Diet Quality Is Associated with Cardiometabolic Outcomes in Survivors of Childhood Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric, Clinical and Biochemical Assessment
2.3. Data Collection and Analysis for Dietary Intake
2.4. Assessment of Diet Quality
2.4.1. Mediterranean Diet Adherence
2.4.2. Healthy Diet Indicator
2.4.3. Healthy Eating Index
2.4.4. Energy-Adjusted-Dietary Inflammatory Index
2.4.5. Ferric Reducing Ability of Plasma Score
2.4.6. NOVA Classification
2.5. Statistical Analysis
3. Results
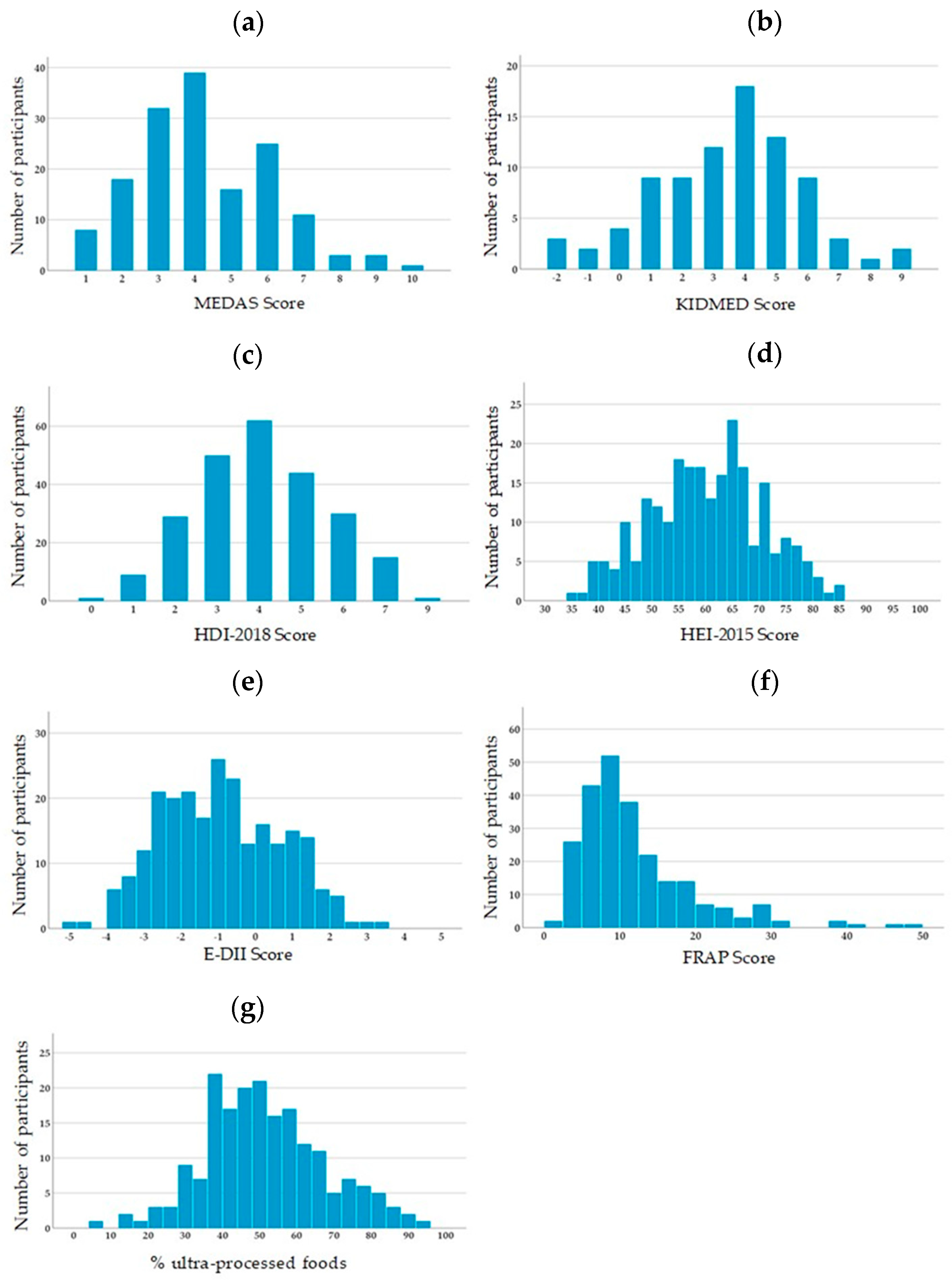
3.1. Descriptive Statistics
3.2. Association between Dietary Scores and Anthropometric and Metabolic Parameters
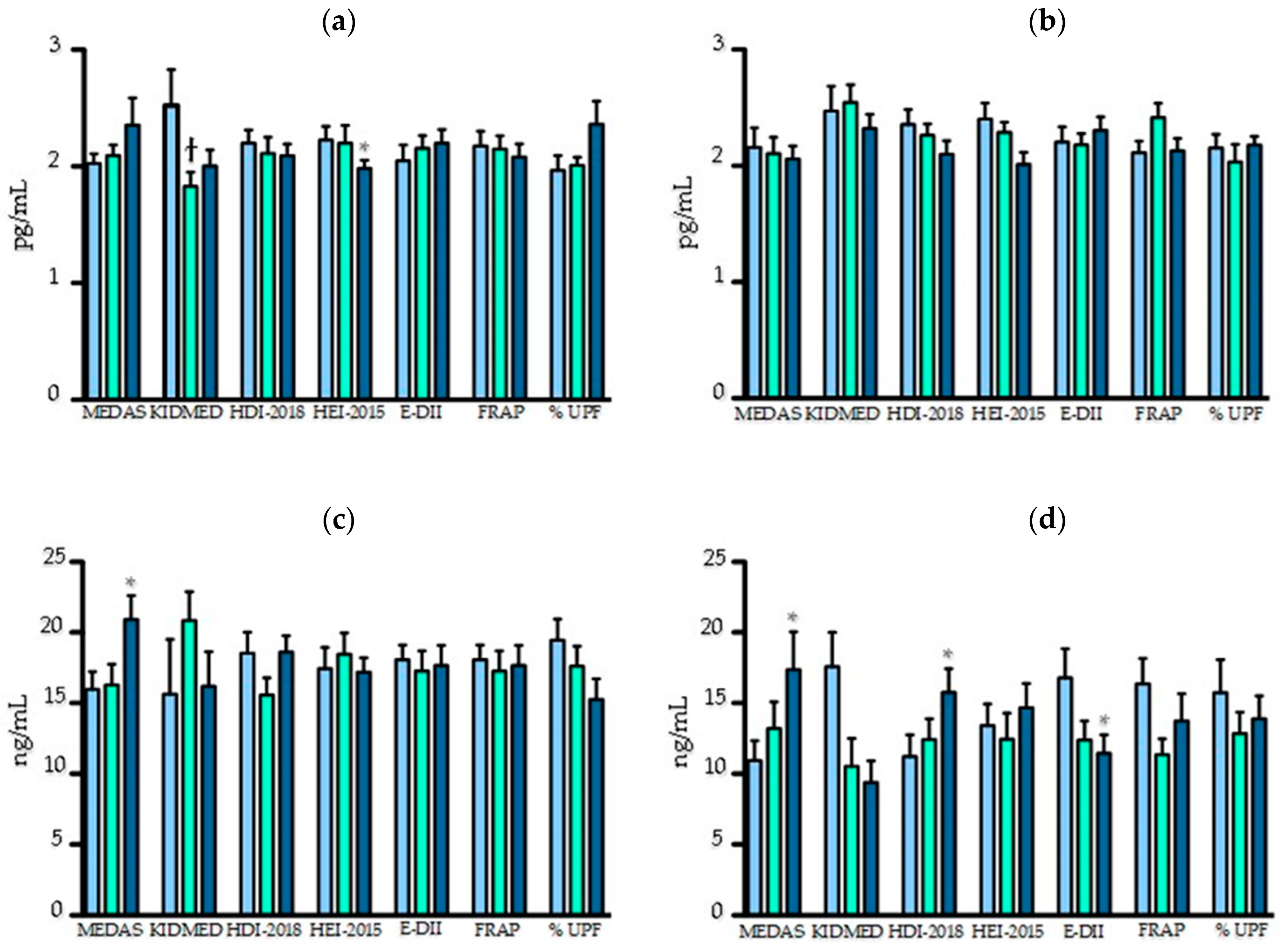
3.3. Association between Dietary Scores and Inflammatory Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclosure
References
- Canadian Vital Death Statistics Database (CVSD) linked to Discharge Abstract Database (DAD) and National Ambulatory Care Reporting System (NACRS). Available online: https://www.statcan.gc.ca/eng/rdc/cvsd-nacrs (accessed on 3 March 2020).
- Government of Canada. Cancer in Children in Canada (0–14 Years). Available online: https://www.canada.ca/en/public-health/services/chronic-diseases/cancer/cancer-children-canada-0-14-years.html (accessed on 3 March 2020).
- Ness, K.K.; Armenian, S.H.; Kadan-Lottick, N.; Gurney, J.G. Adverse effects of treatment in childhood acute lymphoblastic leukemia: General overview and implications for long-term cardiac health. Expert Rev. Hematol. 2011, 4, 185–197. [Google Scholar] [CrossRef]
- Pui, C.H.; Evans, W.E. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin. Hematol. 2013, 50, 185–196. [Google Scholar] [CrossRef]
- Mazzucco, W.; Cusimano, R.; Mazzola, S.; Rudisi, G.; Zarcone, M.; Marotta, C.; Graziano, G.; D’Angelo, P.; Vitale, F. Childhood and Adolescence Cancers in the Palermo Province (Southern Italy): Ten Years (2003–2012) of Epidemiological Surveillance. Int. J. Environ. Res. Public Health 2018, 15, 1344. [Google Scholar] [CrossRef]
- Phillips, S.M.; Padgett, L.S.; Leisenring, W.M.; Stratton, K.K.; Bishop, K.; Krull, K.R.; Alfano, C.M.; Gibson, T.M.; de Moor, J.S.; Hartigan, D.B.; et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol. Biomark. Prev. 2015, 24, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Mody, R.; Li, S.; Dover, D.C.; Sallan, S.; Leisenring, W.; Oeffinger, K.C.; Yasui, Y.; Robison, L.L.; Neglia, J.P. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Blood 2008, 111, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Samoilenko, M.; Morel, S.; England, J.; Amre, D.; Bertout, L.; Drouin, S.; Laverdiere, C.; Krajinovic, M.; Sinnett, D.; et al. Cardiometabolic Risk Factors in Childhood, Adolescent and Young Adult Survivors of Acute Lymphoblastic Leukemia—A Petale Cohort. Sci. Rep. 2017, 7, 17684. [Google Scholar] [CrossRef] [PubMed]
- Janiszewski, P.M.; Oeffinger, K.C.; Church, T.S.; Dunn, A.L.; Eshelman, D.A.; Victor, R.G.; Brooks, S.; Turoff, A.J.; Sinclair, E.; Murray, J.C.; et al. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J. Clin. Endocrinol. Metab. 2007, 92, 3816–3821. [Google Scholar] [CrossRef] [PubMed]
- Chueh, H.W.; Yoo, J.H. Metabolic syndrome induced by anticancer treatment in childhood cancer survivors. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 82–89. [Google Scholar] [CrossRef]
- Meacham, L.R.; Sklar, C.A.; Li, S.; Liu, Q.; Gimpel, N.; Yasui, Y.; Whitton, J.A.; Stovall, M.; Robison, L.L.; Oeffinger, K.C. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: A report for the childhood cancer survivor study. Arch Intern. Med. 2009, 169, 1381–1388. [Google Scholar] [CrossRef]
- Meacham, L.R.; Chow, E.J.; Ness, K.K.; Kamdar, K.Y.; Chen, Y.; Yasui, Y.; Oeffinger, K.C.; Sklar, C.A.; Robison, L.L.; Mertens, A.C. Cardiovascular risk factors in adult survivors of pediatric cancer—A report from the childhood cancer survivor study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 170–181. [Google Scholar] [CrossRef]
