The Effects of Timing of a Leucine-Enriched Amino Acid Supplement on Body Composition and Physical Function in Stroke Patients: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
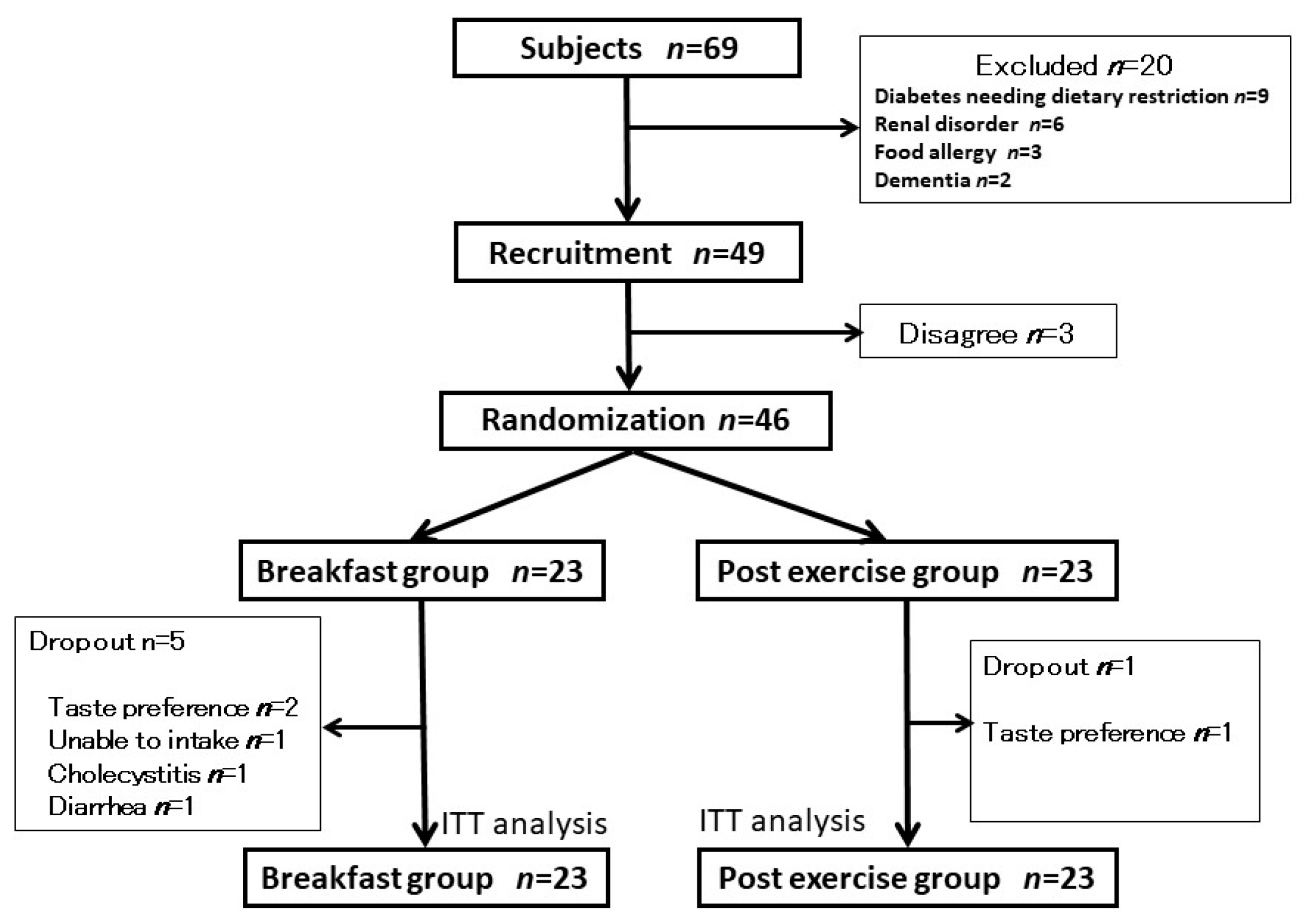
2.1. Subjects
2.2. Experimental Design
2.3. Demographic Data
2.4. Outcome Measures
- •
- Skeletal muscle mass and body fat mass
- •
- Muscle strength
- •
- Balance ability
- •
- Energy consumption and intake
2.5. Interventions
- •
- BCAA supplementation
- •
- Exercise
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, H.K.; Suzuki, T.; Saito, K.; Yoshida, H.; Kobayashi, H.; Kato, H.; Katayama, M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A ramdomized controlled trial. J. Am. Geriatr. Soc. 2012, 17, 1011–1019. [Google Scholar] [CrossRef]
- Ikeda, T.; Aizawa, J.; Nagasawa, H.; Gomi, I.; Kugota, H.; Nanjo, K.; Jinno, T.; Masuda, T.; Morita, S. Effects and feasibility of exercise therapy combined with branched chain amino acid supplementation on muscle strengthening in frail and pre-frail elderly people requiring long-term care: A crossover trial. Appl. Physiol. Nutr. Metab. 2016, 41, 438–445. [Google Scholar] [CrossRef]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The effect of protein supplements on functional frailty in older persons: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 86, 103938. [Google Scholar] [CrossRef]
- Liao, C.D.; Lee, P.H.; Hsiao, D.J.; Huang, S.W.; Tsauo, J.Y.; Chen, H.C.; Liou, T.H. Effects of protein supplementation combined with exercise intervention on frailty indices, body Composition, and physical function in frail older adults. Nutrients 2018, 10, 1916. [Google Scholar] [CrossRef] [Green Version]
- Dedeyne, L.; Deschodt, M.; Verschueren, S.; Tournoy, J.; Gielen, E. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: A systematic review. Clin. Interv. Aging 2017, 12, 873–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for treating sarcopenia: A systematic review and meta-analysis of randomized controlled studies. JAMDA 2017, 18, 553. [Google Scholar] [CrossRef]
- Liao, C.D.; Chen, H.C.; Huang, S.W.; Liou, T.H. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: A systematic review and meta-regression analysis of randomized trials. Nutrients 2019, 11, 1713. [Google Scholar] [CrossRef] [Green Version]
- Damanti, S.; Azzolino, D.; Roncaglione, C.; Arosio, B.; Rossi, P.; Ceari, M. Efficacy of Nutritional Interventions as Stand-Alone or Synergistic Treatments with Exercise for the Management of Sarcopenia. Nutrients 2019, 11, 1991. [Google Scholar] [CrossRef] [Green Version]
- Foley, N.; Finestone, H.; Woodbury, M.G.; Teasell, R.; Finestone, G.L. Energy and protein intakes of acute stroke patients. J. Nutr. Health Aging 2006, 10, 171–175. [Google Scholar] [PubMed]
