Insulin-Method—A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. The SAMMAS Components
2.4. Measurements
2.5. Statistical Analysis
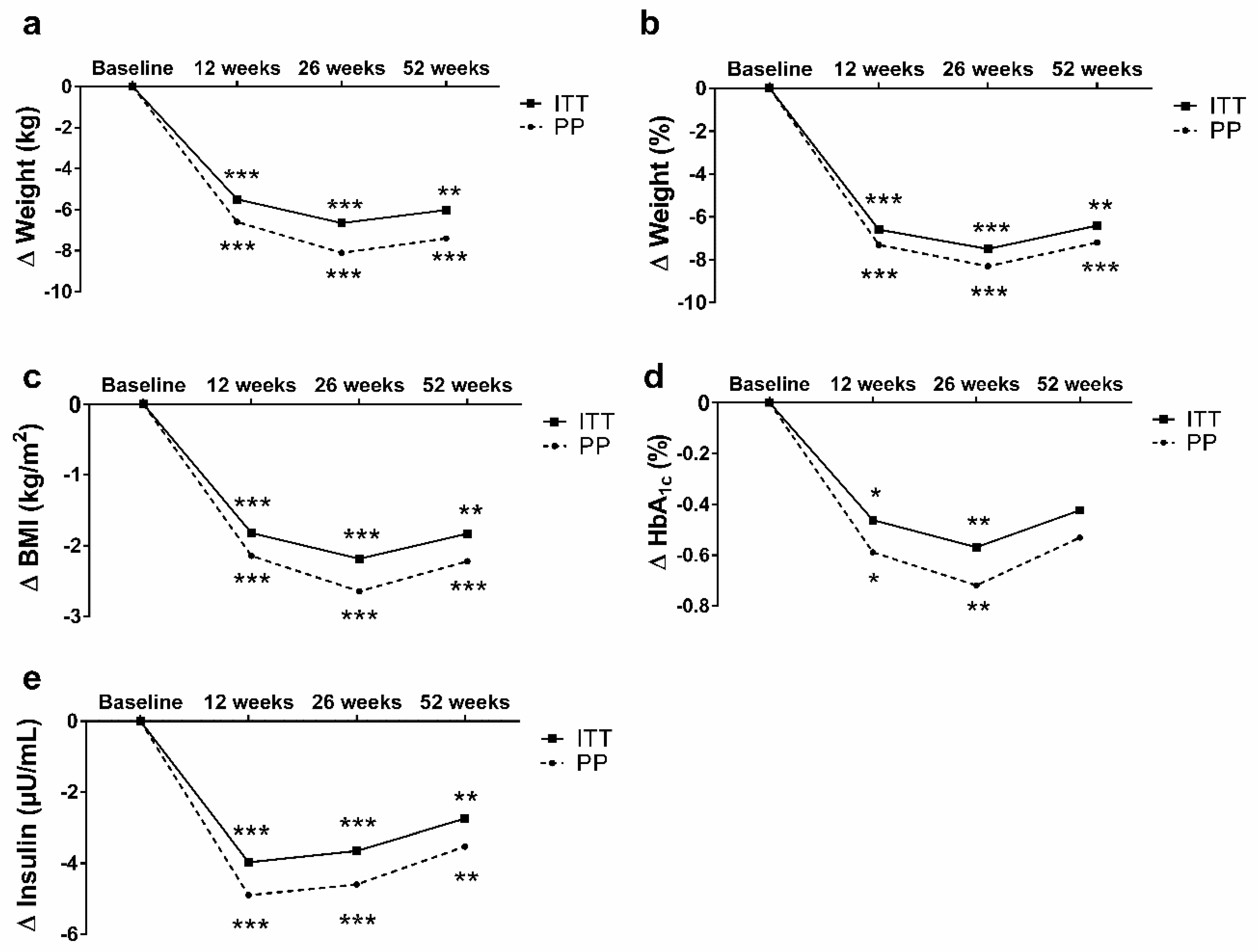
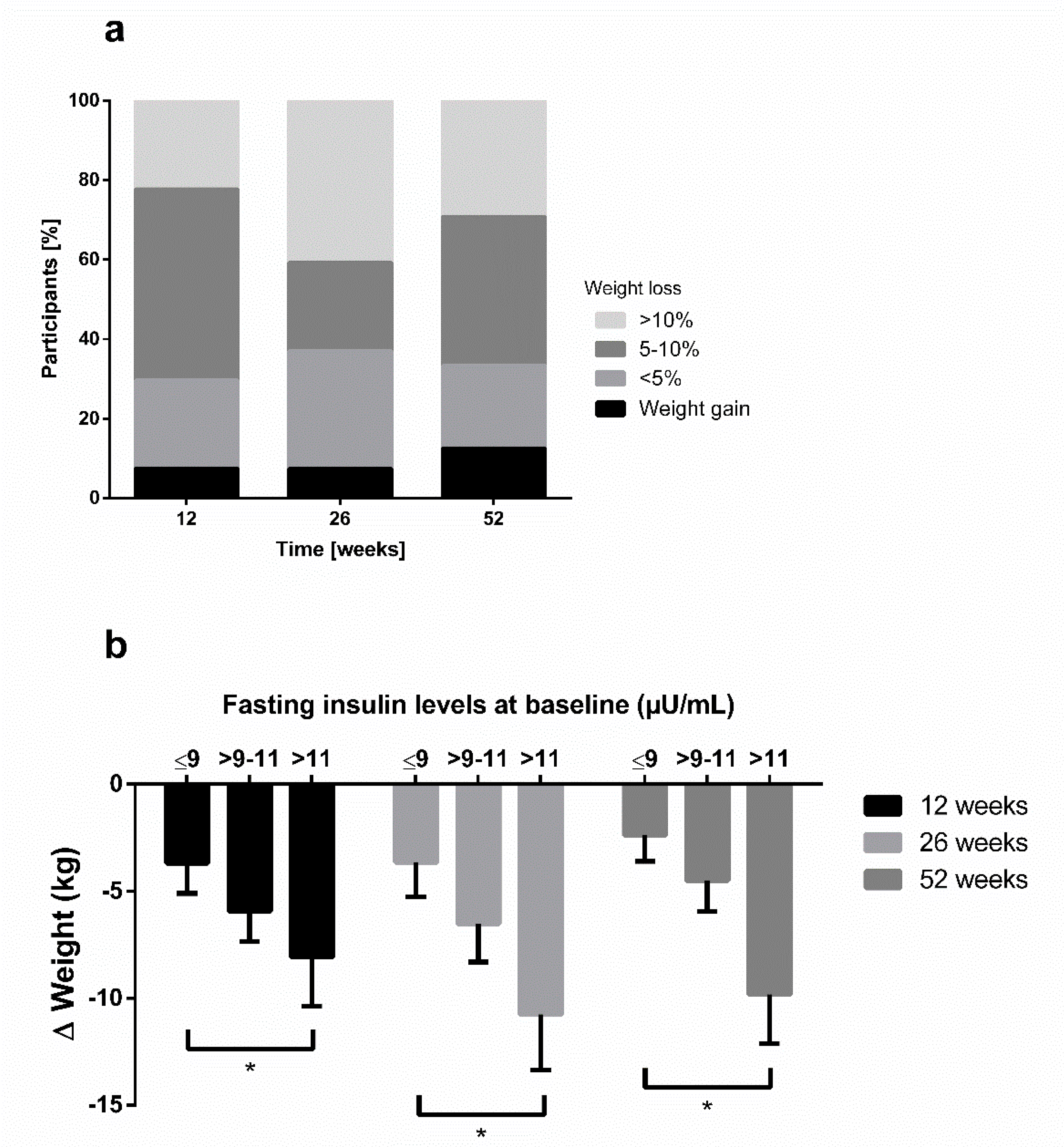
3. Results
3.1. Results Report of SI and WL (First 12 Weeks)
3.2. Results Report of the Total Sample (Until 52 Weeks)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Jacob, S.; Hauer, B.; Becker, R.; Artzner, S.; Grauer, P.; Löblein, K.; Nielsen, M.; Renn, W.; Rett, K.; Wahl, H.G.; et al. Lipolysis in skeletal muscle is rapidly regulated by low physiological doses of insulin. Diabetologia 1999, 42, 1171–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer-Gerspach, A.C.; Cajacob, L.; Riva, D.; Herzog, R.; Drewe, J.; Beglinger, C.; Wölnerhanssen, B.K. Mechanisms Regulating Insulin Response to Intragastric Glucose in Lean and Non-Diabetic Obese Subjects: A Randomized, Double-Blind, Parallel-Group Trial. PLoS ONE 2016, 11, e0150803. [Google Scholar] [CrossRef]
- Kolb, H.; Stumvoll, M.; Kramer, W.; Kempf, K.; Martin, S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018, 16, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corkey, B.E. Banting lecture 2011: Hyperinsulinemia: Cause or consequence? Diabetes 2012, 61, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Pories, W.J.; Dohm, G.L. Diabetes: Have we got it all wrong? Hyperinsulinism as the culprit: Surgery provides the evidence. Diabetes Care 2012, 35, 2438–2442. [Google Scholar] [CrossRef] [Green Version]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. 4. Lifestyle Management: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchini, M.; Sassi, F.; Lauer, J.A.; Lee, Y.Y.; Guajardo-Barron, V.; Chisholm, D. Tackling of unhealthy diets, physical inactivity, and obesity: Health effects and cost-effectiveness. Lancet 2010, 376, 1775–1784. [Google Scholar] [CrossRef]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; McGuckin, B.G.; Brill, C.; Mohammed, B.S.; Szapary, P.O.; Rader, D.J.; Edman, J.S.; Klein, S. A randomized trial of a low-carbohydrate diet for obesity. N. Engl. J. Med. 2003, 348, 2082–2090. [Google Scholar] [CrossRef] [Green Version]
