Effects of Daily Consumption of an Aqueous Dispersion of Free-Phytosterols Nanoparticles on Individuals with Metabolic Syndrome: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
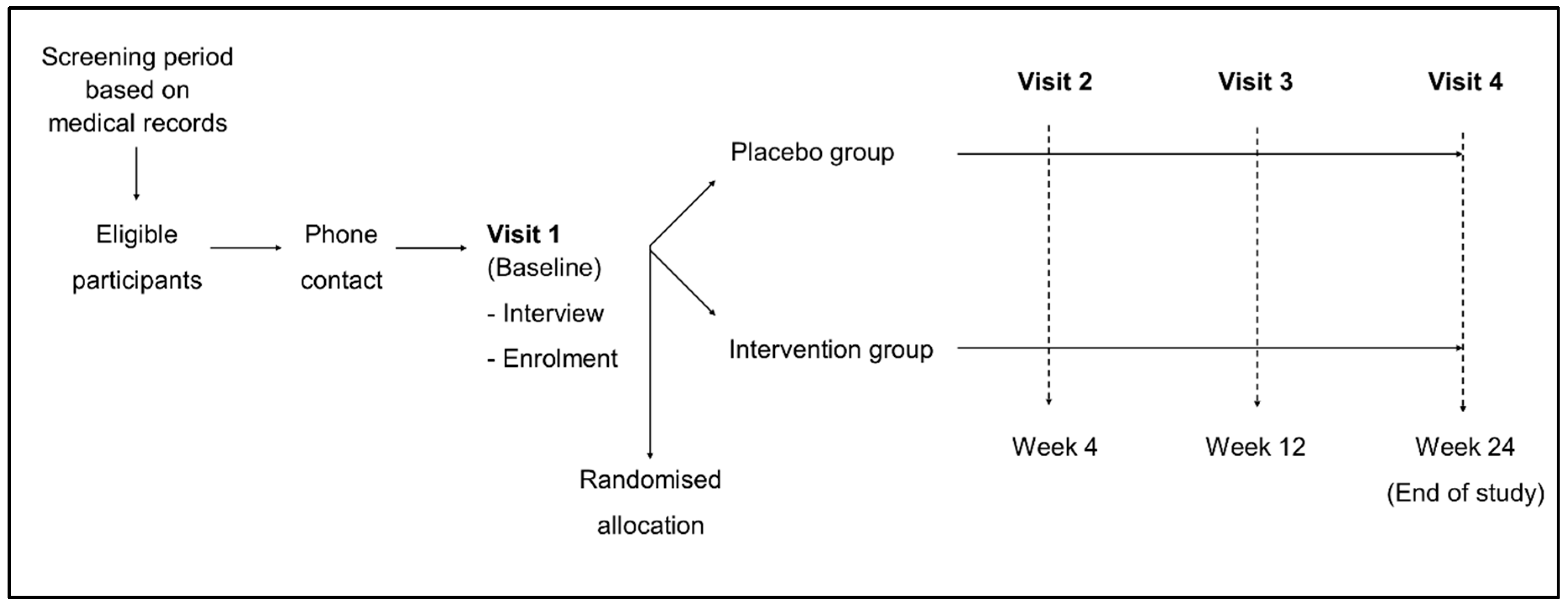
2.1. Design
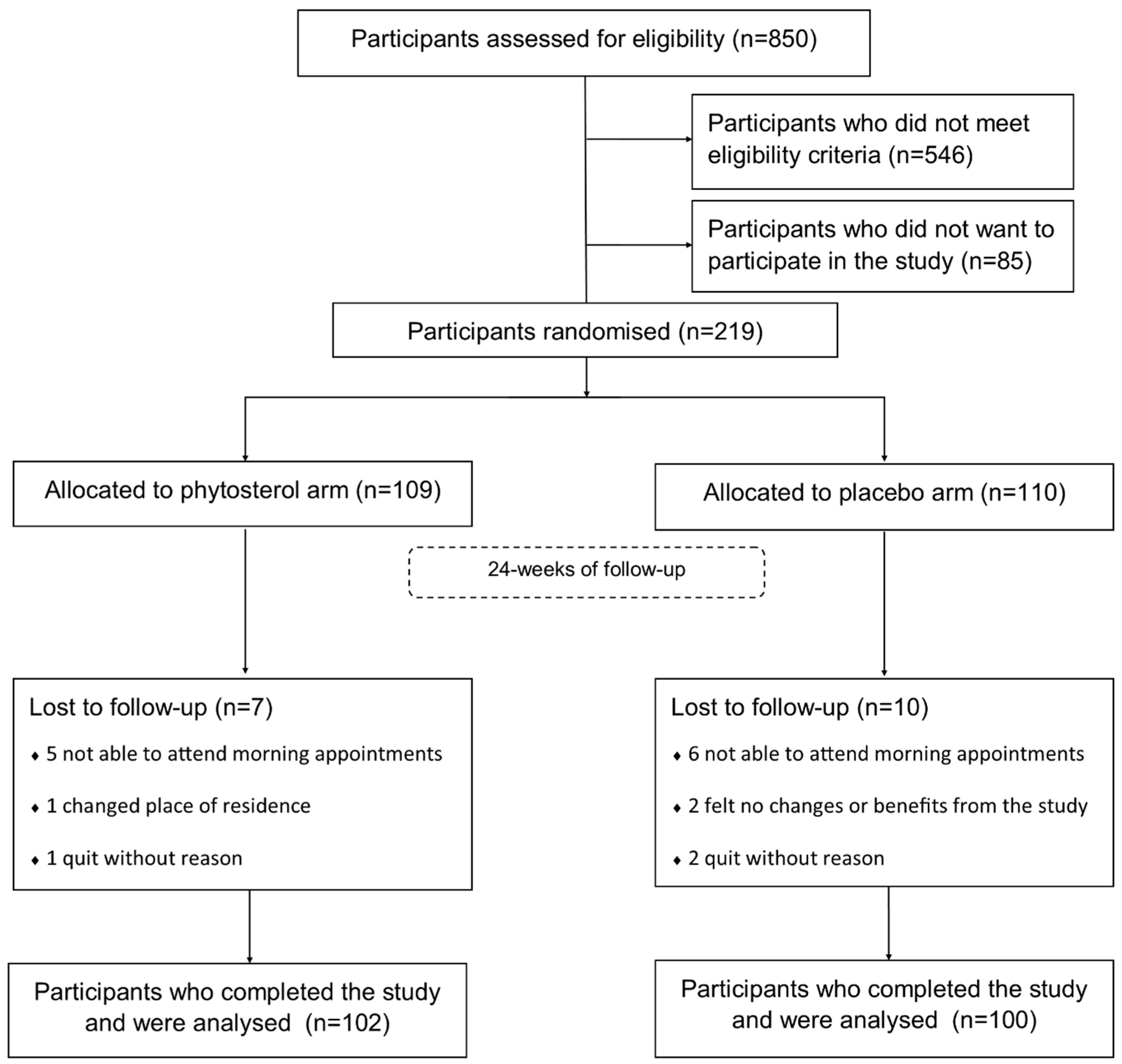
2.2. Participants
2.3. Procedures and Assessments
2.4. Randomisation and Masking
2.5. Intervention
2.6. Outcomes
2.7. Statistical Analyses
3. Results
3.1. Sample Characteristics
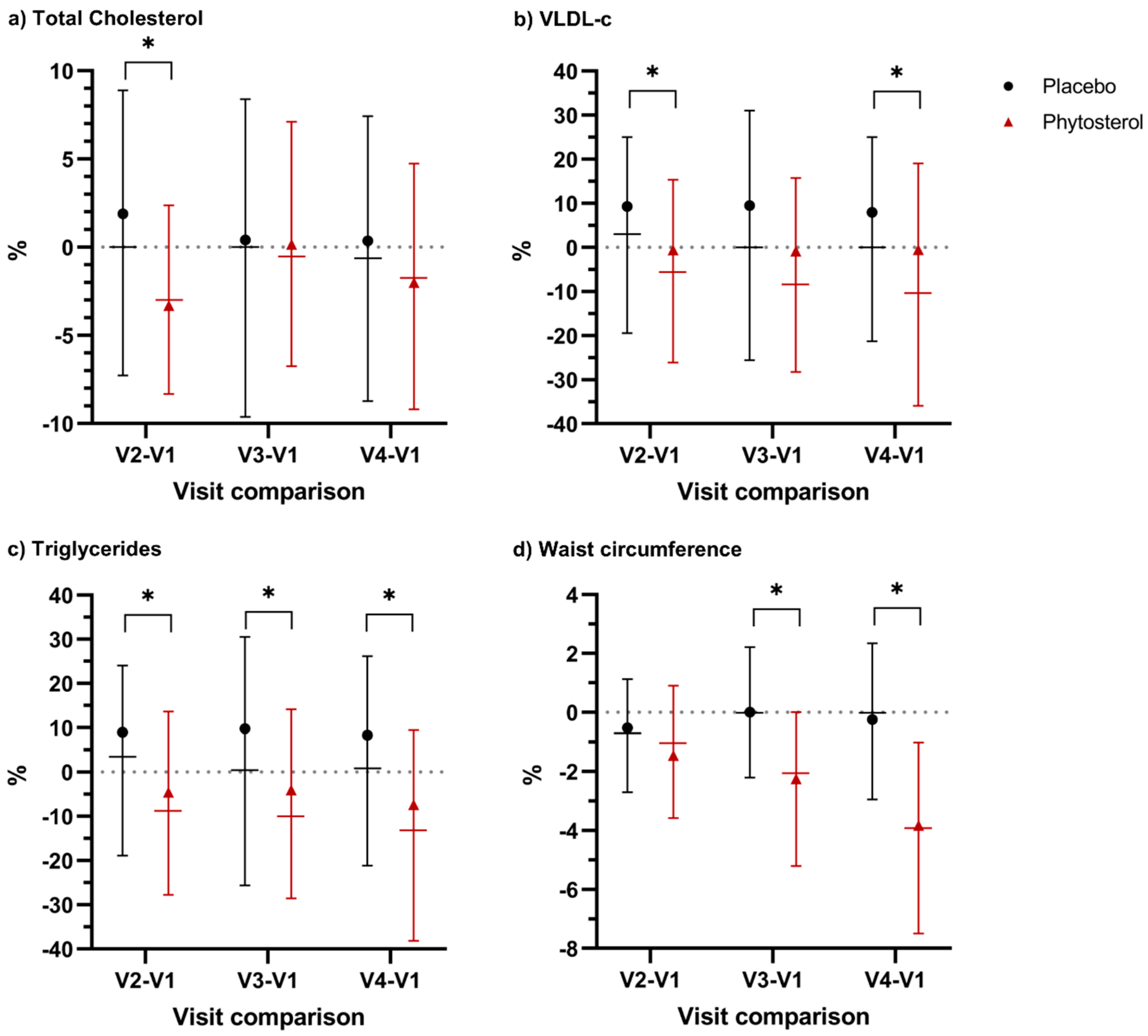
3.2. Primary Outcome
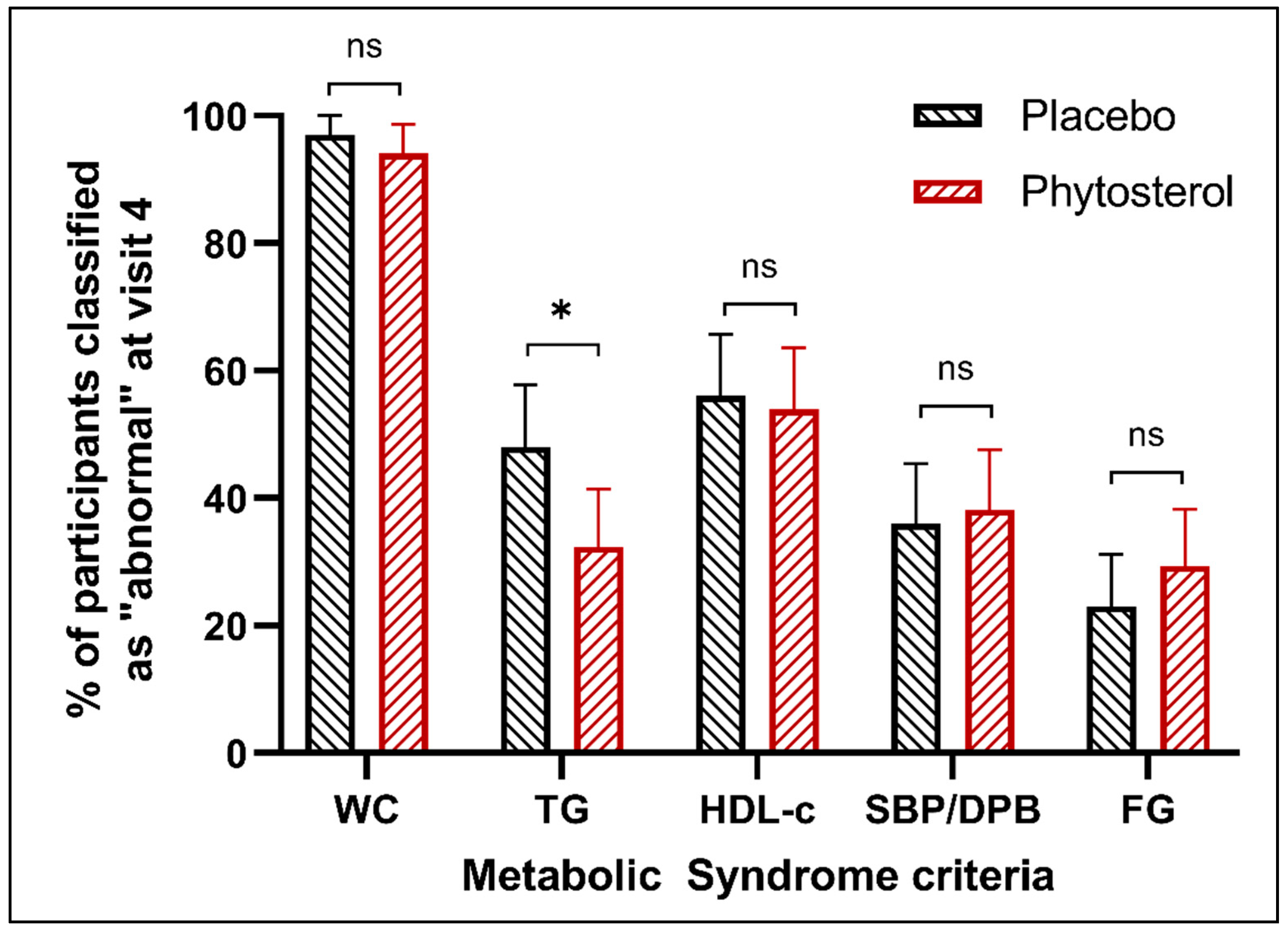
3.3. Secondary Outcomes
3.4. Safety Outcomes
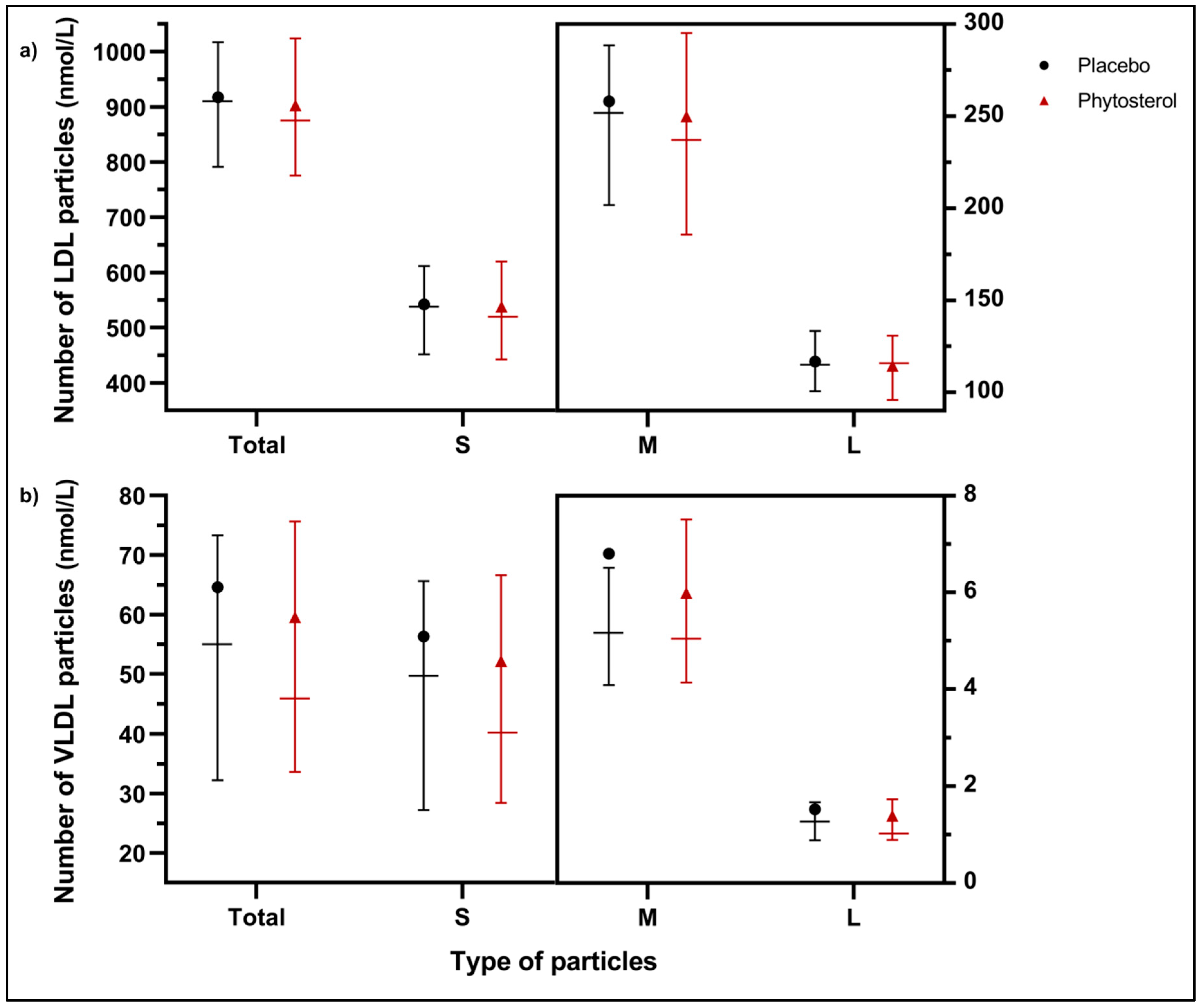
3.5. Exploratory Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2018, 14, 160287. [Google Scholar] [CrossRef] [Green Version]
- Cameron, A.J.; Magliano, D.J.; Zimmet, P.Z.; Welborn, T.; Shaw, J.E. The Metabolic Syndrome in Australia: Prevalence using four definitions. Diabetes Res. Clin. Pract. 2007, 77, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Yu, D.; Wang, Z.; Ding, G. Metabolic syndrome prevalence and its risk factors among adults in China: A nationally representative cross-sectional study. PLoS ONE 2018, 13, e0199293. [Google Scholar] [CrossRef] [PubMed]
- Sigit, F.S.; Tahapary, D.L.; Trompet, S.; Sartono, E.; Willems Van Dijk, K.; Rosendaal, F.R.; De Mutsert, R. The prevalence of metabolic syndrome and its association with body fat distribution in middle-aged individuals from Indonesia and the Netherlands: A cross-sectional analysis of two population-based studies. Diabetol. Metab. Syndr. 2020, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet-Ostaptchouk, J.V.; Nuotio, M.L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Cleeman, J.I.; Grundy, S.M.; Becker, D.; Clark, L. Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; IDF Communications: Brussels, Belgium, 2006. [Google Scholar]
- Balkau, B.; Charles, M.A. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443. [Google Scholar]
- National Cholesterol Education Program Coordinating Committee. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Einhorn, D.; Reaven, G.M.; Cobin, R.H.; Ford, E.; Ganda, O.P.; Handelsman, Y.; Hellman, R.; Jellinger, P.S.; Kendall, D.; Krauss, R.M.; et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003, 9, 237–252. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Berwick, Z.C.; Dick, G.M.; Tune, J.D. Heart of the matter: Coronary dysfunction in metabolic syndrome. J. Mol. Cell. Cardiol. 2012, 52, 848–856. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Hassen Abate, K.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease S. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Metabolic Syndrome: A Multiplex Cardiovascular Risk Factor. J. Clin. Endocrinol. Metab. 