Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis
Abstract
1. Introduction
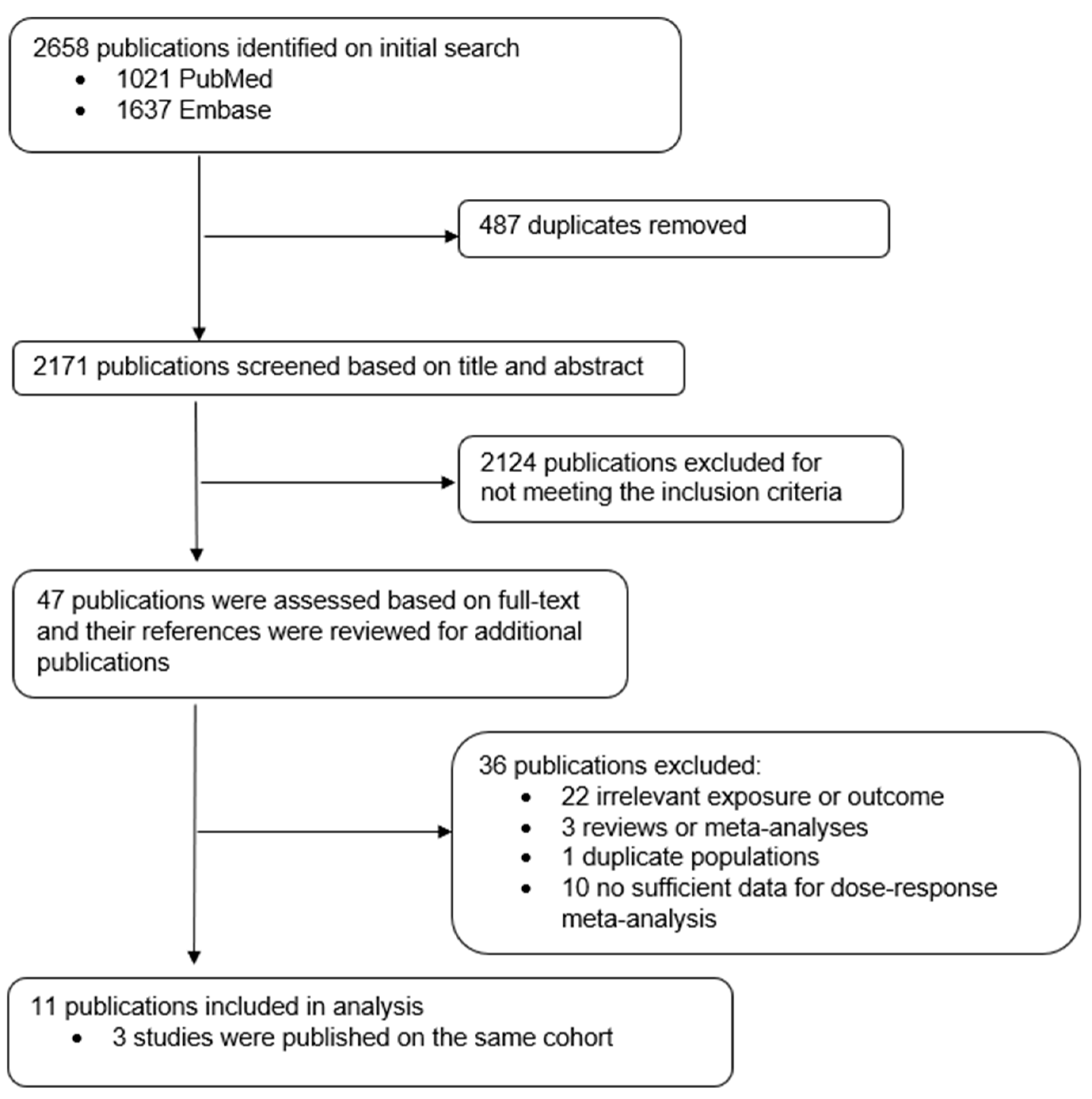