- Rosen, G.P.; Nguyen, H.T.; Shaibi, G.Q. Metabolic syndrome in pediatric cancer survivors: A mechanistic review. Pediatr. Blood Cancer 2013, 60, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Westerink, N.L.; Nuver, J.; Lefrandt, J.D.; Vrieling, A.H.; Gietema, J.A.; Walenkamp, A.M. Cancer treatment induced metabolic syndrome: Improving outcome with lifestyle. Crit. Rev. Oncol. Hematol. 2016, 108, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley des Varannes, S.; Le Vacon, F.; de La Cochetiere, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, M.J.; Tissing, W.J.; Dun, C.A.; Meessen, N.E.; Kamps, W.A.; de Bont, E.S.; Harmsen, H.J. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef]
- Zwielehner, J.; Lassl, C.; Hippe, B.; Pointner, A.; Switzeny, O.J.; Remely, M.; Kitzweger, E.; Ruckser, R.; Haslberger, A.G. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS ONE 2011, 6, e28654. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Ferranti, S.D.; Després, J.P. Heart Disease and Stroke Statistics—2015 Update. A Report From the American Heart Association. Circulation 2015, 131, e29–e32. [Google Scholar]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. High diet quality is associated with a lower risk of cardiovascular disease and all-cause mortality in older men. J. Nutr. 2014, 144, 673–680. [Google Scholar] [CrossRef]
- Mora, S.; Lee, I.-M.; Buring, J.E.; Ridker, P.M. Association of Physical Activity and Body Mass Index with Novel and Traditional Cardiovascular Biomarkers in Women. JAMA 2006, 295, 1412–1419. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A Systematic Review of the Evidence Supporting a Causal Link Between Dietary Factors and Coronary Heart Disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef]
- Cunha, C.M.; Costa, P.R.F.; de Oliveira, L.P.M.; Queiroz, V.A.O.; Pitangueira, J.C.D.; Oliveira, A.M. Dietary patterns and cardiometabolic risk factors among adolescents: Systematic review and meta-analysis. Br. J. Nutr. 2018, 119, 859–879. [Google Scholar] [CrossRef]
- Shang, X.; Li, Y.; Xu, H.; Zhang, Q.; Liu, A.; Ma, G. The Clustering of Low Diet Quality, Low Physical Fitness, and Unhealthy Sleep Pattern and Its Association with Changes in Cardiometabolic Risk Factors in Children. Nutrients 2020, 12, 591. [Google Scholar] [CrossRef]
- Castro, M.A.; Baltar, V.T.; Marchioni, D.M.; Fisberg, R.M. Examining associations between dietary patterns and metabolic CVD risk factors: A novel use of structural equation modelling. Br. J. Nutr. 2016, 115, 1586–1597. [Google Scholar] [CrossRef]
- Papadaki, A.; Johnson, L.; Toumpakari, Z.; England, C.; Rai, M.; Toms, S.; Penfold, C.; Zazpe, I.; Martinez-Gonzalez, M.A.; Feder, G. Validation of the English Version of the 14-Item Mediterranean Diet Adherence Screener of the PREDIMED Study, in People at High Cardiovascular Risk in the UK. Nutrients 2018, 10, 138. [Google Scholar] [CrossRef]
- Schroder, H.; Fito, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Burrows, T.; Collins, C.E. Systematic review of diet quality indices and their associations with health-related outcomes in children and adolescents. J. Hum. Nutr. Diet 2014, 27, 577–598. [Google Scholar] [CrossRef]
- Lazarou, C.; Newby, P.K. Use of dietary indexes among children in developed countries. Adv. Nutr. 2011, 2, 295–303. [Google Scholar] [CrossRef]
- Belle, F.; Wengenroth, L.; Weiss, A.; Sommer, G.; Beck Popovic, M.; Ansari, M.; Bochud, M.; Kuehni, C.; Swiss Paediatric Oncology Group. Low adherence to dietary recommendations in adult childhood cancer survivors. Clin. Nutr. 2017, 36, 1266–1274. [Google Scholar] [CrossRef]