2007, 92, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Drug therapy of the metabolic syndrome: Minimizing the emerging crisis in polypharmacy. Nat. Rev. Drug Discov. 2006, 5, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Visioli, F. Pharmacology of nutraceuticals with lipid lowering properties. High Blood Press. Cardiovasc. Prev. 2019, 26, 113–118. [Google Scholar] [CrossRef]
- Rivellese, A.A.; Ciciola, P.; Costabile, G.; Vetrani, C.; Vitale, M. The Possible Role of Nutraceuticals in the Prevention of Cardiovascular Disease. High Blood Press. Cardiovasc. Prev. 2019, 26, 101–111. [Google Scholar] [CrossRef]
- Pollak, J. Reduction of Blood Cholesterol in Man. Circulation 1953, 7, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Reaver, A.; Hewlings, S.; Westerman, K.; Blander, G.; Schmeller, T.; Heer, M.; Rein, D. A randomized, placebo-controlled, double-blind crossover study to assess a unique phytosterol ester formulation in lowering LDL cholesterol utilizing a novel virtual tracking tool. Nutrients 2019, 11, 2108. [Google Scholar] [CrossRef] [Green Version]
- Ostlund, R.E. Phytosterols in Human Nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef]
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Plat, J.; Brufau, G.; Dallinga-Thie, G.M.; Dasselaar, M.; Mensink, R.P. A Plant Stanol Yogurt Drink Alone or Combined with a Low-Dose Statin Lowers Serum Triacylglycerol and Non-HDL Cholesterol in Metabolic Syndrome Patients. J. Nutr. 2009, 139, 1143–1149. [Google Scholar] [CrossRef] [Green Version]
- Sialvera, T.E.; Pounis, G.D.; Koutelidakis, A.E.; Richter, D.J.; Yfanti, G.; Kapsokefalou, M.; Goumas, G.; Chiotinis, N.; Diamantopoulos, E.; Zampelas, A. Phytosterols supplementation decreases plasma small and dense LDL levels in metabolic syndrome patients on a westernized type diet. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mijares, A.; Bañuls, C.; Jover, A.; Solá, E.; Bellod, L.; Martínez-Triguero, M.L.; Lagarda, M.J.; Víctor, V.M.; Rocha, M. Low intestinal cholesterol absorption is associated with a reduced efficacy of phytosterol esters as hypolipemic agents in patients with metabolic syndrome. Clin. Nutr. 2011, 30, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Huang, P.H.; Chiang, C.H.; Leu, H.B.; Chen, J.W.; Lin, S.J. Phytosterols increase circulating endothelial progenitor cells and insulin-like growth factor-1 levels in patients with nonalcoholic fatty liver disease: A randomized crossover study. J. Funct. Foods 2015, 13, 148–157. [Google Scholar] [CrossRef]