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
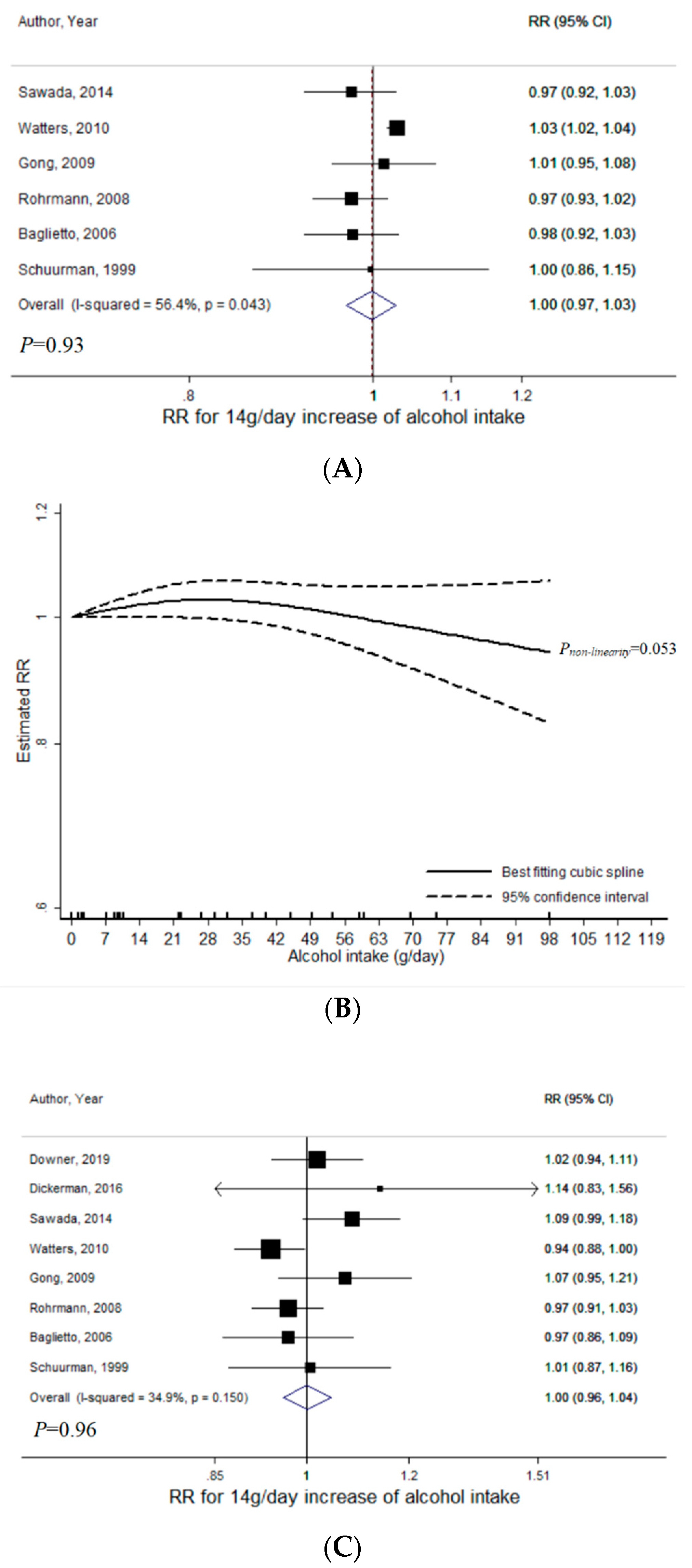