- Astrup, A.; Meinert Larsen, T.; Harper, A. Atkins and other low-carbohydrate diets: Hoax or an effective tool for weight loss? Lancet 2004, 364, 897–899. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Ciotola, M.; Di Palo, C.; Scognamiglio, P.; Gicchino, M.; Petrizzo, M.; Saccomanno, F.; Beneduce, F.; Ceriello, A.; et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: A randomized trial. Ann. Intern. Med. 2009, 151, 306–314. [Google Scholar] [CrossRef]
- Pitt, C.E. Cutting through the Paleo hype: The evidence for the Palaeolithic diet. Aust. Fam. Physician 2016, 45, 35–38. [Google Scholar]
- Walczyk, T.; Wick, J.Y. The Ketogenic Diet: Making a Comeback. Consult. Pharm. 2017, 32, 388–396. [Google Scholar] [CrossRef]
- Kempf, K.; Altpeter, B.; Berger, J.; Reuss, O.; Fuchs, M.; Schneider, M.; Gartner, B.; Niedermeier, K.; Martin, S. Efficacy of the Telemedical Lifestyle intervention Program TeLiPro in Advanced Stages of Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2017, 40, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Kempf, K.; Röhling, M.; Niedermeier, K.; Gärtner, B.; Martin, S. Individualized Meal Replacement Therapy Improves Clinically Relevant Long-Term Glycemic Control in Poorly Controlled Type 2 Diabetes Patients. Nutrients 2018, 10, 1022. [Google Scholar] [CrossRef] [Green Version]
- Röhling, M.; Kempf, K.; Banzer, W.; Berg, A.; Braumann, K.M.; Tan, S.; Halle, M.; McCarthy, D.; Pinget, M.; Predel, H.G.; et al. Prediabetes Conversion to Normoglycemia Is Superior Adding a Low-Carbohydrate and Energy Deficit Formula Diet to Lifestyle Intervention-A 12-Month Subanalysis of the ACOORH Trial. Nutrients 2020, 12, 2022. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Kruse, J.; Martin, S. ROSSO-in-praxi follow-up: Long-term effects of self-monitoring of blood glucose on weight, hemoglobin A1c, and quality of life in patients with type 2 diabetes mellitus. Diabetes Technol. Ther. 2012, 14, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Kruse, J.; Martin, S. ROSSO-in-praxi: A self-monitoring of blood glucose-structured 12-week lifestyle intervention significantly improves glucometabolic control of patients with type 2 diabetes mellitus. Diabetes Technol. Ther. 2010, 12, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Röhling, M.; Stichert, M.; Fischer, G.; Boschem, E.; Könner, J.; Martin, S. Telemedical Coaching Improves Long-Term Weight Loss in Overweight Persons: A Randomized Controlled Trial. Int. J. Telemed. Appl. 2018, 2018, 7530602. [Google Scholar] [CrossRef] [Green Version]
