Abscisic Acid Treatment in Patients with Prediabetes
Abstract
:1. Introduction
2. Materials and Methods
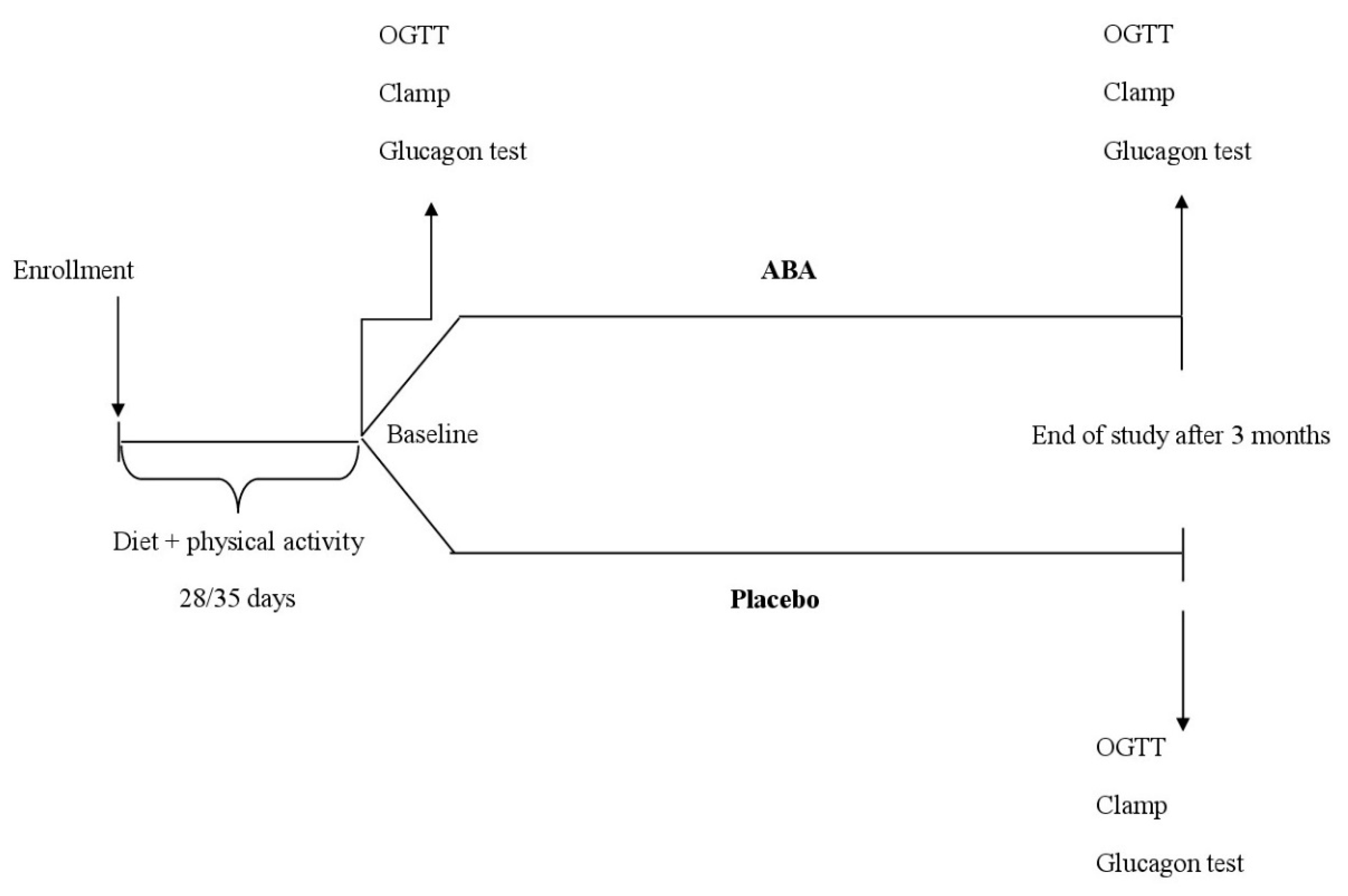
2.1. Study Design
2.2. Patients
2.3. Treatments
2.4. Assessments
2.5. Oral Glucose Tolerance Test
2.6. Euglycemic Hyperinsulinemic Clamp Technique
2.7. Glucagon Stimulation Test Technique
2.8. Statistical Analysis
3. Results
3.1. Study Sample
3.2. Anthropometric Parameters
3.3. Glyco-Metabolic Parameters
3.4. Lipid Profile
3.5. Inflammation Parameter
3.6. OGTT Results
3.7. M Value during Euglycemic Hyperinsulinemic Clamp
3.8. Glucagon Test Results
3.9. Safety and Treatment Acceptance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kanat, M.; DeFronzo, R.A.; Abdul-Ghani, M.A. Treatment of prediabetes. World J. Diabetes 2015, 6, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2017, 40 (Suppl. S1), S11–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, D.M.; Davidson, M.B.; DeFronzo, R.A.; Heine, R.J.; Henry, R.R.; Pratley, R.; Zinman, B. American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: Implications for care. Diabetes Care 2007, 30, 753–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertram, M.Y.; Vos, T. Quantifying the duration of pre-diabetes. Aust. N. Z. J. Public Health 2010, 34, 311–314. [Google Scholar] [CrossRef]
- Phillips, L.S.; Ratner, R.E.; Buse, J.B.; Kahn, S.E. We can change the natural history of type 2 diabetes. Diabetes Care 2014, 37, 2668–2676. [Google Scholar] [CrossRef] [Green Version]
- Glechner, A.; Keuchel, L.; Affengruber, L.; Titscher, V.; Sommer, I.; Matyas, N.; Wagner, G.; Kien, C.; Klerings, I.; Gartlehner, G. Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2018, 12, 393–408. [Google Scholar] [CrossRef]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [Green Version]
- Wing, R.R.; Goldstein, M.G.; Acton, K.J.; Birch, L.L.; Jakicic, J.M.; Sallis, J.F.; Smith-West, D.; Jeffery, R.W.; Surwit, R.S. Behavioral science research in diabetes: Lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care 2001, 24, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Middleton, K.R.; Anton, S.D.; Perri, M.G. Long-Term adherence to health behavior change. Am. J. Lifestyle Med. 2013, 7, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Derosa, G.; D’Angelo, A.; Vanelli, A.; Maffioli, P. An