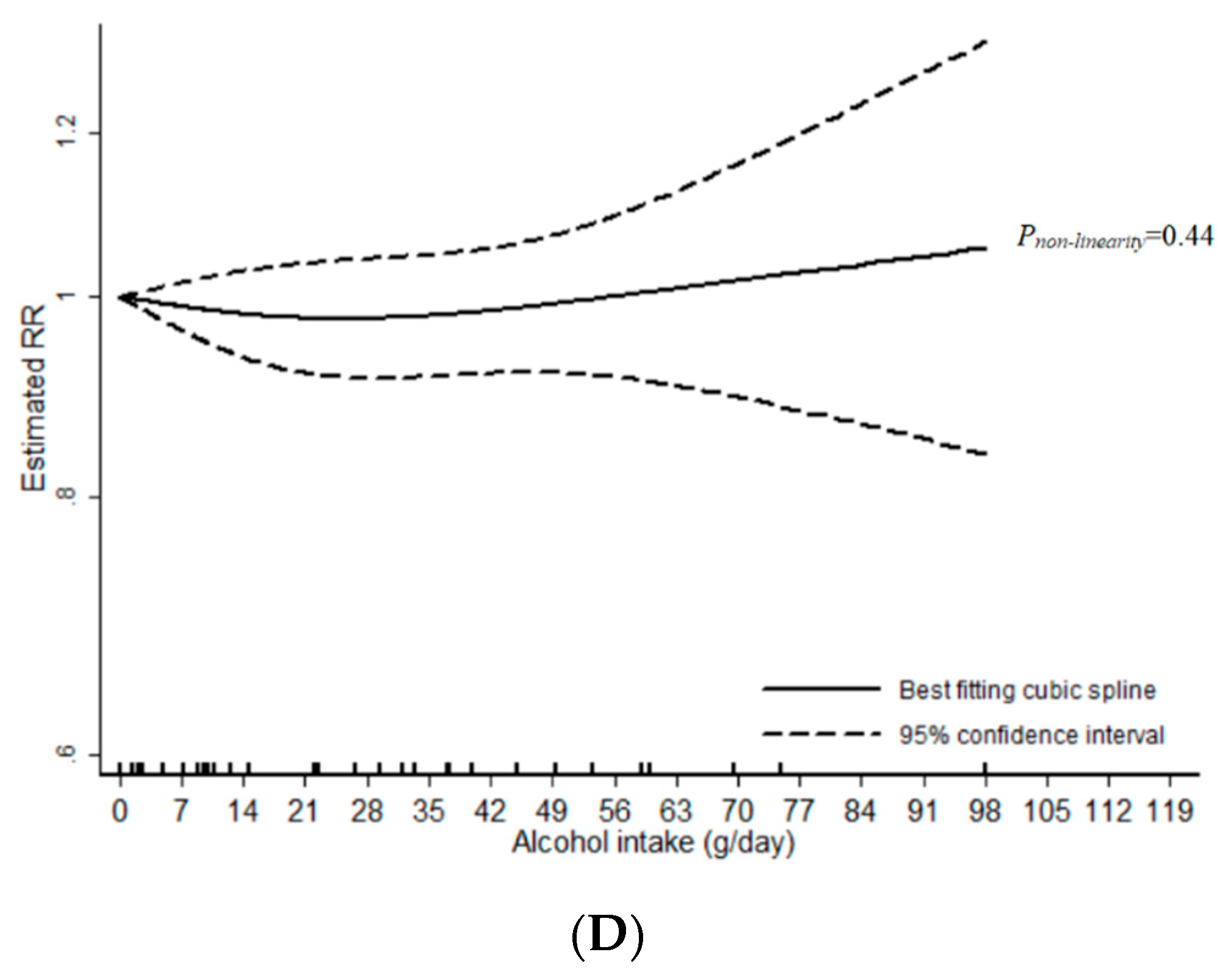
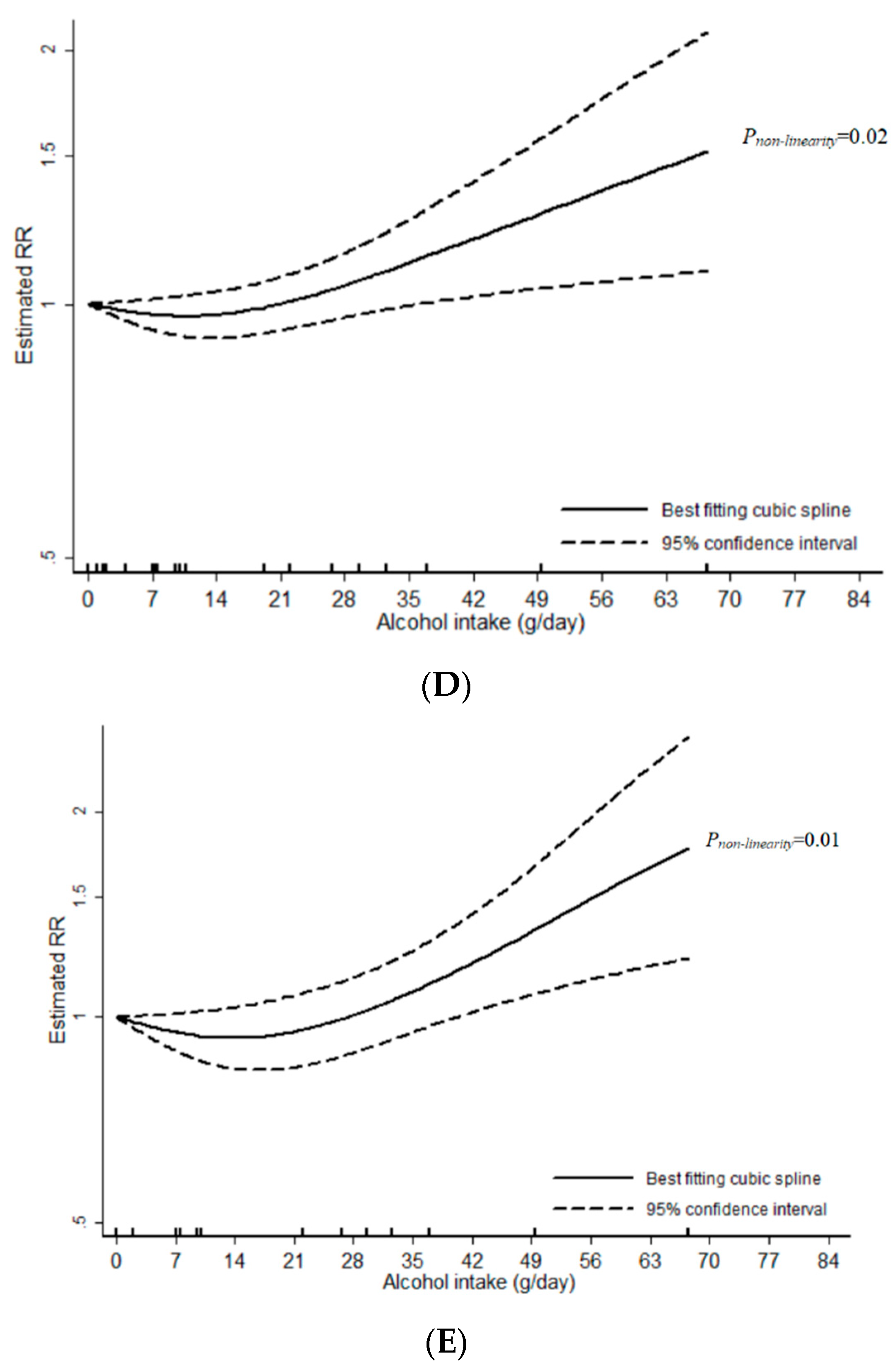
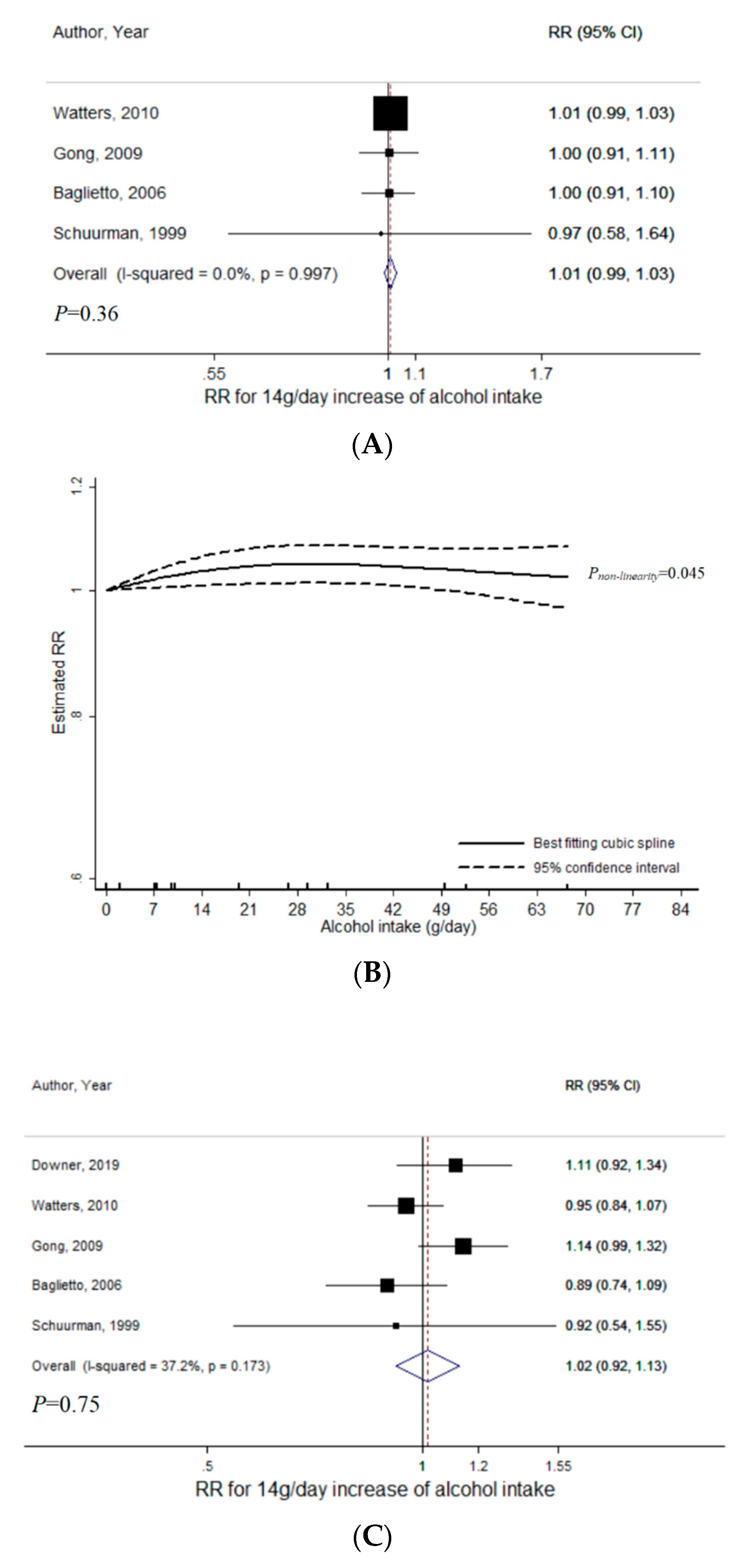