- Robien, K.; Ness, K.K.; Klesges, L.M.; Baker, K.S.; Gurney, J.G. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2008, 30, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Berdan, C.A.; Tangney, C.C.; Scala, C.; Stolley, M. Childhood cancer survivors and adherence to the American Cancer Society Guidelines on Nutrition and Physical Activity. J. Cancer Surviv. 2014, 8, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Saltzman, E.; Kelly, M.J.; Liu, S.; Must, A.; Parsons, S.K.; Roberts, S.B. Comparison of childhood cancer survivors’ nutritional intake with US dietary guidelines. Pediatr. Blood Cancer 2015, 62, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Landy, D.C.; Lipsitz, S.R.; Kurtz, J.M.; Hinkle, A.S.; Constine, L.S.; Adams, M.J.; Lipshultz, S.E.; Miller, T.L. Dietary quality, caloric intake, and adiposity of childhood cancer survivors and their siblings: An analysis from the cardiac risk factors in childhood cancer survivors study. Nutr. Cancer 2013, 65, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Tylavsky, F.A.; Smith, K.; Surprise, H.; Garland, S.; Yan, X.; McCammon, E.; Hudson, M.M.; Pui, C.H.; Kaste, S.C. Nutritional intake of long-term survivors of childhood acute lymphoblastic leukemia: Evidence for bone health interventional opportunities. Pediatr. Blood Cancer 2010, 55, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, S.; Drouin, S.; Laverdiere, C.; Alos, N.; Andelfinger, G.U.; Bertout, L.; Curnier, D.; Friedrich, M.G.; Kritikou, E.A.; Lefebvre, G.; et al. The PETALE study: Late adverse effects and biomarkers in childhood acute lymphoblastic leukemia survivors. Pediatr. Blood Cancer 2017, 64, e26361. [Google Scholar] [CrossRef]
- Morel, S.; Amre, D.; Teasdale, E.; Caru, M.; Laverdiere, C.; Krajinovic, M.; Sinnett, D.; Curnier, D.; Levy, E.; Marcil, V. Dietary Intakes Are Associated with HDL-Cholesterol in Survivors of Childhood Acute Lymphoblastic Leukaemia. Nutrients 2019, 11, 2977. [Google Scholar] [CrossRef]
- Genest, J.; McPherson, R.; Frohlich, J.; Anderson, T.; Campbell, N.; Carpentier, A.; Couture, P.; Dufour, R.; Fodor, G.; Francis, G.A.; et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can. J. Cardiol. 2009, 25, 567–579. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart, A.; National Heart, L.; Blood, I. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- De Jesus, J.M. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128, S213–S256. [Google Scholar] [CrossRef]
- Morel, S.; Portolese, O.; Chertouk, Y.; Leahy, J.; Bertout, L.; Laverdiere, C.; Krajinovic, M.; Sinnett, D.; Levy, E.; Marcil, V. Development and relative validation of a food frequency questionnaire for French-Canadian adolescent and young adult survivors of acute lymphoblastic leukemia. Nutr. J. 2018, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; Garcia, A.; Perez-Rodrigo, C.; Aranceta, J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, C.; Caballero-Perez, P. An update of the KIDMED questionnaire, a Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2019, 22, 2543–2547. [Google Scholar] [CrossRef]
- Amine, E.K.; Baba, N.H.; Belhadj, M.; Deurenberg-Yap, M.; Djazayery, A. Diet, Nutrition and the Prevention of Chronic Diseases. World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- World Health Organization. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 4 March 2020).
- Kanauchi, M.; Kanauchi, K. The World Health Organization’s Healthy Diet Indicator and its associated factors: A cross-sectional study in central Kinki, Japan. Prev. Med. Rep. 2018, 12, 198–202. [Google Scholar] [CrossRef]
- 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services, U.S. Department of Agriculture: Washington, DC, USA, 2015.
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Wilson, M.M.; Lerman, J.L.; Tooze, J.A. Applications of the Healthy Eating Index for Surveillance, Epidemiology, and Intervention Research: Considerations and Caveats. J. Acad. Nutr. Diet 2018, 118, 1603–1621. [Google Scholar] [CrossRef]