- Ghaedi, E.; Foshati, S.; Ziaei, R.; Beigrezaei, S.; Kord-Varkaneh, H.; Ghavami, A.; Miraghajani, M. Effects of phytosterols supplementation on blood pressure: A systematic review and meta-analysis. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Trautwein, E.; Vermeer, M.; Hiemstra, H.; Ras, R. LDL-Cholesterol Lowering of Plant Sterols and Stanols—Which Factors Influence Their Efficacy? Nutrients 2018, 10, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.J.A.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Fat type in phytosterol products influence their cholesterol-lowering potential: A systematic review and meta-analysis of RCTs. Prog. Lipid Res. 2016, 64, 16–29. [Google Scholar] [CrossRef]
- AbuMweis, S.S.; Barake, R.; Jones, P.J.H. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food Nutr. Res. 2008, 52. [Google Scholar] [CrossRef]
- Jones, P.J.H.; Shamloo, M.; MacKay, D.S.; Rideout, T.C.; Myrie, S.B.; Plat, J.; Roullet, J.-B.; Baer, D.J.; Calkins, K.L.; Davis, H.R.; et al. Progress and prospective of plant sterol and plant stanol research. Nutr. Rev. 2018, 76, 725–746. [Google Scholar] [CrossRef] [Green Version]
- Amir Shaghaghi, M.; Harding, S.V.; Jones, P.J.H. Water dispersible plant sterol formulation shows improved effect on lipid profile compared to plant sterol esters. J. Funct. Foods 2014, 6, 280–289. [Google Scholar] [CrossRef]
- Gurka, M.J.; Lilly, C.L.; Oliver, M.N.; DeBoer, M.D. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: A confirmatory factor analysis and a resulting continuous severity score. Metabolism 2014, 63, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Deboer, M.D.; Gurka, M.J. Clinical utility of metabolic syndrome severity scores: Considerations for practitioners. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, S.; Ras, R.T.; Trautwein, E.A.; Mensink, R.P.; Plat, J. Plasma fat-soluble vitamin and carotenoid concentrations after plant sterol and plant stanol consumption: A meta-analysis of randomized controlled trials. Eur. J. Nutr. 2017, 56, 909–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Ye, X.; Kong, W.; Zafar, M.I.; Chen, L.L. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Cardiovasc. Diabetol. 2019, 18, 48. [Google Scholar] [CrossRef] [Green Version]
- Castelli, W.P. Epidemiology of triglycerides: A view from Framingham. Am. J. Cardiol. 1992, 70, H3. [Google Scholar] [CrossRef]
- Assmann, G.; Schulte, H.; Von Eckardstein, A. Hypertriglyceridemia and elevated lipoprotein(a) are risk factors for major coronary events in middle-aged men. Am. J. Cardiol. 1996, 77, 1179–1184. [Google Scholar] [CrossRef]
- Visscher, T.L.S.; Seidell, J.C.; Molarius, A.; van der Kuip, D.; Hofman, A.; Witteman, J.C.M. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: The Rotterdam study. Int. J. Obes. 2001, 25, 1730–1735. [Google Scholar] [CrossRef] [Green Version]