- Kokura, Y.; Wakabayashi, H.; Nishioka, S.; Maeda, K. Nutritional intake is associated with activities of daily living and complications in older inpatients with stroke. Geriatr. Gerontol. Int. 2018, 18, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Wakabayashi, H.; Nishioka, S.; Nagano, A.; Momosaki, R. Energy intake at admission for improving activities of daily living and nutritional status among convalescent stroke patients. Neurol. Med. Chir. 2019, 59, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.M.; Hawley, J.A.; Ross, M.L.; Moore, D.R.; Phillips, S.M.; Slater, G.R.; Stellingwerff, T.; Tipton, K.D.; Garnham, A.P.; Coffey, V.G. Pre-exercise amino acidemia and muscle protein synthesis after resistance exercise. Med. Sci. Sports Exerc. 2012, 44, 1968–1977. [Google Scholar] [CrossRef]
- Chanet, A.; Verlaan, S.; Salles, J.; Giraudet, C.; Patrac, V.; Pidouet, V.; Pouyet, C.; Hafnaoui, N.; Blot, A.; Cano, N.; et al. Supplementing breakfast with a vitamin D and leucine–enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J. Nutr. 2017, 147, 2262–2271. [Google Scholar] [CrossRef] [Green Version]
- Pelmez, C.T.; Bjørnshave, A.; Pratico, G.; Hermansen, K.; Dragsted, L.O. Pre-meal protein intake alters postprandial plasma metabolome in subjects with metabolic syndrome. Eur. J. Nutr. 2019, in press. [Google Scholar] [CrossRef]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D. Evidence-based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein Intake and Exercise for Optimal Muscle Function With Aging: Recommendations From the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, M.; De Sire, A.; D’Andrea, F.; Carrera, D.; Renò, F.; Migliaccio, S.; Iolascon, G.; Cisari, C. Effects of essential amino acid supplementation and rehabilitation on functioning in hip fracture patients: A pilot randomized controlled trial. Aging Clin. Exp. 2019, 31, 1517–1524. [Google Scholar] [CrossRef]
- Dreyer, H.C.; Owen, E.C.; Strycker, L.A.; Smolkowski, K.; Muyskens, J.B.; Kirkpatrick, T.K.; Christie, A.D.; Kuehl, K.S.; Lantz, B.A.; Shah, S.N.; et al. Essential amino acid supplementation mitigates muscle atrophy after total knee arthroplasty: A randomized, double-blind, placebo-controlled trial. JB JS Open Access 2018, 3, e006. [Google Scholar] [CrossRef]
- Ikeda, T.; Matsunaga, Y.; Kanbara, M.; Kamono, A.; Masuda, T.; Watanabe, M.; Nakanishi, R.; Jinno, T. Effect of exercise therapy combined with branched-chain amino acid supplementation on muscle strength in elderly women after total hip arthroplasty: A randomized controlled trial. Asia Pac. J. Clin. Nutr. 2019, 28, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Cintineo, H.P.; Arent, M.A.; Antonio, J.; Arent, S.M. Effects of protein supplementation on performance and recovery in resistance and endurance training. Front. Nutr. 2018, 5, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Nabuco, H.C.G.; Tomeleri, C.M.; Sugihara, P.J.; Fernandes, R.R.; Cavalcante, E.F.; Antunes, M. Effects of whey protein supplementation pre- or post-resistance training on muscle mass, muscular strength, and functional capacity in pre-conditioned older women: A randomized clinical trial. Nutrients. 2018, 10, 563. [Google Scholar] [CrossRef] [Green Version]