- Basiotis, P.P.; Carlson, A.; Gerrior, S.A.; Juan, W.Y.; Lino, M. The Healthy Eating Index: 1999–2000; U.S. Department of Agriculture, Center for Nutrition Policy and Promotion: Washington, DC, USA, 2002.
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hebert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bohn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1999, 239, 70–76. [Google Scholar] [CrossRef]
- Benzie, I.F.; Choi, S.W. Antioxidants in food: Content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food Nutr. Res. 2014, 71, 1–53. [Google Scholar] [CrossRef]
- Monteiro, C.; Cannon, G.; Levy, R.B.; Claro, R.; Moubarac, J.-C.; Martins, A.P.; Louzada, M.L.; Baraldi, L.; Canella, D. The Food System. Ultra-processing. The big issue for nutrition, disease, health, well-being. World Nutr. 2012, 3, 527–569. [Google Scholar]
- Moubarac, J.C.; Parra, D.C.; Cannon, G.; Monteiro, C.A. Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr. Obes. Rep. 2014, 3, 256–272. [Google Scholar] [CrossRef]
- Jessri, M.; Nishi, S.K.; L’Abbe, M.R. Assessing the nutritional quality of diets of Canadian children and adolescents using the 2014 Health Canada Surveillance Tool Tier System. BMC Public Health 2016, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, L.M.; Whitton, J.A.; Friedman, D.L.; Hammond, S.; Armstrong, G.T.; Leisenring, W.; Robison, L.L.; Neglia, J.P. Risk of Subsequent Neoplasms During the Fifth and Sixth Decades of Life in the Childhood Cancer Survivor Study Cohort. J. Clin. Oncol. 2015, 33, 3568–3575. [Google Scholar] [CrossRef]
- Mazidi, M.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Mikhailidis, D.P.; Kengne, A.P.; Banach, M. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis 2018, 276, 23–27. [Google Scholar] [CrossRef]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Goncalves, D.C. Reverse Cholesterol Transport: Molecular Mechanisms and the Non-medical Approach to Enhance HDL Cholesterol. Front. Physiol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Rye, K.A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid. Res. 2014, 55, 168–179. [Google Scholar] [CrossRef]
- Soran, H.; Schofield, J.D.; Durrington, P.N. Antioxidant properties of HDL. Front. Pharmacol. 2015, 6, 222. [Google Scholar] [CrossRef]
- Camont, L.; Lhomme, M.; Rached, F.; Le Goff, W.; Negre-Salvayre, A.; Salvayre, R.; Calzada, C.; Lagarde, M.; Chapman, M.J.; Kontush, A. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: Relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arter. Thromb Vasc. Biol. 2013, 33, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, A.; Fernandez-Castillejo, S.; Farras, M.; Catalan, U.; Subirana, I.; Montes, R.; Sola, R.; Munoz-Aguayo, D.; Gelabert-Gorgues, A.; Diaz-Gil, O.; et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arter. Thromb Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef]
- Tanaka, N.; Ishida, T.; Nagao, M.; Mori, T.; Monguchi, T.; Sasaki, M.; Mori, K.; Kondo, K.; Nakajima, H.; Honjo, T.; et al. Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis 2014, 237, 577–583. [Google Scholar] [CrossRef]
- Nottage, K.A.; Ness, K.K.; Li, C.; Srivastava, D.; Robison, L.L.; Hudson, M.M. Metabolic syndrome and cardiovascular risk among long-term survivors of acute lymphoblastic leukaemia—From the St. Jude Lifetime Cohort. Br. J. Haematol. 2014, 165, 364–374. [Google Scholar] [CrossRef]
- Link, K.; Moell, C.; Garwicz, S.; Cavallin-Stahl, E.; Bjork, J.; Thilen, U.; Ahren, B.; Erfurth, E.M. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J. Clin. Endocrinol. Metab. 2004, 89, 5003–5012. [Google Scholar] [CrossRef]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-processed Food Intake and Obesity: What Really Matters for Health-Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef]