- Dobbelsteyn, C.J.; Joffres, M.R.; MacLean, D.R.; Flowerdew, G.; Balram, C.; Blair, L.; Butler-Jones, D.; Cameron, R.; Collins-Nakai, R.; Connelly, P.W.; et al. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian heart health surveys. Int. J. Obes. 2001, 25, 652–661. [Google Scholar] [CrossRef] [Green Version]
- German, C.A.; Laughey, B.; Bertoni, A.G.; Yeboah, J. Associations between BMI, waist circumference, central obesity and outcomes in type II diabetes mellitus: The ACCORD Trial. J. Diabetes Complicat. 2020, 34, 107499. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Wareham, N.J.; Khaw, K.T. Changes in waist circumference and risk of all-cause and CVD mortality: Results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. BMC Cardiovasc. Disord. 2019, 19, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Gaskill, S.P.; Haffner, S.M.; Stern, M.P. Waist Circumference as the Best Predictor of Noninsulin Dependent Diabetes Mellitus (NIDDM) Compared to Body Mass Index, Waist/hip Ratio and Other Anthropometric Measurements in Mexican Americans-A 7-Year Prospective Study. Obes. Res. 1997, 5, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Su, J.; Liu, H. A study of the correlation of waist circumference with metabolic risks among non-obese populations. Eur. Rev. Medican Pharmacol. Sci. 2019, 23, 4391–4397. [Google Scholar]
- Naumann, E.; Plat, J.; Kester, A.D.M.; Mensink, R.P. The Baseline Serum Lipoprotein Profile Is Related to Plant Stanol Induced Changes in Serum Lipoprotein Cholesterol and Triacylglycerol Concentrations. J. Am. Coll. Nutr. 2008, 27, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Demonty, I.; Ras, R.T.; Van Der Knaap, H.C.M.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: A pooled analysis of 12 randomised controlled trials. Eur. J. Nutr. 2013, 52, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Rideout, T.C.; Marinangeli, C.P.F.; Harding, S. V Triglyceride-Lowering Response to Plant Sterol and Stanol Consumption. J. AOAC Int. 2015, 98, 707. [Google Scholar] [CrossRef] [Green Version]
- Plat, J.; Mensink, R.P. Plant stanol esters lower serum triacylglycerol concentrations via a reduced hepatic VLDL-1 production. Lipids 2009, 44, 1149–1153. [Google Scholar] [CrossRef] [Green Version]
- Smet, E.D.; Mensink, R.P.; Plat, J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol. Nutr. Food Res. 2012, 56, 1058–1072. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Duchateau, G.S.M.J.E.; Lin, Y.; Mel’nikov, S.M.; Molhuizen, H.O.F.; Ntanios, F.Y. Proposed mechanisms of cholesterol-lowering action of plant sterols. Eur. J. Lipid Sci. Technol. 2003, 105, 171–185. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Gong, L.; Li, Q.; Hu, J.; Zhang, S.; Wang, Y.; Zhou, H.; Yang, S.; Wang, Z. A Novel Visceral Adiposity Index for Prediction of Type 2 Diabetes and Pre-diabetes in Chinese adults: A 5-year prospective study. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.-K.; Cho, A.-R.; Kwon, Y.-J.; Lee, H.-S.; Lee, J.-W. Derivation and validation of a new visceral adiposity index for predicting visceral obesity and cardiometabolic risk in a Korean population. PLoS ONE 2018, 13, e0203787. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Tamborlane, W.V.; Maggs, D.G.; Enoksson, S.; Sherwin, R.S.; Silver, D.; Shulman, G.I.; Caprio, S. Effect of insulin on glycerol production in obese adolescents. Am. J. Physiol. Endocrinol. Metab. 1998, 274, E737–E743. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Bernard, S.; Näslund, E.; Salehpour, M.; Possnert, G.; Appelsved, L.; Fu, K.Y.; Alkass, K.; Druid, H.; Thorell, A.; et al. Impact of fat mass and distribution on lipid turnover in human adipose tissue. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Ginsberg, H.N. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol. Metab. 2011, 22, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Adiels, M.; Olofsson, S.-O.; Taskinen, M.-R.; Borén, J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1225–1236. [Google Scholar] [CrossRef]
- Ebbert, J.; Jensen, M. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef] [Green Version]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.; Guo, Z.K.; Johnson, C.M.; Hensrud, D.D.; Jensen, M.D. Splanchnic lipolysis in human obesity. J. Clin. Investig. 2004, 113, 1582–1588. [Google Scholar] [CrossRef] [Green Version]
- Riches, F.M.; Watts, G.F.; Hua, J.; Stewart, G.R.; Naoumova, R.P.; Barrett, P.H.R. Reduction in Visceral Adipose Tissue Is Associated with Improvement in Apolipoprotein B-100 Metabolism in Obese Men. J. Clin. Endocrinol. Metab. 1999, 84, 2854–2861. [Google Scholar] [CrossRef]
- Nielsen, S.; Karpe, F. Determinants of VLDL-triglycerides production. Curr. Opin. Lipidol. 2012, 23, 321–326. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Puska, P.; Gylling, H.; Vanhanen, H.; Vartiainen, E. Reduction of Serum Cholesterol with Sitostanol-Ester Margarine in a Mildly Hypercholesterolemic Population. N. Engl. J. Med. 1995, 333, 1308–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, H.F.J.; Brink, E.J.; Meijer, G.W.; Princen, H.M.G.; Ntanios, F.Y. Safety of long-term consumption of plant sterol esters-enriched spread. Eur. J. Clin. Nutr. 2003, 57, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Hallikainen, M.; Raitakari, O.T.; Laakso, M.; Vartiainen, E.; Salo, P.; Korpelainen, V.; Sundvall, J.; Miettinen, T.A. Long-term consumption of plant stanol and sterol esters, vascular function and genetic regulation. Br. J. Nutr. 2009, 101, 1688–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, E.M.M.; Ng, T.W.K.; Chan, D.C.; Watts, G.F. Plasma markers of cholesterol homeostasis in metabolic syndrome subjects with or without type-2 diabetes. Diabetes Res. Clin. Pract. 2009, 85, 310–316. [Google Scholar] [CrossRef]
