Dairy Consumption and Cardiometabolic Risk Factors in Patients with Type 2 Diabetes and Overweight or Obesity during Intensive Multidisciplinary Weight Management: A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Assessment of Dietary Intake
2.3. Assessment of Clinical and Laboratory Parameters
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Subjects
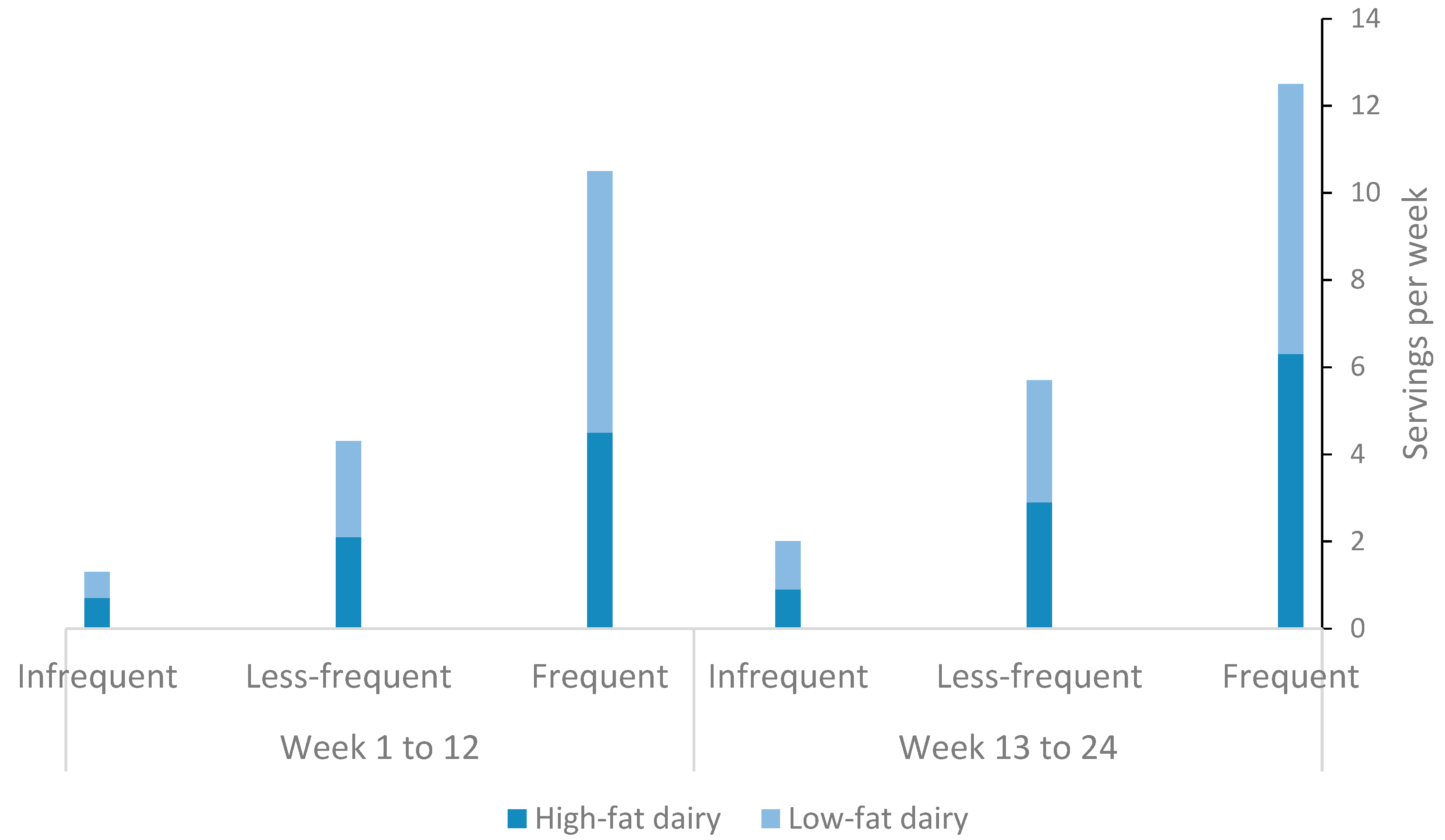
3.2. Dairy Consumption and its Fat Content among Study Subjects during the Intervention and Maintenance Phases
3.3. Changes in Study Outcomes from Baseline to 12 Weeks (Intervention Phase) and 24 Weeks (Maintenance Phase)