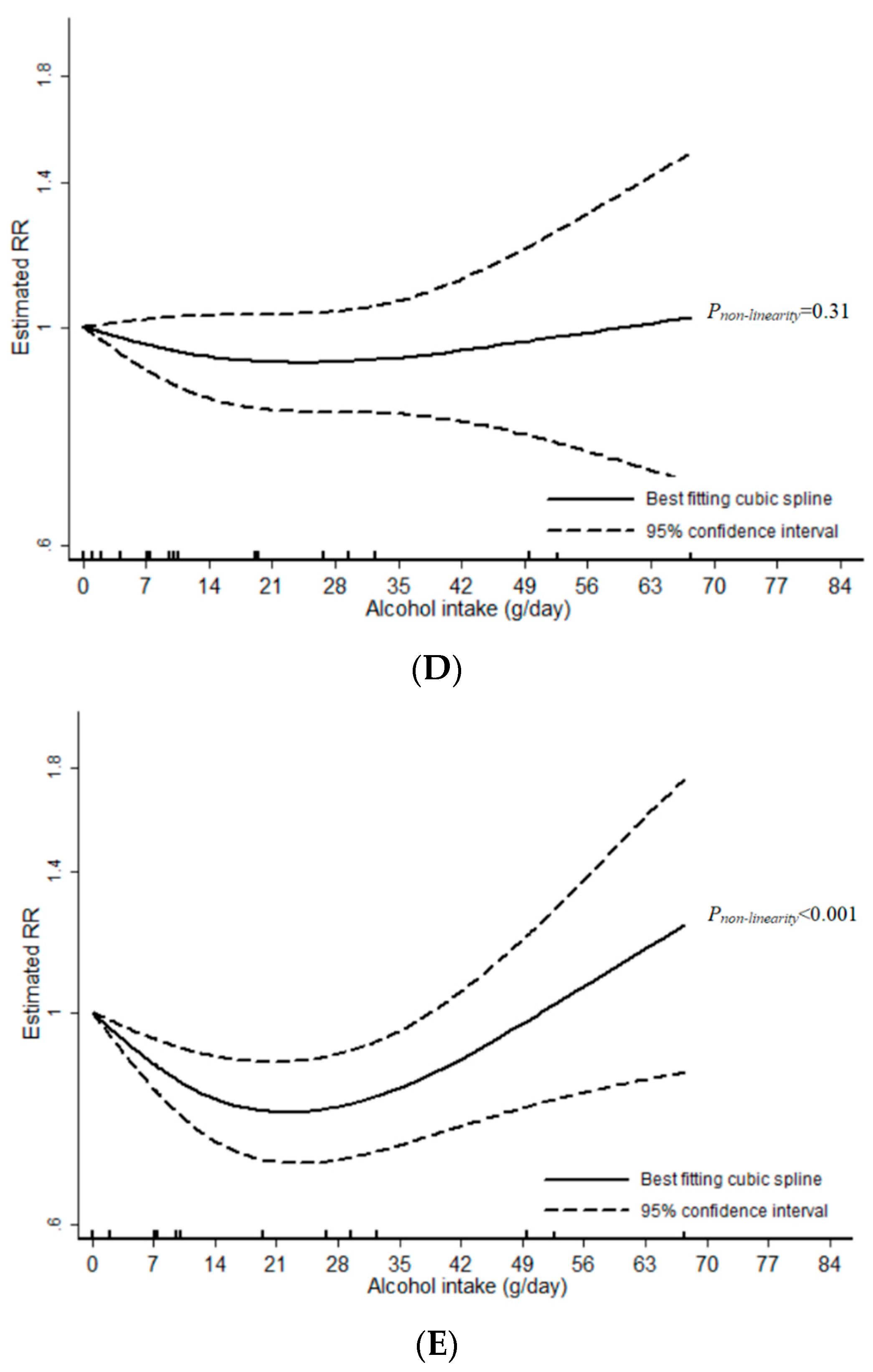
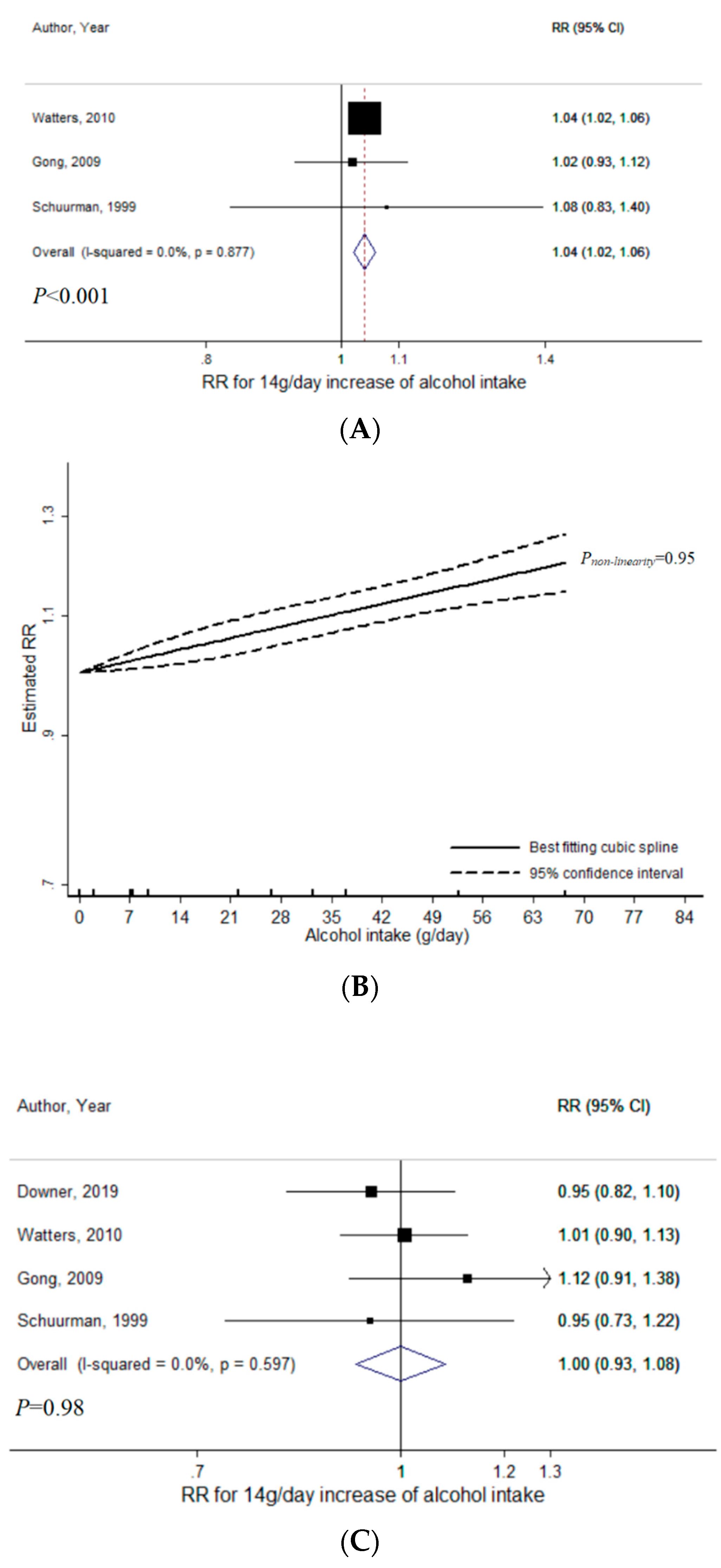
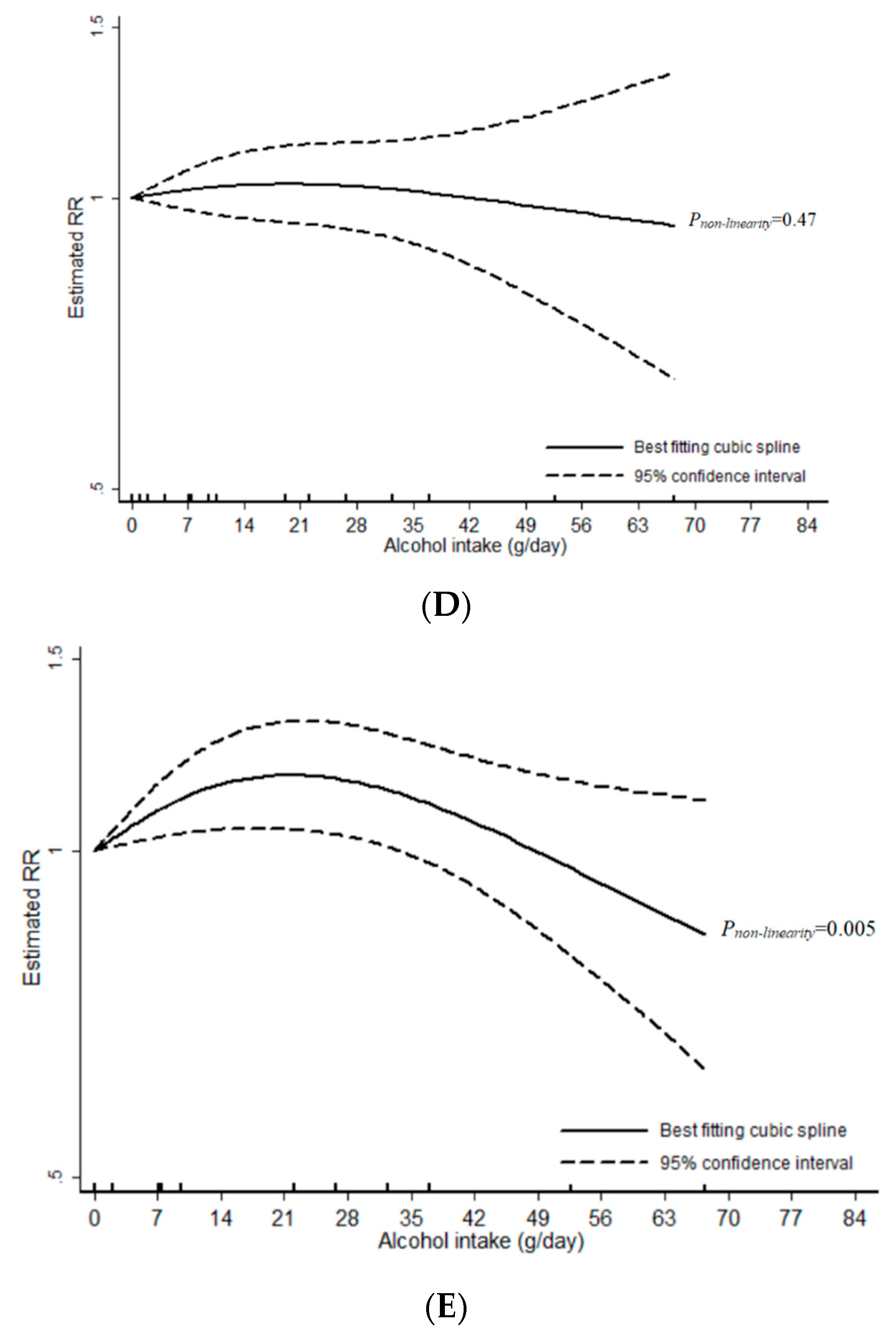