- De Graaf, J.; de Sauvage Nolting, P.R.W.; van Dam, M.; Belsey, E.M.; Kastelein, J.J.P.; Haydn Pritchard, P.; Stalenhoef, A.F.H. Consumption of tall oil-derived phytosterols in a chocolate matrix significantly decreases plasma total and low-density lipoprotein-cholesterol levels. Br. J. Nutr. 2002, 88, 479–488. [Google Scholar] [CrossRef]
- Lin, X.; Racette, S.B.; Lefevre, M.; Spearie, C.A.; Most, M.; Ma, L.; Ostlund, R.E. The effects of phytosterols present in natural food matrices on cholesterol metabolism and LDL-cholesterol: A controlled feeding trial. Eur. J. Clin. Nutr. 2010, 64, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Kathiresan, S.; Otvos, J.D.; Sullivan, L.M.; Keyes, M.J.; Schaefer, E.J.; Wilson, P.W.F.; D’Agostino, R.B.; Vasan, R.S.; Robins, S.J. Increased small low-density lipoprotein particle number: A prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation 2006, 113, 20–29. [Google Scholar] [CrossRef] [Green Version]
| Criterion | Categorical Cut-Off Point |
|---|---|
| Waist circumference | Depends on ethnic and country-specific definitions 1 |
| Triglycerides | ≥150 mg/dL or specific treatment (fibrates or nicotinic acid) |
| High-density lipoprotein cholesterol (HDL-c) | <40 mg/dL in males; <50 mg/dL in females, or specific treatment (fibrates or nicotinic acid) |
| Blood pressure (BP) | Systolic BP ≥ 130 or Diastolic BP ≥ 85 mmHg, or treatment of previously diagnosed hypertension |
| Fasting glycaemia | ≥100 mg/dL, or previously diagnosed with type 2 diabetes |
| KERRYPNX | Phytosterol (n = 102) | Placebo (n = 100) | p-Value |
|---|---|---|---|
| Sociodemographic variables | |||
| Age in months, median (p25–p75) | 520 (407–636) | 527.5 (414–632.5) | 0.9568 |
| Sex | |||
| Men, n (%) | 28 | 26 | |
| Women, n (%) | 74 | 74 | 0.816 |
| Socioeconomic status (self-reported) | |||
| High, n (%) | 3 (2.94) | 2 (2) | |
| Middle, n (%) | 93 (91.18) | 90 (90) | |
| Low, n (%) | 6 (5.88) | 8 (8) | 0.733 |
| General health-related information | |||
| Underlying health condition | |||
| Yes, n (%) | 86 (84.31) | 79 (79) | |
| No, n (%) | 16 (15.69) | 21 (21) | 0.329 |
| Concomitant medication | |||
| Yes, n (%) | 63 (61.76) | 57 (57) | |
| No, n (%) | 39 (38.24) | 43 (43) | 0.491 |
| Smoking | |||
| Yes, n (%) | 55 (53.92) | 44 (44) | |
| No, n (%) | 47 (46.08) | 56 (56) | 0.158 |
| Alcohol consumption | |||
| Yes, n (%) | 67 (65.69) | 72 (72) | |
| No, n (%) | 35 (34.31) | 28 (28) | 0.333 |