- Stark, M.; Lukaszuk, J.; Prawitz, A.; Salacinski, A. Protein timing and its effects on muscular hypertrophy and strength in individuals engaged in weight-training. JISSN 2012, 9, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, C.; Murakami, K.; Fujisawa, H. Change of muscle thickness over time in hemiplegia patients after acute stroke. J. Jpn. Phys. Ther. Assoc. 2016, 43, 136–142. (In Japanese) [Google Scholar] [CrossRef]
- Burd, N.A.; Gorissen, S.H.; Van Vliet, S.; Snijders, T.; Van Loon, L.J.C. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 828–836. [Google Scholar] [CrossRef]
- Trommelen, J.; Van Loon, L.J.C. Pre-Sleep Protein Ingestion to Improve the Skeletal Muscle Adaptive Response to Exercise Training. Nutrients 2016, 8, 763. [Google Scholar] [CrossRef] [Green Version]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle full effect after oral protein: Time dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef] [Green Version]
- Burd, N.A.; West, D.W.; Moore, D.R.; Atherton, P.J.; Staples, A.W.; Prior, T.; Tang, J.E.; Rennie, M.J.; Baker, S.K.; Phillips, S.M. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J. Nutr. 2011, 141, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Hata, J.; Doi, Y.; Ninomiya, T.; Tanizaki, Y.; Yonemoto, K.; Fukuhara, M.; Kubo, M.; Kitazono, T.; Iida, M.; Kiyohara, Y. The effect of metabolic syndrome defined by various criteria on the development of ischemic stroke subtypes ill a general Japanesepopulation. Atherosclerosis 2010, 210, 249–255. [Google Scholar] [CrossRef]
- Ten Haaf, D.M.S.; Nuijten, M.A.H.; Maessen, M.F.H.; Horstman, A.M.H.; Eijsvogels, T.M.H.; Hopman, M.T.E. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 1043–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Breakfast Group | Post-Exercise Group | p-Value | |
|---|---|---|---|
| (n = 23) | (n = 23) | ||
| Age (years) | 65.5 ± 13.1 | 67.5 ± 5 | 0.635 |
| Sex (male/female) | 14:09 | 14:09 | 1.0 |
| Body mass index (kg/m2) | 23.2 ± 3.3 | 21.3 ± 4.3 | 0.093 |
| Charlson co-morbidity index (score) | 4.7 ± 1.7 | 5.0 ± 1.9 | 0.464 |
| Diagnosis: Cerebral infarction | 14 persons | 13 persons | |
| Cerebral hemorrhage | 5 persons | 7 persons | 0.773 |
| Subarachnoid hemorrhage | 4 persons | 3 persons | |
| Brumstrom recovery stage (I:II:III:IV:V:VI) | |||
| Upper limb | 0:3:3:3:5:9 | 0:2:5:3:3:10 | 0.860 |
| Lower limb | 0:3:3:3:4:11 | 0:4:5:3:4:7 | 0.772 |
| Finger | 1:0:4:4:5:9 | 0:2:6:4:2:9 | 0.455 |
| Breakfast Group | Post-Exercise Group | p-Value | |
|---|---|---|---|
| (n = 23) | (n = 23) | ||
| Number of sessions Exercise therapy | 89.0 ± 34.8 | 87.7 ± 32.8 | 0.900 |
| Supplementation | 42.3 ± 19.6 | 43.0 ± 16.3 | 0.893 |
| Nutritional status | |||
| Albumin (g/dL): Pre-intervention | 3.8 ± 0.6 | 3.8 ± 0.6 | 0.980 |
| Post-intervention | 4.0 ± 0.4 | 3.9 ± 0.7 | 0.578 |