3.3.1. Changes in TEI and Macronutrients
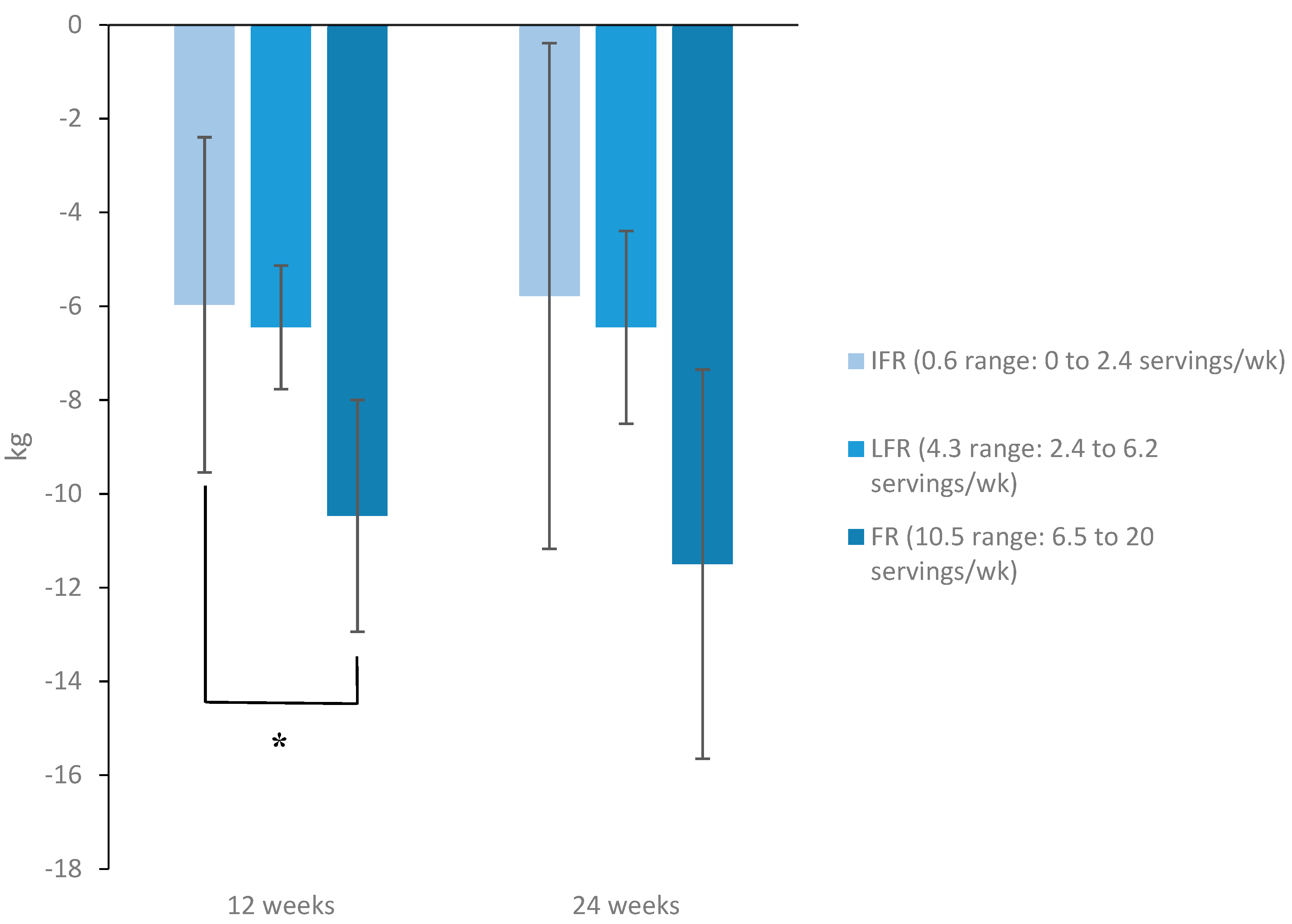
3.3.2. Changes in HbA1c, Body Weight, and Body Composition Parameters
3.3.3. Changes in Markers of Inflammation, Glycemia, and BP
3.3.4. Changes in Lipid Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besançon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020. [Google Scholar] [CrossRef] [Green Version]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 391, 541–551. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. 1), S89–S97.