3.1. Total Alcohol
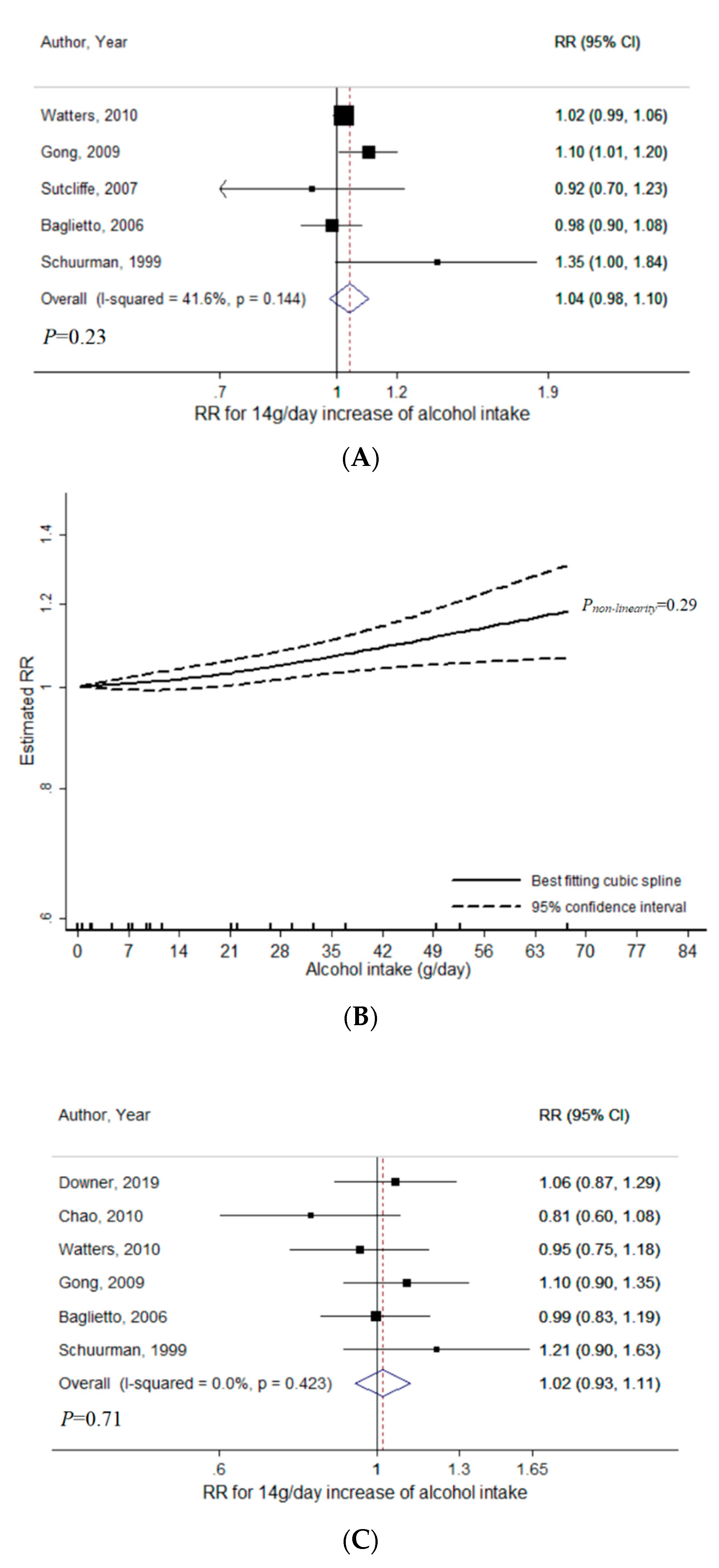
3.2. Wine
3.3. Beer
3.4. Liquor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Globocan 2018, W.H.O. Available online: https://gco.iarc.fr/ (accessed on 16 July 2020).