evaluation of a nutraceutical with Berberine, Curcumin, Inositol, Banaba and Chromium Picolinate in patients with fasting dysglycemia. Diabetes Metab. Syndr. Obes. 2020, 13, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Derosa, G.; Cicero, A.F.G.; D’Angelo, A.; Maffioli, P. Ascophyllum nodosum and Fucus vesiculosus on glycemic status and on endothelial damage markers in dysglicemic patients. Phytother. Res. 2019, 33, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Maffioli, P. Ilex paraguariensis, white mulberry and chromium picolinate in patients with pre-diabetes. Phytother. Res. 2020, 34, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philipson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic acid: A novel nutraceutical for glycemic control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Bruzzone, S.; Bodrato, N.; Usai, C.; Guida, L.; Moreschi, I.; Nano, R.; Antonioli, B.; Fruscione, F.; Magnone, M.; Scarfì, S.; et al. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. J. Biol. Chem. 2008, 283, 32188–32197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruzzone, S.; Ameri, P.; Briatore, L.; Mannino, E.; Basile, G.; Andraghetti, G.; Grozio, A.; Magnone, M.; Guida, L.; Scarfì, S.; et al. The plant hormone abscisic acid increases in human plasma after hyperglycemia and stimulates glucose consumption by adipocytes and myoblasts. FASEB J. 2012, 26, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Ameri, P.; Bruzzone, S.; Mannino, E.; Sociali, G.; Andraghetti, G.; Salis, A.; Ponta, M.L.; Briatore, L.; Adami, G.F.; Ferraiolo, A.; et al. Impaired increase of plasma abscisic acid in response to oral glucose load in type 2 diabetes and in gestational diabetes. PLoS ONE 2015, 10, e0115992. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, F.S.; Villar, A.; Mulà, A.; Zangara, A.; Risco, E.; Smidt, C.R.; Hontecillas, R.; Leber, A.; Bassaganya-Riera, J. Abscisic acid standardized fig (Ficus carica) extracts ameliorate postprandial glycemic and insulinemic responses in healthy adults. Nutrients 2019, 11, 1757. [Google Scholar] [CrossRef] [Green Version]
- Proposed International Guidelines for Biomedical Research Involving Human Subjects; The Council for International Organisation of Medical Sciences: Geneva, Switzerland, 1982.