- Kempf, K.; Röhling, M.; Martin, S.; Schneider, M. Telemedical coaching for weight loss in overweight employees: A three-armed randomised controlled trial. BMJ Open 2019, 9, e022242. [Google Scholar] [CrossRef] [Green Version]
- Kempf, K.; Dirk, M.; Kolb, H.; Hebestreit, A.; Bittner, G.; Martin, S. The Da Vinci Medical-mental motivation program for supporting lifestyle changes in patients with type 2 diabetes. Dtsch Med. Wochenschr. 2012, 137, 362–367. [Google Scholar] [PubMed]
- Martin, S.; Kempf, K. Das Neue Diabetes-Programm: Mit Protein-Shakes den Blutzucker Senken und Abnehmen (Deutsch) Taschenbuch; Trias: Stuttgart, Germany, 2019. [Google Scholar]
- Fuller, N.J.; Jebb, S.A.; Laskey, M.A.; Coward, W.A.; Elia, M. Four-component model for the assessment of body composition in humans: Comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clin. Sci. 1992, 82, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [Green Version]
- Frey, I.; Berg, A.; Grathwohl, D.; Keul, J. [Freiburg Questionnaire of physical activity--development, evaluation and application]. Soz Praventivmed. 1999, 44, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindstrom, J.; Louheranta, A.; Mannelin, M.; Rastas, M.; Salminen, V.; Eriksson, J.; Uusitupa, M.; Tuomilehto, J. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003, 26, 3230–3236. [Google Scholar] [CrossRef] [Green Version]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Kosaka, K.; Noda, M.; Kuzuya, T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGT males. Diabetes Res. Clin. Pract. 2005, 67, 152–162. [Google Scholar] [CrossRef]
- Leslie, W.S.; Taylor, R.; Harris, L.; Lean, M.E. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: Systematic review and meta-analysis. Int. J. Obes. 2017, 41, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; Belinova, L.; Malinska, H.; Oliyarnyk, O.; Trnovska, J.; Skop, V.; Kazdova, L.; Dezortova, M.; Hajek, M.; Tura, A.; et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: A randomised crossover study. Diabetologia 2014, 57, 1552–1560. [Google Scholar] [CrossRef] [Green Version]