| Number of meals per day, median (p25–p75) | 3 (3–3) | 3 (3–3) | 0.1777 |
| Physical activity | |||
| Yes, n (%) | 44 (43.14) | 46 (46) | |
| No, n (%) | 58 (56.86) | 54 (54) | 0.682 |
| Blood test parameters, median (p25–p75) | |||
| Total cholesterol (mg/dL) | 196 (169–224) | 196 (168.5–225) | 0.9789 |
| LDL-c (mg/dL) | 118.5 (99–144) | 118.5 (100.5–147.5) | 0.8069 |
| VLDL-c (mg/dL) | 26.5 (20–42) | 28.5 (20–40.5) | 0.9625 |
| Insulin (µUl/mL) | 15.6 (10.38–23.7) | 13.36 (9.07–18.48) | 0.1065 |
| HOMA-IR index | 3.02 (1.94–4.26) | 3.62 (2.28–5.52) | 0.0684 |
| Glycated haemoglobin - HbA1c (%) | 5.215 (4.98–5.77) | 5.305 (4.96–5.69) | 0.7361 |
| Vitamin D (ng/mL) | 24.6 (19.9–29) | 24.25 (19.7–29.55) | 0.9109 |
| Anthropometric measurements and vital signs, median (p25–p75) | |||
| Weight (kg) | 78.95 (71.2–90.1) | 80.6 (70.5–89.75) | 0.8985 |
| Height (m) | 1.585 (1.55–1.67) | 1.59 (1.545–1.675) | 0.7413 |
| Body mass index (kg/m2) | 30.63 (27.46–34.4) | 30.55 (28–34.46) | 0.5298 |
| Heart rate (beats per minute) | 72 (67–80) | 71 (65–76.5) | 0.0455 |
| Metabolic syndrome variables, median (p25–p75) | |||
| Waist circumference (cm) | 99.5 (93–105) | 100.5 (92–108.5) | 0.4795 |
| Triglycerides (mg/dL) | 138 (105–221) | 143.5 (100.5–202.5) | 0.8303 |
| HDL-c (mg/dL) | 43.5 (37–52) | 43 (37.5–51) | 0.9875 |
| Systolic blood pressure (mm Hg) | 122 (113–133) | 119.5 (110.5–132) | 0.2176 |
| Diastolic blood pressure (mm Hg) | 83 (78–90) | 82 (76–89) | 0.6188 |
| Fasting glycaemia (mg/dL) | 93.5 (88–99) | 91 (85.5–98) | 0.0910 |
| Metabolic syndrome criteria, n (%) | |||
| 3 | 71 (69.61) | 76 (76) | 0.379 |
| 4 | 22 (21.57) | 20 (20) | |
| 5 | 9 (8.82) | 4 (4) | |
| MetS-Z, median (p25–p75) | 0.463 (0.06–0.99) | 0.489 (−0.02–0.93) | 0.5947 |
| N° of Criteria | Visit 4 | N° of Criteria | Difference Between V4 and V1 | ||||
|---|---|---|---|---|---|---|---|
| Phytosterol (n = 102) | Placebo (n = 100) | p-Value | Phytosterol (n = 102) | Placebo (n = 100) | p-Value | ||
| 0, n (%) | 2 (1.96) | 1 (1) | 0.563 | −3, n (%) | 3 (2.94) | 1 (1) | 0.648 |
| 1, n (%) | 16 (15.69) | 16 (16) | −2, n (%) | 24 (23.53) | 19 (19) | ||
| 2, n (%) | 39 (38.24) | 30 (30) | −1, n (%) | 45 (44.12) | 40 (40) | ||
| 3, n (%) | 29 (28.43) | 35 (35) | 0, n (%) | 23 (22.55) | 31 (31) | ||
| 4, n (%) | 9 (8.82) | 14 (14) | 1, n (%) | 6 (5.88) | 8 (8) | ||
| 5, n (%) | 7 (6.86) | 4 (4) | 2, n (%) | 1 (0.98) | 1 (1) | ||
| Visit 4 | Difference Between V4–V1 | |||||
|---|---|---|---|---|---|---|
| Phytosterol (n = 102) | Placebo (n = 100) | p-Value | Phytosterol (n = 102) | Placebo (n = 100) | p-Value | |
| WC (cm) | ||||||
| All | 94 (89–102) | 99 (93–109.5) | 0.0022 | −4 (−7–−1) | 0 (−3–2.5) | 0.0001 |