| Total protein (g/dL): Pre-intervention | 6.8 ± 0.6 | 6.7 ± 0.7 | 0.456 |
| Post-intervention | 7.0 ± 0.5 | 6.9 ± 0.6 | 0.319 |
| Energy status at baseline (kcal/day) | |||
| Energy consumption | 1865.8 ± 422.5 | 1767.9 ± 475.1 | 0.464 |
| Energy intake | 1611.5 ± 331.6 | 1581.6 ± 278.5 | 0.742 |
| Energy sufficient ratio (%) | 88.3 ± 18.1 | 93.6± 23.1 | 0.399 |
| Group | Pre-Intervention | Post-Intervention | Main Effect (Group) | 95% CI | ||
|---|---|---|---|---|---|---|
| p-Value | Lower Limit | Upper Limit | ||||
| Skeletal muscle mass (kg) | Breakfast | 23.7 ± 5.0 | 23.9 ± 4.6 | 0.679 | 21.5 | 26.1 |
| Post-exercise | 23.4 ± 7.4 | 23.1 ± 7.8 | ||||
| Body fat mass (kg) | Breakfast | 17.6 ± 6.6 | 15.1 ± 6.4 | 0.023 * | 13.2 | 17.7 |
| Post-exercise | 13.9 ± 6.6 | 12.9 ± 5.5 | ||||
| Leg press strength (kgf) | Breakfast | 86.3 ± 45.9 | 113.0 ± 35.4 | 0.038 * | 94.5 | 124.6 |
| Post-exercise | 74.9 ± 44.1 | 87.7 ± 41.7 | ||||
| Grip strength (kgf) | Breakfast | 25.7 ± 9.9 | 26.2 ± 8.7 | 0.290 | 22.6 | 29.9 |
| Post-exercise | 23.4 ± 11.3 | 24.0 ± 10.8 | ||||
| Berg balance scale (score) | Breakfast | 41.3 ± 15.4 | 47.2 ± 8.4 | 0.030 * | 40.8 | 53.6 |
| Post-exercise | 34.3 ± 19.4 | 40.0 ± 16.2 | ||||
| Timed up and go test (s) | Breakfast | 18.8 ± 19.6 | 19.0 ± 19.1 | 0.081 † | 2.9 | 33.1 |
| Post-exercise | 36.2 ± 56.7 | 32.5 ± 56.3 | ||||
| Functional independence | Breakfast | 62.9 ± 18.9 | 80.6 ± 10.8 | 0.072 † | 72.6 | 85.4 |
| Measure (score) | Post-exercise | 59.3 ± 19.7 | 70.7 ± 20.1 | |||
| Breakfast Group (n = 23) | Post-Exercise Group (n = 23) | p-Value | |
|---|---|---|---|
| Δ Body fat mass (kg) | –2.5 ± 2.6 | –0.9 ± 2.1 | 0.038 * |
| Δ Leg press strength (kgf) | 26.7 ± 28.5 | 12.8 ± 18.1 | 0.051 † |
| Δ Berg balance scale (score) | 5.9 ± 10.2 | 5.8 ± 9.8 | 0.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, T.; Morotomi, N.; Kamono, A.; Ishimoto, S.; Miyazawa, R.; Kometani, S.; Sako, R.; Kaneko, N.; Iida, M.; Kawate, N. The Effects of Timing of a Leucine-Enriched Amino Acid Supplement on Body Composition and Physical Function in Stroke Patients: A Randomized Controlled Trial. Nutrients 2020, 12, 1928. https://doi.org/10.3390/nu12071928
Ikeda T, Morotomi N, Kamono A, Ishimoto S, Miyazawa R, Kometani S, Sako R, Kaneko N, Iida M, Kawate N. The Effects of Timing of a Leucine-Enriched Amino Acid Supplement on Body Composition and Physical Function in Stroke Patients: A Randomized Controlled Trial. Nutrients. 2020; 12(7):1928. https://doi.org/10.3390/nu12071928
Chicago/Turabian StyleIkeda, Takashi, Nobuo Morotomi, Arinori Kamono, Saki Ishimoto, Ryo Miyazawa, Shogo Kometani, Rikitaro Sako, Naohisa Kaneko, Mamoru Iida, and Nobuyuki Kawate. 2020. "The Effects of Timing of a Leucine-Enriched Amino Acid Supplement on Body Composition and Physical Function in Stroke Patients: A Randomized Controlled Trial" Nutrients 12, no. 7: 1928. https://doi.org/10.3390/nu12071928