- Lavigne-Robichaud, M.; Moubarac, J.C.; Lantagne-Lopez, S.; Johnson-Down, L.; Batal, M.; Laouan Sidi, E.A.; Lucas, M. Diet quality indices in relation to metabolic syndrome in an Indigenous Cree (Eeyouch) population in northern Quebec, Canada. Public Health Nutr. 2018, 21, 172–180. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, J.B.; Kim, S.H.; Jo, M.-W.; Hwang, J.Y.; Bae, S.J.; Jung, C.H.; Lee, W.J.; Park, J.-Y.; Park, G.-M.; et al. Serum Triglyceride Levels and Cardiovascular Disease Events in Koreans. Cardiology 2015, 131, 228–235. [Google Scholar] [CrossRef]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Boren, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A.; et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef]
- Langsted, A.; Jensen, A.M.R.; Varbo, A.; Nordestgaard, B.G. Low High-Density Lipoprotein Cholesterol to Monitor Long-Term Average Increased Triglycerides. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Liu, Q.; Yasui, Y.; Neglia, J.P.; Leisenring, W.; Robison, L.L.; Mertens, A.C. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2328–2338. [Google Scholar] [CrossRef]
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Pahwa, R.; Goyal, A.; Bansal, P.; Jialal, I. Chronic Inflammation; StatPearls Publishing: Treasure Island, FL, USA, 2020; pp. 1–22. [Google Scholar]
- Shivappa, N.; Hebert, J.R.; Marcos, A.; Diaz, L.E.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; Gonzalez-Gil, E.; Frederic, G.; et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol. Nutr. Food Res. 2017, 61, 1600707. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Godos, J.; Hebert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients 2018, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.E.; Akinyemiju, T.F. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int. J. Cancer 2017, 141, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Miao, Q.; Walker, M.; Hebert, J.R.; Aronson, K.J. Association Between a Dietary Inflammatory Index and Prostate Cancer Risk in Ontario, Canada. Nutr. Cancer 2017, 69, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Mtintsilana, A.; Micklesfield, L.K.; Chorell, E.; Olsson, T.; Shivappa, N.; Hebert, J.R.; Kengne, A.P.; Goedecke, J.H. Adiposity Mediates the Association between the Dietary Inflammatory Index and Markers of Type 2 Diabetes Risk in Middle-Aged Black South African Women. Nutrients 2019, 11, 1246. [Google Scholar] [CrossRef]
- Moslehi, N.; Ehsani, B.; Mirmiran, P.; Shivappa, N.; Tohidi, M.; Hebert, J.R.; Azizi, F. Inflammatory Properties of Diet and Glucose-Insulin Homeostasis in a Cohort of Iranian Adults. Nutrients 2016, 8, 735. [Google Scholar] [CrossRef]
- Vlassara, H. Advanced glycation in health and disease: Role of the modern environment. Ann. N. Y. Acad. Sci. 2005, 1043, 452–460. [Google Scholar] [CrossRef]
- Vlassara, H.; Striker, G. Glycotoxins in the Diet Promote Diabetes and Diabetic Complications. Diabet. Complicat. 2007, 7, 235–241. [Google Scholar] [CrossRef]
- Mistretta, A.; Marventano, S.; Antoci, M.; Cagnetti, A.; Giogianni, G.; Nolfo, F.; Rametta, S.; Pecora, G.; Marranzano, M. Mediterranean diet adherence and body composition among Southern Italian adolescents. Obes. Res. Clin. Pract. 2017, 11, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, A.; Bes-Rastrollo, M.; Sanchez-Villegas, A.; Basterra-Gortari, F.J.; Nunez-Cordoba, J.M.; Martinez-Gonzalez, M.A. Mediterranean diet inversely associated with the incidence of metabolic syndrome: The SUN prospective cohort. Diabetes Care 2007, 30, 2957–2959. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Roswall, N.; Strom, P.; Sandin, S.; Adami, H.O.; Weiderpass, E. Mediterranean and Nordic diet scores and long-term changes in body weight and waist circumference: Results from a large cohort study. Br. J. Nutr. 2015, 114, 2093–2102. [Google Scholar] [CrossRef]
- Mendonca, R.D.; Lopes, A.C.; Pimenta, A.M.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. Am. J. Hypertens. 2017, 30, 358–366. [Google Scholar] [CrossRef]