- Jackness, C.; Karmally, W.; Febres, G.; Conwell, I.M.; Ahmed, L.; Bessler, M.; McMahon, D.J.; Korner, J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell Function in type 2 diabetic patients. Diabetes 2013, 62, 3027–3032. [Google Scholar] [CrossRef] [Green Version]
- Appel, L.J.; Clark, J.M.; Yeh, H.C.; Wang, N.Y.; Coughlin, J.W.; Daumit, G.; Miller, E.R., 3rd; Dalcin, A.; Jerome, G.J.; Geller, S.; et al. Comparative effectiveness of weight-loss interventions in clinical practice. N. Engl. J. Med. 2011, 365, 1959–1968. [Google Scholar] [CrossRef] [Green Version]
- Holzapfel, C.; Merl, M.; Stecher, L.; Hauner, H. One-Year Weight Loss with a Telephone-Based Lifestyle Program. Obes. Facts 2016, 9, 230–240. [Google Scholar] [CrossRef]
- Loffler, A.; Luck, T.; Then, F.S.; Sikorski, C.; Kovacs, P.; Bottcher, Y.; Breitfeld, J.; Tonjes, A.; Horstmann, A.; Loffler, M.; et al. Eating Behaviour in the General Population: An Analysis of the Factor Structure of the German Version of the Three-Factor-Eating-Questionnaire (TFEQ) and Its Association with the Body Mass Index. PLoS ONE 2015, 10, e0133977. [Google Scholar] [CrossRef]
- Miller, C.K.; Kristeller, J.L.; Headings, A.; Nagaraja, H. Comparison of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: A randomized controlled trial. Health Educ. Behav. 2014, 41, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurkkala, M.; Kaikkonen, K.; Vanhala, M.L.; Karhunen, L.; Keranen, A.M.; Korpelainen, R. Lifestyle intervention has a beneficial effect on eating behavior and long-term weight loss in obese adults. Eat. Behav. 2015, 18, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Luley, C.; Blaik, A.; Gotz, A.; Kicherer, F.; Kropf, S.; Isermann, B.; Stumm, G.; Westphal, S. Weight loss by telemonitoring of nutrition and physical activity in patients with metabolic syndrome for 1 year. J. Am. Coll. Nutr. 2014, 33, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef] [Green Version]