- Derosa, G.; Carbone, A.; Franzetti, I.; Querci, F.; Fogari, E.; Bianchi, L. Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, β-cell function and insulin resistance in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2012, 98, 51–60. [Google Scholar] [CrossRef]
- Derosa, G.; Cicero, A.F.; D’Angelo, A.; Borghi, C.; Maffioli, P. Effects of n-3 pufas on fasting plasma glucose and insulin resistance in patients with impaired fasting glucose or impaired glucose tolerance. Biofactors 2016, 42, 316–322. [Google Scholar]
- De Fronzo, R.A.; Tobin, J.A.; Andres, B. Glucose clamp technique, a method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, 214–223. [Google Scholar]
- Miki, H.; Matsuyama, T.; Fujii, S.; Komatsu, R.; Nishioeda, Y.; Omae, T. Glucagon-glucose (GG) test for the estimation of the insulin reserve in diabetes. Diabetes Res. Clin. Pract. 1992, 18, 99–105. [Google Scholar] [CrossRef]
- Winer, B.J. Statistical Principles in Experimental Design, 2nd ed.; McGraw-Hill: New York, NY, USA, 1971. [Google Scholar]
- Magnone, M.; Leoncini, G.; Vigliarolo, T.; Emionite, L.; Sturla, L.; Zocchi, E.; Murialdo, G. Chronic intake of micrograms of abscisic acid improves glycemia and lipidemia in a human study and in high-glucose fed mice. Nutrients 2018, 10, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnone, M.; Ameri, P.; Salis, A.; Andraghetti, G.; Emionite, L.; Murialdo, G.; De Flora, A.; Zocchi, E. Microgram amounts of abscisic acid in fruit extracts improve glucose tolerance and reduce insulinemia in rats and in humans. FASEB J. 2015, 29, 4783–4793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guri, A.J.; Hontecillas, R.; Si, H.; Liu, D.; Bassaganya-Riera, J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/ db mice fed high-fat diets. Clin. Nutr. 2007, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.; Fresia, C.; Guida, L.; Bruzzone, S.; Scarfi, S.; Usai, C.; Fruscione, F.; Magnone, M.; Millo, E.; Basile, G.; et al. LANCL2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J. Biol. Chem. 2009, 284, 28045–28057. [Google Scholar] [CrossRef] [Green Version]
- Bassaganya-Riera, J.; Guri, A.J.; Lu, P.; Climent, M.; Carbo, A.; Sobral, B.W.; Horne, W.T.; Lewis, S.N.; Bevan, D.R.; Hontecillas, R. Abscisic acid regulates inflammation via ligand-binding domain-independent activation of peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2011, 286, 2504–2516. [Google Scholar] [CrossRef] [Green Version]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. 2), S1–S45. [Google Scholar] [PubMed] [Green Version]
- Guri, A.J.; Hontecillas, R.; Bassaganya-Riera, J. Abscisic acid synergizes with rosiglitazone to improve glucose tolerance and down-modulate macrophage accumulation in adipose tissue: Possible action of the cAMP/PKA/PPAR γ axis. Clin. Nutr. 2010, 29, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Derosa, G.; Bonaventura, A.; Bianchi, L.; Romano, D.; D’Angelo, A.; Fogari, E.; Maffioli, P. Berberis aristata/Silybum marianum fixed combination on lipid profile and insulin secretion in dyslipidemic patients. Expert Opin. Biol. Ther. 2013, 13, 1495–1506. [Google Scholar] [CrossRef]
| Parameters | ABA | Placebo | ||
|---|---|---|---|---|
| Baseline | 3 Months | Baseline | 3 Months | |
| Patients (n) | 33 | 30 | 32 | 30 |
| M/F | 15/18 | 14/16 | 16/16 | 15/15 |
| Age (years) | 51.9 ± 6.5 | - | 52.2 ± 6.8 | - |
| Smoking status (M/F) | 7/6 | 6/5 | 8/6 | 6/6 |