- Thomson, J.L.; Landry, A.S.; Tussing-Humphreys, L.M.; Goodman, M.H. Diet quality of children in the United States by body mass index and sociodemographic characteristics. Obes. Sci. Pract. 2020, 6, 84–98. [Google Scholar] [CrossRef]
- Jakicic, J.M. The Effect of Physical Activity on Body Weight. Obes. J. 2009, 17, S34–S38. [Google Scholar] [CrossRef]
- Beccuti, G.; Pannain, S. Sleep and obesity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 402–412. [Google Scholar] [CrossRef]
- Rampersaud, E.; Mitchell, B.D.; Pollin, T.I.; Fu, M.; Shen, H.; O’Connell, J.R.; Ducharme, J.L.; Hines, S.; Sack, P.; Naglieri, R.; et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch. Intern. Med. 2008, 168, 1791–1797. [Google Scholar] [CrossRef]
- Pouliot, M.C.; Després, J.P.; Lemieux, S.; Moorjani, S.; Bouchard, C.; Tremblay, A.; Nadeau, A.; Lupien, P.J. Waist Circumference and Abdominal Sagittal Diameter: Best Simple Anthropometric Indexes of Abdominal Visceral Adipose Tissue Accumulation and Related Cardiovascular Risk in Men and Women. Am. J. Cardiol. 1994, 73, 460–468. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Urschel, K. TNF-&alpha in the cardiovascular system: From physiology to therapy. Int. J. Interferon Cytokine Mediat. Res. 2015, 7, 9–25. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martinez, P.; Salas-Salvado, J.; Covas, M.I.; Toledo, E.; Andres-Lacueva, C.; Llorach, R.; et al. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J. Nutr. 2012, 142, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Liu, J.; Lau, C.W.; Huang, Y. IL-6 in diabetes and cardiovascular complications. Br. J. Pharmacol. 2014, 171, 3595–3603. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Pischon, T.; Hankinson, S.E.; Rifai, N.; Joshipura, K.; Willett, W.C.; Rimm, E.B. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 2004, 79, 606–612. [Google Scholar] [CrossRef]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef]
- Sahin-Efe, A.; Katsikeris, F.; Mantzoros, C.S. Advances in adipokines. Metabolism 2012, 61, 1659–1665. [Google Scholar] [CrossRef]
- Katsiki, N.; Mantzoros, C.; Mikhailidis, D.P. Adiponectin, lipids and atherosclerosis. Curr. Opin. Lipidol. 2017, 28, 347–354. [Google Scholar] [CrossRef]
- Sparrenberger, K.; Sbaraini, M.; Cureau, F.V.; Telo, G.H.; Bahia, L.; Schaan, B.D. Higher adiponectin concentrations are associated with reduced metabolic syndrome risk independently of weight status in Brazilian adolescents. Diabetol. Metab. Syndr. 2019, 11, 40. [Google Scholar] [CrossRef]
- Shafiee, G.; Ahadi, Z.; Qorbani, M.; Kelishadi, R.; Ziauddin, H.; Larijani, B.; Heshmat, R. Association of adiponectin and metabolic syndrome in adolescents: The caspian-III study. J. Diabetes Metab. Disord. 2015, 14, 89. [Google Scholar] [CrossRef]
- Izadi, V.; Azadbakht, L. Specific dietary patterns and concentrations of adiponectin. J. Res. Med. Sci. 2015, 20, 178–184. [Google Scholar] [PubMed]
- Berger, S.; Polotsky, V.Y. Leptin and Leptin Resistance in the Pathogenesis of Obstructive Sleep Apnea: A Possible Link to Oxidative Stress and Cardiovascular Complications. Oxid. Med. Cell. Longev. 2018, 2018, 5137947. [Google Scholar] [CrossRef]
- Kohler, J.A.; Moon, R.J.; Wright, S.; Willows, E.; Davies, J.H. Increased adiposity and altered adipocyte function in female survivors of childhood acute lymphoblastic leukaemia treated without cranial radiation. Horm. Res. Paediatr. 2011, 75, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Kafai, M.R.; McCarthy, E. Serum leptin concentrations are not related to dietary patterns but are related to sex, age, body mass index, serum triacylglycerol, serum insulin, and plasma glucose in the US population. Nutr. Metab. (Lond.) 2009, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cook, N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation 2004, 109, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Vandermorris, A.; Sampson, L.; Korenblum, C. Promoting adherence in adolescents and young adults with cancer to optimize outcomes: A developmentally oriented narrative review. Pediatr. Blood Cancer 2020, 67, e28128. [Google Scholar] [CrossRef]