| Men | 99.5 (94.5–105) | 103 (94–109) | 0.5732 | −3 (−5–0) | 0 (−3–3) | 0.0875 |
| Women | 92 (86–99) | 99 (93–110) | 0.0007 | −5 (−8–−1) | 0 (−3–2) | 0.0001 |
| TG (mg/dL) | ||||||
| All | 123 (87–175) | 145 (99.5–191.5) | 0.1137 | −16.5 (−57–15) | 1.5 (−40–32) | 0.0245 |
| Men | 150 (123–243.5) | 188 (122–245) | 0.4566 | −21.5 (−87–3.5) | −37 (−54–13) | 0.9724 |
| Women | 112.5 (82–152) | 134.5 (86–168) | 0.1176 | −14 (−57–17) | 3.5 (−32–32) | 0.0074 |
| HDL-c (mg/dL) | ||||||
| All | 46 (40–55) | 45 (39–53.5) | 0.6839 | 1 (−1–5) | 1 (−2–5) | 0.6563 |
| Men | 39.5 (35.5–46) | 37.5 (31–45) | 0.3721 | 0 (3.5–−2) | 0 (−3–2) | 0.5037 |
| Women | 48 (42–58) | 47 (41–55) | 0.6071 | 2 (−1–6) | 2 (−2–5) | 0.8102 |
| SBP (mm Hg) | ||||||
| All | 119 (112–129) | 119 (112.4–128) | 0.9242 | −2 (−12–5) | −1 (−10.5–7.5) | 0.2753 |
| Men | 125 (118–131) | 127.5 (117–136) | 0.6093 | 0 (−12.5–7) | 2.5 (−9–8) | 0.7095 |
| Women | 116.5 (112–126) | 118 (108–126) | 0.7735 | −2.5 (−12–4) | −1 (−11–7) | 0.2939 |
| DBP (mm Hg) | ||||||
| All | 79.5 (73–87) | 80 (72.5–86) | 0.6820 | −2.5 (−9–3) | −3 (3.5–−10) | 0.9099 |
| Men | 85 (78.5–91) | 84 (75–91) | 0.6398 | −2 (2.5–−9) | −5 (−15–2) | 0.3630 |
| Women | 78 (72–85) | 79 (70–85) | 0.9480 | −3 (−9–3) | −2.5 (−10–5) | 0.4508 |
| FG (mg/dL) | ||||||
| All | 95 (90–102) | 92 (87–98) | 0.1712 | 2 (−3–7) | 1.5 (−3–6) | 0.9769 |
| Men | 96 (90–99.5) | 94 (92–105) | 0.7947 | 2 (−2–12) | 3.5 (0–7) | 0.9378 |
| Women | 94.5 (86–103) | 91.5 (85–97) | 0.1179 | 1 (−4–6) | 1 (−3–6) | 0.9480 |
| Phytosterol (n = 102) | Placebo (n = 100) | |
|---|---|---|
| Bowel habit improved from V1, n (%) | 69 (67.65) | 4 (4) |
| Bowel habit did not change from V1, n (%) | 31 (30.39) | 90 (90) |
| Bowel habit worsened from V1, n (%) | 2 (1.96) | 6 (6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmeiro-Silva, Y.K.; Aravena, R.I.; Ossio, L.; Parro Fluxa, J. Effects of Daily Consumption of an Aqueous Dispersion of Free-Phytosterols Nanoparticles on Individuals with Metabolic Syndrome: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2020, 12, 2392. https://doi.org/10.3390/nu12082392
Palmeiro-Silva YK, Aravena RI, Ossio L, Parro Fluxa J. Effects of Daily Consumption of an Aqueous Dispersion of Free-Phytosterols Nanoparticles on Individuals with Metabolic Syndrome: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2020; 12(8):2392. https://doi.org/10.3390/nu12082392
Chicago/Turabian StylePalmeiro-Silva, Yasna K., Raúl I. Aravena, Lisette Ossio, and Javiera Parro Fluxa. 2020. "Effects of Daily Consumption of an Aqueous Dispersion of Free-Phytosterols Nanoparticles on Individuals with Metabolic Syndrome: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 12, no. 8: 2392. https://doi.org/10.3390/nu12082392