- Kantor, L.S. A Dietary Assessment of the U.S. Food Supply: Comparing Per Capita Food Consumption with Food Guide Pyramid Serving Recommendations; United States Department of Agriculture and Center for Nutrition Policy and Promotion: Washington, DC, USA, 2018.
- United States Department of Agriculture. Dietary guidelines for Americans 2015–2020; United States Department of Agriculture and Center for Nutrition Policy and Promotion: Washington, DC, USA, 2018.
- Micha, R.; Mozaffarian, D.J.L. Saturated Fat and Cardiometabolic Risk Factors, Coronary Heart Disease, Stroke, and Diabetes: A Fresh Look at the Evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D. Dairy foods, dairy fat, diabetes, and death: What can be learned from 3 large new investigations? Am. J. Clin. Nutr. 2019, 110, 1053–1054. [Google Scholar] [CrossRef]
- Ardisson Korat, A.V.; Li, Y.; Sacks, F.; Rosner, B.; Willett, W.C.; Hu, F.B.; Sun, Q. Dairy fat intake and risk of type 2 diabetes in 3 cohorts of US men and women. Am. J. Clin. Nutr. 2019, 110, 1192–1200. [Google Scholar] [CrossRef] [Green Version]
- Drouin-Chartier, J.P.; Li, Y.; Ardisson Korat, A.V.; Ding, M.; Lamarche, B.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in dairy product consumption and risk of type 2 diabetes: Results from 3 large prospective cohorts of US men and women. Am. J. Clin. Nutr. 2019, 110, 1201–1212. [Google Scholar] [CrossRef]
- Chen, M.; Pan, A.; Malik, V.S.; Hu, F.B. Effects of dairy intake on body weight and fat: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Hoffmann, G.; Schwedhelm, C.; Kalle-Uhlmann, T.; Missbach, B.; Knuppel, S.; Boeing, H. Consumption of Dairy Products in Relation to Changes in Anthropometric Variables in Adult Populations: A Systematic Review and Meta-Analysis of Cohort Studies. PLoS ONE 2016, 11, e0157461. [Google Scholar] [CrossRef]
- Stonehouse, W.; Wycherley, T.; Luscombe-Marsh, N.; Taylor, P.; Brinkworth, G.; Riley, M. Dairy Intake Enhances Body Weight and Composition Changes during Energy Restriction in 18-50-Year-Old Adults-A Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 394. [Google Scholar] [CrossRef] [Green Version]
- Rozenberg, S.; Body, J.J.; Bruyere, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.P.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; et al. Effects of Dairy Products Consumption on Health: Benefits and Beliefs--A Commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Palacios, C.; Bertrán, J.J.; Ríos, R.E.; Soltero, S. No effects of low and high consumption of dairy products and calcium supplements on body composition and serum lipids in Puerto Rican obese adults. Nutrition 2011, 27, 520–525. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, O.; Carver, C. The Why WAIT program: Improving clinical outcomes through weight management in type 2 diabetes. Curr. Diabetes Rep. 2008, 8, 413. [Google Scholar] [CrossRef]
- Hamdy, O.; Mottalib, A.; Morsi, A.; El-Sayed, N.; Goebel-Fabbri, A.; Arathuzik, G.; Shahar, J.; Kirpitch, A.; Zrebiec, J. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: A 5-year longitudinal study. BMJ Open Diabetes Res. Care 2017, 5, e000259. [Google Scholar] [CrossRef]
- Tomah, S.; Mahmoud, N.; Mottalib, A.; Pober, D.M.; Tasabehji, M.W.; Ashrafzadeh, S.; Hamdy, O. Frequency of self-monitoring of blood glucose in relation to weight loss and A1C during intensive multidisciplinary weight management in patients with type 2 diabetes and obesity. BMJ Open Diabetes Res. Care 2019, 7, e000659. [Google Scholar] [CrossRef] [Green Version]
- US Department of Agriculture. Agriculture USDo. ChooseMyPlate. Available online: https://www.choosemyplate.gov/eathealthy/dairy (accessed on 1 April 2020).