| IFG (n; %) | 6/7 (39.4) | 3/3 (26.7) | 5/6 (34.4) | 4/5 (30.0) |
| IGT (n; %) | 9/11 (60.6) | 3/10 (46.7) | 11/10 (65.6) | 10/9 (63.3) |
| EU from IFG (n; %) | - | 5/3 (61.5) | - | 0/0 |
| EU from IGT (n; %) | - | 0 | - | 0/0 |
| IFG from IGT (n; %) | - | 0/0 | - | 1/0 (4.8) |
| IGT from IFG (n; %) | - | 3/0 (23.1) | - | 0/0 |
| D from IFG (n; %) | - | 0/0 | - | 0/0 |
| D from IGT (n; %) | - | 0/0 | - | 1/1 (9.5) |
| Lost to FU from IFG (n; %) | - | 1/1 (15.4) | - | 0/1 (9.1) |
| Lost to FU from IGT (n; %) | - | 0/1 (7.7) | - | 1/0 (4.7) |
| Parameters | ABA | Placebo | ||
|---|---|---|---|---|
| Baseline | 3 Months | Baseline | 3 Months | |
| Height (cm) | 1.69 ± 0.05 | - | 1.68 ± 0.04 | - |
| Weight (kg) | 77.4 ± 6.1 | 76.5 ± 5.9 | 77.1 ± 5.9 | 76.2 ± 5.7 |
| BMI (kg/m2) | 27.1 ± 1.3 | 26.8 ± 1.1 | 27.3 ± 1.5 | 27.0 ± 1.2 |
| WC (cm) | 86.5 ± 4.9 | 86.4 ± 4.8 | 86.8 ± 5.0 | 86.7 ± 4.9 |
| HC (cm) | 89.2 ± 5.2 | 89.0 ± 5.0 | 88.9 ± 4.9 | 88.7 ± 4.7 |
| AC (cm) | 97.2 ± 5.8 | 97.0 ± 5.6 | 97.4 ± 6.0 | 97.2 ± 5.8 |
| FPG (mg/dL) | 109.4 ± 6.5 | 104.5 ± 6.1 *,^ | 112.8 ± 5.6 | 110.7 ± 5.5 |
| PPG (mg/dL) | 144.0 ± 12.8 | 130.1 ± 12.8 *,^ | 153.3 ± 18.4 | 149.7 ± 18.5 |
| HbA1c (%) | 5.9 ± 0.4 | 5.5 ± 0.2 * | 5.8 ± 0.3 | 5.7 ± 0.2 |
| FPI (μU/mL) | 10.3 ± 6.7 | 9.2 ± 5.8 *,^ | 10.1 ± 6.5 | 10.5 ± 6.9 |
| HOMA-IR | 2.80 ± 0.7 | 2.39 ± 0.4 *,^ | 2.84 ± 0.8 | 2.89 ± 0.9 |
| TC (mg/dL) | 215.1 ± 15.8 | 211.0 ± 14.2 | 218.6 ± 16.9 | 220.2 ± 18.1 |
| LDL-C (mg/dL) | 146.9 ± 18.4 | 143.4 ± 17.7 | 150.9 ± 19.2 | 152.6 ± 20.7 |
| HDL-C (mg/dL) | 43.8 ± 5.0 | 44.0 ± 5.1 | 43.6 ± 4.9 | 43.7 ± 4.8 |
| Tg (mg/dL) | 122.1 ± 24.2 | 117.3 ± 22.0 | 120.4 ± 23.5 | 119.5 ± 23.1 |
| AST (UI/L) | 18.8 ± 10.8 | 18.5 ± 10.4 | 18.2 ± 10.3 | 18.4 ± 10.5 |
| ALT (UI/L) | 28.3 ± 14.2 | 28.9 ± 14.5 | 26.8 ± 13.1 | 26.1 ± 12.8 |
| γ-GT (UI/L) | 24.5 ± 8.1 | 24.1 ± 7.7 | 25.8 ± 8.7 | 25.3 ± 8.4 |
| Creatinine (mg/dL) | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.8 ± 0.4 |
| Hs-CRP (mg/L) | 1.3 ± 0.5 | 1.0 ± 0.2 *,^ | 1.3 ± 0.5 | 1.4 ± 0.6 |
| N | Baseline | End of Treatment | Delta End of Treatment vs. Baseline | |
|---|---|---|---|---|
| ABA | 33 | 6.09 ± 0.51 | 7.38 ± 0.89 *,° | 1.29 ± 0.59 * |
| Placebo | 32 | 6.02 ± 0.37 | 6.03 ± 0.76 | 0.01 ± 0.006 |
| Baseline | 3 Months | |||
|---|---|---|---|---|
| Time 0 | 6 min | Time 0 | 6 min | |
| FPG (mg/dL) | 110.6 ± 7.3 | 148.6 ± 21.3 ^ | 103.8 ± 5.9 *,£ | 131.2 ± 16.1 ^,*,£ |
| C-peptide (ng/mL) | 7.15 ± 2.38 | 20.45 ± 7.59 ° | 9.02 ± 4.72 *,£ | 31.07 ± 10.15 °,$,§ |
| Baseline | 3 Months | |||
|---|---|---|---|---|
| Time 0 | 6 min | Time 0 | 6 min | |
| FPG (mg/dL) | 111.5 ± 7.9 | 146.1 ± 20.2 ^ | 112.1 ± 8.4 | 148.5 ± 21.4 ^ |
| C-peptide (ng/mL) | 7.28 ± 2.51 | 21.33 ± 7.91 ° | 7.02 ± 2.37 | 20.15 ± 6.88 ° |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derosa, G.; Maffioli, P.; D’Angelo, A.; Preti, P.S.; Tenore, G.; Novellino, E. Abscisic Acid Treatment in Patients with Prediabetes. Nutrients 2020, 12, 2931. https://doi.org/10.3390/nu12102931
Derosa G, Maffioli P, D’Angelo A, Preti PS, Tenore G, Novellino E. Abscisic Acid Treatment in Patients with Prediabetes. Nutrients. 2020; 12(10):2931. https://doi.org/10.3390/nu12102931
Chicago/Turabian StyleDerosa, Giuseppe, Pamela Maffioli, Angela D’Angelo, Paola S. Preti, Giancarlo Tenore, and Ettore Novellino. 2020. "Abscisic Acid Treatment in Patients with Prediabetes" Nutrients 12, no. 10: 2931. https://doi.org/10.3390/nu12102931