- Lehnert, T.; Stuhldreher, N.; Streltchenia, P.; Riedel-Heller, S.G.; Konig, H.H. Sick leave days and costs associated with overweight and obesity in Germany. J. Occup. Environ. Med. 2014, 56, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, D.E.; Maciejewski, M.L.; Tsevat, J. Impact of morbid obesity on medical expenditures in adults. Int. J. Obes. 2005, 29, 334–339. [Google Scholar] [CrossRef] [Green Version]

| SI-Group (n = 15) | WL-Group (n = 15) | p | |
|---|---|---|---|
| Anthropometrics | |||
| Sex [n] (male%) | 3 (20%) | 2 (13%) | 0.624 |
| Age [years] | 44 ± 9 | 49 ± 7 | 0.085 |
| Weight [kg] | 104 ± 25 | 92 ± 14 | 0.095 |
| Body Mass Index [kg/m2] | 35.1 ± 6.9 | 32.8 ± 6.1 | 0.346 |
| Waist circumference [cm] | 106 ± 19 | 101 ± 12 | 0.407 |
| Fat mass [%] | 43 ± 7 | 44 ± 8 | 0.486 |
| SBP [mmHg] | 121 ± 14 | 112 ± 31 | 0.354 |
| DBP [mmHg] | 69 ± 8 | 68 ± 7 | 0.636 |
| Laboratory parameters | |||
| Triglycerides [mg/dL] | 180 ± 199 | 111 ± 48 | 0.208 |
| Total cholesterol [mg/dL] | 210 ± 46 | 214 ± 33 | 0.765 |
| HDL cholesterol [mg/dL] | 61 ± 16 | 62 ± 11 | 0.776 |
| LDL cholesterol [mg/dL] | 129 ± 38 | 140 ± 27 | 0.340 |
| HbA1c [%] [mmol/mol] | 6.2 ± 1.7 44 ± 19 | 5.5 ± 0.3 37 ± 3 | 0.167 |
| Fasting blood glucose [mg/dL] | 113 ± 41 | 95 ± 10 | 0.128 |
| Fasting insulin [µU/mL] | 11.8 ± 3.9 | 13.1 ± 7.9 | 0.576 |
| HOMA-IR | 3.4 ± 2.2 | 3.2 ± 2.2 | 0.928 |
| Cardiometabolic risk | |||
| FRS | 7.6 ± 5.9 | 10.2 ± 5.2 | 0.250 |
| Criteria of metabolic syndrome | 2.1 ± 0.7 | 1.5 ± 1.0 | 0.540 |
| Eating behaviour | |||
| TFEQ—Cognitive control [au] | 8.6 ± 3.0 | 8.9 ± 3.0 | 0.768 |
| TFEQ—Suggestibility [au] | 9.9 ± 3.6 | 8.6 ± 3.3 | 0.303 |
| TFEQ—Hunger [au] | 6.9 ± 3.2 | 6.1 ± 4.1 | 0.562 |
| Quality of life | |||
| SF12—Physical health [au] | 48 ± 9 | 50 ± 9 | 0.385 |
| SF12—Mental health [au] | 35 ± 7 | 39 ± 5 | 0.131 |
| Physical activity | |||
| FFkA—Sports per week [min/week] | 26 ± 47 | 113 ± 219 | 0.147 |
| FFkA—Physically active per week [min/week] | 338 ± 350 | 628 ± 941 | 0.276 |
| ETD | PP | PP | p | ETD | ITT | ITT | p | |
|---|---|---|---|---|---|---|---|---|
| SI-Group (n = 14) | WL-Group (n = 14) | SI-Group (n = 15) | WL-Group (n = 15) | |||||
| Anthropometrics | ||||||||
| Weight [kg] | −6.3 [−7.4; −4.5] | −7.8 [−9.7; −5.9] | −1.5 [−3.4; 0.5] | <0.001 | −5.9 [−8.1; −4.1] | −7.3 [−9.3; −5.4] | −1.4 [−3.3; 0.6] | 0.001 |