- Monzillo, L.U.; Hamdy, O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr. Rev. 2003, 61, 397–412. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Kratz, M.; Marcovina, S.; Nelson, J.E.; Yeh, M.M.; Kowdley, K.V.; Callahan, H.S.; Song, X.; Di, C.; Utzschneider, K.M. Dairy fat intake is associated with glucose tolerance, hepatic and systemic insulin sensitivity, and liver fat but not β-cell function in humans. Am. J. Clin. Nutr. 2014, 99, 1385–1396. [Google Scholar] [CrossRef] [Green Version]
- Chartrand, D.; da Silva, M.S.; Julien, P.; Rudkowska, I. Influence of amino acids in dairy products on glucose homeostasis: The clinical evidence. Can. J. Diabetes 2017, 41, 329–337. [Google Scholar] [CrossRef]
- Krachler, B.; Norberg, M.; Eriksson, J.W.; Hallmans, G.; Johansson, I.; Vessby, B.; Weinehall, L.; Lindahl, B. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Cao, H.; King, I.B.; Lemaitre, R.N.; Song, X.; Siscovick, D.S.; Hotamisligil, G.K.S. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in US adults: A cohort study. Ann. Intern. Med. 2010, 153, 790–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakoob, M.Y.; Shi, P.; Willett, W.C.; Rexrode, K.M.; Campos, H.; Orav, E.J.; Hu, F.B.; Mozaffarian, D. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation 2016, 133, 1645–1654. [Google Scholar] [CrossRef] [Green Version]

| All Subjects (N = 45) | IFR (n = 15) | LFR (n = 15) | FR (n = 15) | P Value 1 | |
|---|---|---|---|---|---|
| Age (years) | 56 (9) | 57 (7) | 58 (9) | 53 (10) | 0.3 |
| Female sex (%) | 40% | 46.7% | 26.7% | 46.7% | 0.5 |
| Race/ethnicity (%) | |||||
| Non-Hispanic White | 82% | 66.7% | 92.9% | 84.6% | 0.2 |
| African American | 12.8% | 25% | 7.1% | 7.7% | 0.3 |
| Asian | 0% | 0% | 0% | 0% | - |
| Hispanic | 2.6% | 0% | 0% | 7.7% | 0.3 |
| Other/Not reported | 2.6% | 8.3% | 0% | 0% | 0.3 |
| Diabetes duration (years) | 10.9 (7.1) | 11 (6) | 12 (7) | 9 (8) | 0.7 |
| Total energy intake (kcal/d) | 1880 (544) | 1496 (540) | 1962 (448) | 2106 (498) | 0.008 |
| Energy from total fat (%) | 37.5 (5.6) | 37.4 (4.4) | 36.2 (6.1) | 38.8 (5.8) | 0.9 |
| Energy from saturated fat (%) | 11.8 (3) | 11.2 (2.4) | 11 (2.9) | 13.1 (3.2) | 0.1 |
| Energy from carbohydrate (%) | 40.3 (6.5) | 41.3 (5.8) | 40 (5.8) | 39.9 (7.7) | 0.8 |
| Energy from protein (%) | 20.7 (5) | 21 (5.5) | 20.8 (4.3) | 20.2 (5.6) | 0.9 |
| Body weight (kg) | 106.3 (19.1) | 102.3 (15.9) | 102.4 (20.6) | 114.1 (19.3) | 0.15 |