- Kleinke, A.M.; Classen, C.F. Adolescents and young adults with cancer: Aspects of adherence—A questionnaire study. Adolesc. Health Med. Ther. 2018, 9, 77–85. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Werner, C.; Clipp, E.C.; Guill, A.B.; Bonner, M.; Jones, L.W.; Rosoff, P.M. Survivors of childhood cancer and their guardians. Cancer 2005, 103, 2171–2180. [Google Scholar] [CrossRef]
- Banfield, E.C.; Liu, Y.; Davis, J.S.; Chang, S.; Frazier-Wood, A.C. Poor Adherence to US Dietary Guidelines for Children and Adolescents in the National Health and Nutrition Examination Survey Population. J. Acad. Nutr. Diet 2016, 116, 21–27. [Google Scholar] [CrossRef]
- Hebert, J.R.; Clemow, L.; Pbert, L.; Ockene, I.S.; Ockene, J.K. Social Desirability Bias in Dietary Self-Report May Compromise the Validity of Dietary Intake Measures. Int. J. Epidemiol. 1995, 24, 389–398. [Google Scholar] [CrossRef]
- Hebert, J.R.; Ma, Y.; Clemow, L.; Ockene, I.S.; Saperia, G.; Stanek, E.J.; Merriam, P.A.; Ockene, J.K. Gender Differences in Social Desirability and Social Approval Bias in Dietary Self-Report. Am. J. Epidemiol. 1997, 146, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
| Total N = 241 | Adults N = 156 | Children N = 85 | |
|---|---|---|---|
| Median (range or interquartile range 1) | |||
| Age at visit, years (range) | 21.3 (8.5–40.9) | 24.6 (18.0–40.9) | 16.2 (8.5–17.9) |
| Age at cancer diagnosis, years (range) | 4.7 (0.9–18.0) | 6.5 (0.9–18.0) | 3.5 (1.3–10.9) |
| Time since end of treatment, years (range) | 12.9 (3.3–26.1) | 16.11 (3.9–26.1) | 9.6 (3.3–13.4) |
| Gender (males, %) | 49.4 | 49.4 | 49.4 |
| CRT exposure (n, %) | 142 (58.9%) | 108 (69.2%) | 34 (40.0%) |
| BMI (kg/m2) | 23.5 (20.9–26.1) | 24.3 (21.7–27.4) | 21.8 (19.2–24.1) |
| WHtR | 0.50 (0.46–0.55) | 0.51 (0.48–0.58) | 0.49 (0.45–0.52) |
| WC (cm) | 85.7 (76.0–93.0) | 89.0 (79.5–97.0) | 79.9 (72.0–84.0) |
| Glucose (mmol/L) | 5.0 (4.8–5.3) | 5.0 (4.8–5.4) | 5.0 (4.8–5.3) |
| Insulin (pmol/L) | 53.3 (37.6–75.8) | 50.1 (35.2–68.6) | 58.1 (42.9–81.1) |
| HOMA-IR | 1.7 (1.2–2.5) | 1.7 (1.1–2.3) | 1.9 (1.3–2.7) |
| TC (mmol/L) | 4.37 (3.87–5.01) | 4.59 (4.10–5.15) | 4.18 (3.61–4.63) |
| TG (mmol/L) | 0.91 (0.66–1.25) | 0.97 (0.72–1.38) | 0.82 (0.62–1.07) |
| LDL-C (mmol/L) | 2.57 (2.13–3.16) | 2.73 (2.22–3.32) | 2.36 (2.04–2.82) |
| HDL-C (mmol/L) | 1.30 (1.12–1.49) | 1.31 (1.13–1.52) | 1.29 (1.09–1.45) |
| SBP (mmHg) | 115 (108–124) | 117 (110–125) | 112 (104–119) |
| DBP (mmHg) | 65 (59–70) | 67 (63–72) | 62 (57–65) |
| n = 78 | n = 38 | n = 40 | |
| Apo A1 (mg/mL) | 2.32 (1.94–2.65) | 2.46 (1.98–2.72) | 2.18 (1.90–2.52) |
| Apo B100 (g/L) | 0.82 (0.71–0.94) | 0.85 (0.72–0.97) | 0.79 (0.68–0.92) |
| Score (Range) | N | Mean | Median | SD | Min | Max |
|---|---|---|---|---|---|---|
| MEDAS (0–14) | 156 | 4.26 | 4.00 | 1.87 | 1.00 | 10.00 |
| KIDMED (−4–12) | 85 | 3.45 | 4.00 | 2.36 | −2.00 | 9.00 |
| HDI-2018 (0–9) | 241 | 4.06 | 4.00 | 1.56 | 0.00 | 9.00 |
| HEI-2015 (0–100) | 241 | 59.78 | 60.00 | 10.58 | 35.00 | 85.00 |
| E-DII (−8.87–7.98) | 241 | −0.92 | −1.06 | 1.61 | −4.80 | +3.23 |
| FRAP (≥0 mmol/day) | 241 | 11.89 | 9.78 | 7.64 | 1.96 | 48.79 |
| % UPF (0–100%) 1 | 191 | 51.33 | 50.69 | 16.11 | 6.93 | 93.36 |
| E-DII Score | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | Tertile 1 | Tertile 2 | Tertile 3 | ||||
| −2.67 (−4.80–−1.75) 1 | −1.05 (−1.71–−0.35) 1 | 0.97 (−0.34–3.23) 1 | |||||
| OR (95% CI) | OR (95% CI) 2 Tertile 2 vs. 1 | p | OR (95% CI) 2 Tertile 3 vs. 1 | p | OR (95% CI) 2 Tertiles 2 and 3 vs. 1 | p | |