| Weight [%] | −6.8 [−8.0; −3.9] | −8.4 [−12.2; −4.6] | −1.6 [−3.9; 0.8] | <0.001 | −6.3 [−7.8; −3.7] | −8.0 [−11.5; −3.5] | −1.5 [−3.6; 0.6] | <0.001 |
| BMI [kg/m2] | −2.3 [−3.4; −1.2] | −2.5 [−3.1; −1.9] | −0.5 [−1.1; 0.1] | <0.001 | −2.1 [−3.1; −1.0] | −2.4 [−3.0; −1.8] | −0.4 [−1.1; 0.2] | <0.001 |
| Waist circum-ference [cm] | −6.4 [−10.0; −2.8] | −7.7 [−10.1; −5.2] | −1.9 [−4.4; 0.5] | <0.001 | −6.2 [−9.5; −2.3] | −7.6 [−9.8; −6.0] | −1.8 [−3.7; 0.1] | <0.001 |
| Fat mass [%] | −2.7 [−4.3; −1.2] | −3.8 [−4.7; −2.8] | −0.3 [−1.3; 0.7] | <0.001 | −2.2 [−3.1; −0.9] | −3.5 [−4.5; −2.5] | −0.3 [−1.3; 0.7] | <0.001 |
| SBP [mmHg] | − 1.0 [−12; 10] | −11 [−22; −1] | −7 [−17; 6] | 0.522 | − 0.5 [−10; 8] | −10 [−20; −1] | −6 [−16; 3] | 0.530 |
| DBP [mmHg] | 0 [−2;2] | −3 [−9; 4] | −3 [−9; 4] | 1.000 | −1 [−3;2] | −2 [−7; 3] | −3 [−8; 3] | 0.990 |
| Laboratory parameters | ||||||||
| Triglycerides [mg/dL] | −40 [−70; 10] | −62 [−134; 9] | −1 [−72; 72] | 0.218 | −25 [−55; 20] | −58 [−125; 9] | −1 [−67; 66] | 0.220 |
| Total cholesterol [mg/dL] | −6 [−32; 20] | −23 [−37; −9] | −11 [−25; 3] | 0.200 | −4 [−25; 16] | −21 [−35; −8] | −11 [−24; 3] | 0.248 |
| HDL cholesterol [mg/dL] | 3 [−6; 9] | −3 [−8; 2] | −6 [−11; −1] | 0.350 | 2 [−5; 7] | −3 [−7; 2] | −5 [−10; −1] | 0.443 |
| LDL cholesterol [mg/dL] | −9 [−14; 5] | −12 [−25; 2] | −2 [−15; 12] | 0.161 | −8 [−16; 4] | −11 [−24; 1] | −1 [−14; 11] | 0.280 |
| HbA1c [%] [mmol/mol] | −0.10 [−0.40; 0.30] −1.0 [−4.4; 3.3] | −0.57 [−1.08; −0.07] −6.2 [−11.8; −0.8] | −0.06 [−0.57; 0.44] −0.7 [−6.2; 4.8] | 0.356 | −0.08 [−0.32; 0.16] −0.9 [−3.5; 1.7] | −0.53 [−1.00; −0.06] −5.8 [−10.9; −0.7] | −0.06 [−0.53; 0.41] −0.7 [−5.3; 4.5] | 0.167 |
| Fasting blood glucose [mg/dL] | −3 [−16; 11] | −15 [−29; −1] | −1 [−15; 13] | 0.104 | −2 [−13; 9] | −13 [−26; −1] | −1 [−14; 12] | 0.178 |
| Fasting insulin [µU/mL] | −0.5 [−3.7; 2.7] | −3.5 [−5.9; −1.1] | −3.0 [−5.4; −0.6] | 0.777 | −0.3 [−3.0; 2.4] | −3.2 [−5.5; −1.0] | −2.8 [−5.1; −0.5] | 0.780 |
| HOMA-IR | −0.5 [−1.4; 0.7] | −1.5 [−2.4; −0.5] | −0.8 [−1.7; 0.2] | 0.139 | −0.4 [−1.2; 0.6] | −1.4 [−2.3; −0.4] | −0.7 [−1.6; 0.1] | 0.217 |
| Cardiometabolic risk | ||||||||