- International Agency for Research on Cancer. Globocan 2018: Cancer Fact Sheets—Prostate Cancer. IARC. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf (accessed on 16 July 2020).
- Prostate Cancer, A.I.C.R. 2014. Available online: https://www.wcrf.org/sites/default/files/Prostate-Cancer-2014-Report.pdf (accessed on 15 July 2020).
- Connor, J. Alcohol consumption as a cause of cancer. Addiction 2017, 112, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, B.A.; Markt, S.C.; Koskenvuo, M.; Pukkala, E.; Mucci, L.A.; Kaprio, J. Alcohol intake, drinking patterns, and prostate cancer risk and mortality: A 30-year prospective cohort study of Finnish twins. Cancer Causes Control 2016, 27, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Paffenbarger, R.S., Jr.; Lee, I.M. Alcohol consumption and risk of prostate cancer: The Harvard alumni health study. Int. J. Epidemiol. 2001, 30, 749–755. [Google Scholar] [CrossRef]
- Breslow, R.A.; Wideroff, L.; Graubard, B.I.; Erwin, D.; Reichman, M.E.; Ziegler, R.G.; Ballard-Barbash, R. Alcohol and prostate cancer in the NHANES I epidemiologic follow-up study. First National Health and Nutrition Examination Survey of the United States. Ann. Epidemiol. 1999, 9, 254–261. [Google Scholar] [CrossRef]
- Baglietto, L.; Severi, G.; English, D.R.; Hopper, J.L.; Giles, G.G. Alcohol consumption and prostate cancer risk: Results from the Melbourne collaborative cohort study. Int. J. Cancer 2006, 119, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Downer, M.K.; Kenfield, S.A.; Stampfer, M.J.; Wilson, K.M.; Dickerman, B.A.; Giovannucci, E.L.; Rimm, E.B.; Wang, M.; Mucci, L.A.; Willett, W.C.; et al. Alcohol Intake and Risk of Lethal Prostate Cancer in the Health Professionals Follow-Up Study. J. Clin. Oncol. 2019, 37, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, K.; Gronbaek, M. Does amount or type of alcohol influence the risk of prostate cancer. Prostate 2002, 52, 297–304. [Google Scholar] [CrossRef]
- Severson, R.K.; Nomura, A.M.; Grove, J.S.; Stemmermann, G.N. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989, 49, 1857–1860. [Google Scholar]
- Lund Nilsen, T.I.; Johnsen, R.; Vatten, L.J. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br. J. Cancer 2000, 82, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, W.M.; Salinas, C.A.; Kiemeney, L.A.; Stanford, J.L. Alcohol consumption and risk of prostate cancer in middle-aged men. Int. J. Cancer 2005, 113, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Watters, J.L.; Park, Y.; Hollenbeck, A.; Schatzkin, A.; Albanes, D. Alcoholic beverages and prostate cancer in a prospective US cohort study. Am. J. Epidemiol. 2010, 172, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stockwell, T.; Roemer, A.; Chikritzhs, T. Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysis. BMC Cancer 2016, 16, 845. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Platz, E.A.; Stampfer, M.J.; Willett, W.C. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int. J. Cancer 2007, 121, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Linseisen, J.; Key, T.J.; Jensen, M.K.; Overvad, K.; Johnsen, N.F.; Tjonneland, A.; Kaaks, R.; Bergmann, M.M.; Weikert, C.; et al. Alcohol consumption and the risk for prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1282–1287. [Google Scholar] [CrossRef]
- Sutcliffe, S.; Giovannucci, E.; Leitzmann, M.F.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Platz, E.A. A prospective cohort study of red wine consumption and risk of prostate cancer. Int. J. Cancer 2007, 120, 1529–1535. [Google Scholar] [CrossRef]
- Schuurman, A.G.; Goldbohm, R.A.; van den Brandt, P.A. A prospective cohort study on consumption of alcoholic beverages in relation to prostate cancer incidence (The Netherlands). Cancer Causes Control 1999, 10, 597–605. [Google Scholar] [CrossRef]
- Jahn, J.L.; Giovannucci, E.L.; Stampfer, M.J. The high prevalence of undiagnosed prostate cancer at autopsy: Implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. Int. J. Cancer 2015, 137, 2795–2802. [Google Scholar] [CrossRef]
- National Institutes of Health. What Is a Standard Drink. Available online: https://www.niaaa.nih.gov/what-standard-drink (accessed on 15 July 2020).