| High BMI | - | 1.297 (0.50–3.34) | 0.59 | 1.260 (0.47–3.41) | 0.65 | 1.280 (0.55–3.01) | 0.57 |
| High WC | - | 1.089 (0.54–2.18) | 0.81 | 1.574 (0.76–3.26) | 0.22 | 1.283 (0.69–2.37) | 0.43 |
| High BP | - | 3.029 (1.01–9.11) | 0.049 | 1.135 (0.35–3.71) | 0.83 | 1.928 (0.68–5.44) | 0.21 |
| High HOMA-IR | - | 2.667 (1.11–6.43) | 0.03 | 1.349 (0.50–3.68) | 0.56 | 2.047 (0.89–4.70) | 0.09 |
| Low HDL-C | - | 2.318 (1.04–5.16) | 0.04 | 2.414 (1.04–5.58) | 0.04 | 2.359 (1.13–4.92) | 0.02 |
| High LDL-C | - | 1.200 (0.50–2.89) | 0.68 | 1.183 (0.48–2.93) | 0.72 | 1.192 (0.54–2.62) | 0.66 |
| High TG | - | 0.937 (0.34–2.59) | 0.90 | 1.658 (0.62–4.41) | 0.31 | 1.240 (0.52–2.94) | 0.63 |
| % Ultra-Processed Foods According to the NOVA Classification | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | Tertile 1 | Tertile 2 | Tertile 3 | ||||
| 34.2% (6.9–43.0%) 1 | 50.0% (43.7–56.5%) 1 | 69.3% (56.6–93.4%) 1 | |||||
| OR (95% CI) | OR (95% CI) 2 Tertile 2 vs. 1 | p | OR (95% CI) 2 Tertile 3 vs. 1 | p | OR (95% CI) 2 Tertiles 2 and 3 vs. 1 | p | |
| High BMI | - | 0.360 (0.10–1.24) | 0.11 | 0.929 (0.34–2.58) | 0.89 | 0.619 (0.25–1.54) | 0.30 |
| High WC | - | 0.622 (0.28–1.37) | 0.24 | 0.968 (0.44–2.13) | 0.94 | 0.772 (0.39–1.51) | 0.45 |
| High BP | - | 0.781 (0.24–2.57) | 0.68 | 1.078 (0.36–3.33) | 0.89 | 0.934 (0.35–2.53) | 0.89 |
| High HOMA-IR | - | 0.341 (0.11–1.03) | 0.06 | 0.763 (0.30–1.97) | 0.58 | 0.533 (0.23–1.23) | 0.14 |
| Low HDL-C | - | 1.410 (0.55–3.64) | 0.48 | 3.885 (1.54–9.80) | 0.004 | 2.323 (1.02–5.28) | 0.04 |
| High LDL-C | - | 0.407 (0.15–1.13) | 0.09 | 0.728 (0.29–1.84) | 0.50 | 0.556 (0.25–1.26) | 0.16 |
| High TG | - | 2.998 (0.74–12.1) | 0.12 | 5.434 (1.38–21.4) | 0.02 | 4.021 (1.12–14.5) | 0.03 |
| Presence of 2 or More Cardiometabolic Complications | |||||||
|---|---|---|---|---|---|---|---|
| Scores | OR (95% CI) Tertile 1 | OR (95% CI) Tertile 2 vs. 1 | p | OR (95% CI) Tertile 3 vs. 1 | p | OR (95% CI) Tertiles 2 and 3 vs. 1 | p |
| MEDAS 2 | - | 0.800 (0.33–1.97) | 0.63 | 1.380 (0.54–3.50) | 0.30 | 1.279 (0.58–2.80) | 0.54 |
| KIDMED 2 | - | 0.424 (0.12–1.57) | 0.20 | 0.735 (0.23–2.40) | 0.61 | 0.728 (0.25–2.13) | 0.56 |
| HDI-2018 2 | - | 1.079 (0.52–2.23) | 0.84 | 1.191 (0.58–2.43) | 0.63 | 0.728 (0.25–2.13) | 0.56 |
| HEI-2015 3 | - | 1.053 (0.52–2.12) | 0.88 | 0.750 (0.36–1.55) | 0.44 | 0.911 (0.49–1.68) | 0.77 |
| E-DII 3 | - | 2.506 (1.22–5.15) | 0.01 | 1.613 (0.74–3.50) | 0.23 | 2.076 (1.07–4.07) | 0.03 |
| FRAP 2 | - | 1.509 (0.73–3.13) | 0.27 | 1.245 (0.57–2.73) | 0.58 | 1.391 (0.71–2.71) | 0.33 |
| % UPF 2,4 | - | 0.647 (0.29–1.47) | 0.30 | 1.128 (0.51–2.49) | 0.77 | 0.856 (0.43–1.70) | 0.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bérard, S.; Morel, S.; Teasdale, E.; Shivappa, N.; Hebert, J.R.; Laverdière, C.; Sinnett, D.; Levy, E.; Marcil, V. Diet Quality Is Associated with Cardiometabolic Outcomes in Survivors of Childhood Leukemia. Nutrients 2020, 12, 2137. https://doi.org/10.3390/nu12072137
Bérard S, Morel S, Teasdale E, Shivappa N, Hebert JR, Laverdière C, Sinnett D, Levy E, Marcil V. Diet Quality Is Associated with Cardiometabolic Outcomes in Survivors of Childhood Leukemia. Nutrients. 2020; 12(7):2137. https://doi.org/10.3390/nu12072137
Chicago/Turabian StyleBérard, Sophie, Sophia Morel, Emma Teasdale, Nitin Shivappa, James R. Hebert, Caroline Laverdière, Daniel Sinnett, Emile Levy, and Valérie Marcil. 2020. "Diet Quality Is Associated with Cardiometabolic Outcomes in Survivors of Childhood Leukemia" Nutrients 12, no. 7: 2137. https://doi.org/10.3390/nu12072137
APA StyleBérard, S., Morel, S., Teasdale, E., Shivappa, N., Hebert, J. R., Laverdière, C., Sinnett, D., Levy, E., & Marcil, V. (2020). Diet Quality Is Associated with Cardiometabolic Outcomes in Survivors of Childhood Leukemia. Nutrients, 12(7), 2137. https://doi.org/10.3390/nu12072137