| Framingham Risk score | −0.4 [−1.0; 0.6] | −1.4 [−2.6; −0.2] | −0.8 [−2.0; 0.5] | 0.152 | −0.3 [−0.8; 0.5] | −1.3 [−2.1; −0.3] | −0.6 [−1.4; 0.2] | 0.248 |
| Criteria of metabolic syndrome | −0.2 [−0.6; 0.2] | −0.5 [−1.0; 0.1] | −0.1 [−0.6; 0.5] | 0.277 | −0.1 [−0.5; 0.4] | −0.4 [−0.9; 0.5] | 0 [−0.4; 0.4] | 0.420 |
| Eating behaviour | ||||||||
| TFEQ—Cognitive control [au] | 3.5 [2.7; 4.1] | 5.7 [3.9; 7.5] | 1.6 [−0.2; 3.4] | 0.003 | 3.0 [2.1; 3.8] | 5.3 [3.5; 7.1] | 1.5 [−0.3; 3.3] | 0.004 |
| TFEQ—Suggestibility [au] | −2.2 [−3.0; −0.5] | −2.4 [−3.8; −0.9] | 0.2 [−1.7; 1.2] | 0.017 | −1.6 [−2.4; −1.0] | −2.2 [−3.6; −0.8] | 0.2 [−1.2; 1.6] | 0.025 |
| TFEQ—Hunger [au] | −1.7 [−2.5; −1.0] | −2.1 [−3.0; −1.3] | 0.1 [−0.7; 1.0] | 0.001 | −1.2 [−1.9; −0.7] | −2.0 [−2.8; −1.2] | 0.1 [−0.7; 0.9] | 0.001 |
| Quality of life | ||||||||
| SF12—Physical health [au] | 0.4 [−1.0; 1.8] | 1.6 [−1.6; 4.9] | 0.9 [−2.4; 4.2] | 0.748 | 0.2 [−0.7; 1.2] | 1.5 [−1.5; 4.6] | 0.9 [−2.2; 3.9] | 0.755 |
| SF12—Mental health [au] | −0.2 [−2.4; 2.1] | −0.1 [−4.2; 4.1] | 0.2 [−4.0; 4.4] | 0.928 | −0.1 [−2.0; 1.9] | −0.1 [−4.2; 4.1] | −0.1 [−3.9; 3.8] | 1.000 |
| Physical activity | ||||||||
| FFkA—Sports per week [min/week] | 40 [−60; 100] | 61 [−16; 139] | −20 [−98; 57] | 0.139 | 30 [−45; 85] | 57 [−15; 129] | −19 [−91; 53] | 0.147 |
| FFkA—Physically active per week [min/week] | 315 [−50; 452] | 505 [193; 817] | 79 [−233; 391] | 0.058 | 225 [−70; 320] | 471 [177; 765] | 73 [−220; 367] | 0.060 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röhling, M.; Martin, K.; Ellinger, S.; Schreiber, M.; Martin, S.; Kempf, K. Weight Reduction by the Low-Insulin-Method—A Randomized Controlled Trial. Nutrients 2020, 12, 3004. https://doi.org/10.3390/nu12103004
Röhling M, Martin K, Ellinger S, Schreiber M, Martin S, Kempf K. Weight Reduction by the Low-Insulin-Method—A Randomized Controlled Trial. Nutrients. 2020; 12(10):3004. https://doi.org/10.3390/nu12103004
Chicago/Turabian StyleRöhling, Martin, Katharina Martin, Sabine Ellinger, Michael Schreiber, Stephan Martin, and Kerstin Kempf. 2020. "Weight Reduction by the Low-Insulin-Method—A Randomized Controlled Trial" Nutrients 12, no. 10: 3004. https://doi.org/10.3390/nu12103004