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef]
- Nuesch, E.; Trelle, S.; Reichenbach, S.; Rutjes, A.W.; Tschannen, B.; Altman, D.G.; Egger, M.; Juni, P. Small study effects in meta-analyses of osteoarthritis trials: Meta-epidemiological study. BMJ 2010, 341, 3515. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Lee, K.L.; Pollock, B.G. Regression models in clinical studies: Determining relationships between predictors and response. J. Natl. Cancer Inst. 1988, 80, 1198–1202. [Google Scholar] [CrossRef]
- Orsini, N.; Greenland, S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J. 2011, 11, 1–29. [Google Scholar] [CrossRef]
- White, I.R. Multivariate random-effects meta-analysis. Stata J. 2009, 9, 40–56. [Google Scholar] [CrossRef]
- Sawada, N.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Yamaji, T.; Shimazu, T.; Tsugane, S. Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: The Japan Public Health Center-based prospective study. Int. J. Cancer 2014, 134, 971–978. [Google Scholar] [CrossRef]
- Gong, Z.; Kristal, A.R.; Schenk, J.M.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M. Alcohol consumption, finasteride, and prostate cancer risk: Results from the Prostate Cancer Prevention Trial. Cancer 2009, 115, 3661–3669. [Google Scholar] [CrossRef]
- Chao, C.; Haque, R.; Van Den Eeden, S.K.; Caan, B.J.; Poon, K.Y.; Quinn, V.P. Red wine consumption and risk of prostate cancer: The California men’s health study. Int. J. Cancer 2010, 126, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Farris, M.S.; Courneya, K.S.; Kopciuk, K.A.; McGregor, S.E.; Friedenreich, C.M. Post-diagnosis alcohol intake and prostate cancer survival: A population-based cohort study. Int. J. Cancer 2018, 143, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Casey, G.; Silverman, R. Genetic susceptibility and oxidative stress in prostate cancer: Integrated model with implications for prevention. Urology 2006, 68, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, J.; Yunxia, Z.; Zhu, H.; Liu, J.; Pumill, C. The role of prostatitis in prostate cancer: Meta-analysis. PLoS ONE 2013, 8, 85179. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef]
- Le Magnen, C.; Virk, R.K.; Dutta, A.; Kim, J.Y.; Panja, S.; Lopez-Bujanda, Z.A.; Califano, A.; Drake, C.G.; Mitrofanova, A.; Abate-Shen, C. Cooperation of loss of NKX3.1 and inflammation in prostate cancer initiation. Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Zhang, J.; Dhakal, I.B.; Greene, G.; Lang, N.P.; Kadlubar, F.F. Polymorphisms in hOGG1 and XRCC1 and risk of prostate cancer: Effects modified by plasma antioxidants. Urology 2010, 75, 779–785. [Google Scholar] [CrossRef][Green Version]
- Victor, R.; Preedy, R.R.W. Comprehensive Handbook of Alcohol Related Pathology; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us. Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Ida, Y.; Tsujimaru, S.; Nakamaura, K.; Shirao, I.; Mukasa, H.; Egami, H.; Nakazawa, Y. Effects of acute and repeated alcohol ingestion on hypothalamic-pituitary-gonadal and hypothalamic-pituitary-adrenal functioning in normal males. Drug Alcohol. Depend. 1992, 31, 57–64. [Google Scholar] [CrossRef]
- Milligan, S.R.; Kalita, J.C.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Sawagado, L.; Houdebine, L.M. Identification of the lactogenic compound present in beer. Ann. Biol. Clin. (Paris) 1988, 46, 129–134. [Google Scholar] [PubMed]
- Greger, M. Beer Phytoestrogens. Available online: https://nutritionfacts.org/2019/06/04/beer-phytoestrogens/ (accessed on 17 July 2020).
- Gaya, P.; Medina, M.; Sanchez-Jimenez, A.; Landete, J.M. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules 2016, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lehner, F. Beer and breastfeeding. Adv. Exp. Med. Biol. 2000, 478, 23–28. [Google Scholar] [CrossRef]
- Carlson, H.E.; Wasser, H.L.; Reidelberger, R.D. Beer-induced prolactin secretion: A clinical and laboratory study of the role of salsolinol. J. Clin. Endocrinol. Metab. 1985, 60, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Watts, E.L.; Appleby, P.N.; Perez-Cornago, A.; Bueno-de-Mesquita, H.B.; Chan, J.M.; Chen, C.; Cohn, B.A.; Cook, M.B.; Flicker, L.; Freedman, N.D.; et al. Low Free Testosterone and Prostate Cancer Risk: A Collaborative Analysis of 20 Prospective Studies. Eur. Urol. 2018, 74, 585–594. [Google Scholar] [CrossRef]
- American, D.G. Dietary Guidelines Advisory Commitee: Draft Report Meeting: Session 2. Available online: https://www.dietaryguidelines.gov/draft-report-meeting/archived-webcast (accessed on 18 July 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Khil, H.; Lee, D.H.; Keum, N.; Giovannucci, E.L. Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Nutrients 2020, 12, 2188. https://doi.org/10.3390/nu12082188
Hong S, Khil H, Lee DH, Keum N, Giovannucci EL. Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Nutrients. 2020; 12(8):2188. https://doi.org/10.3390/nu12082188
Chicago/Turabian StyleHong, SungEun, Hayeong Khil, Dong Hoon Lee, NaNa Keum, and Edward L. Giovannucci. 2020. "Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis" Nutrients 12, no. 8: 2188. https://doi.org/10.3390/nu12082188
APA StyleHong, S., Khil, H., Lee, D. H., Keum, N., & Giovannucci, E. L. (2020). Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Nutrients, 12(8), 2188. https://doi.org/10.3390/nu12082188