| Body Mass Index (kg/m2) | 36 (5) | 36 (4) | 35 (6) | 37 (5) | 0.5 |
| HbA1c (%) | 7.9 (1.3) | 8 (1.4) | 7.6 (1.2) | 8.1 (1.4) | 0.6 |
| Total Cholesterol (mg/dL) | 162 (35) | 165 (33) | 156 (31) | 167 (41) | 0.7 |
| HDL-Cholesterol (mg/dL) | 46 (12) | 48 (12) | 44 (14) | 45 (11) | 0.7 |
| LDL-Cholesterol (mg/dL) | 85 (30) | 83 (22) | 80 (28) | 91 (38) | 0.6 |
| VLDL-Cholesterol (mg/dL) | 32 (21) | 40 (30) | 28 (15) | 27 (9) | 0.2 |
| Triglycerides (mg/dL) | 173 (145) | 165 (88) | 167 (105) | 187 (217) | 0.9 |
| Waist circumference (cm) | 120 (13) | 119 (8.3) | 117 (13) | 124 (16) | 0.3 |
| Hip circumference (cm) | 120.7 (14) | 120 (7.6) | 116 (16) | 126 (15.7) | 0.2 |
| Waist/hip ratio | 1 (0.07) | 1 (0.07) | 1 (0.07) | 1 (0.07) | 0.6 |
| Body fat (%) | 40.1 (8) | 41.8 (7.6) | 37.7 (7.4) | 40.9 (9) | 0.4 |
| Fat mass (kg) | 38 (15) | 38.8 (11.7) | 34 (13) | 41 (19) | 0.4 |
| Fat free mass (kg) | 57.2 (20.4) | 55.3 (18) | 57 (19.3) | 59 (24.4) | 0.9 |
| Total body water (kg) | 42 (15) | 40.5 (13.2) | 42 (14.2) | 43.3 (18) | 0.9 |
| Trunk fat (%) | 46.2 (8.4) | 45 (6.5) | 44 (9.9) | 50 (8) | 0.2 |
| Visceral fat (arbitrary units) | 20.2 (8.6) | 18.9 (7.1) | 20.8 (9) | 21 (10.4) | 0.8 |
| CRP (mg/L) | 4.3 (6.7) | 2.6 (2.2) | 3.5 (3.6) | 6.6 (10.7) | 0.2 |
| Fasting glucose (mg/dL) | 152 (51) | 147 (54) | 143 (42) | 166 (56) | 0.4 |
| HOMA-IR | 6.9 (5.6) | 7.7 (6.6) | 6.1 (5.4) | 7.1 (4.8) | 0.7 |
| Systolic blood pressure (mmHg) | 128 (16) | 124 (12) | 130 (16) | 131 (21) | 0.45 |
| Diastolic blood pressure (mmHg) | 72 (10) | 70 (9) | 74 (12) | 72 (9) | 0.56 |
| Diabetes medications (%) | |||||
| Metformin | 84.4% | 86.7% | 73.3% | 93.3% | 0.3 |
| SFUs | 26.6% | 20% | 33.3% | 26.7% | 0.7 |
| DPP-4 inhibitors | 4.4% | 0% | 6.7% | 6.7% | 0.6 |
| SGLT-2 inhibitors | 26.6% | 26.7% | 26.7% | 26.7% | 1 |
| GLP-1 analogues | 37.7% | 33.3% | 53.3% | 26.7% | 0.29 |
| TZDs | 2.2% | 0% | 0% | 6.7% | 0.36 |
| Other | 44.4% | 26.7% | 53.3% | 53.3% | 0.23 |
| IFR (n = 15) | LFR (n = 15) | FR (n = 15) | P3 | P4 | ||||
|---|---|---|---|---|---|---|---|---|
| 12 Weeks | 24 Weeks | 12 Weeks | 24 Weeks | 12 Weeks | 24 Weeks | |||
| Total energy intake (kcal/d) | −123 (−500, 254) | 30 (−352, 413) | −312 (−596, −28) 1 | −73 (−394, 247) | −379 (−760, 2.7) | −209 (−574, 155) | 0.4 | 0.6 |
| Energy from total fat (%) | −10 (−29, 9) | −2 (−21, 18) | −11 (−30, 7) | −6 (−22, 10) | −19 (−42, 4) | −9 (−31, 14) | 0.4 | 0.6 |
| Energy from saturated fat (%) | −1.8 (−3.8, 0.3) | −0.3 (−2.2, 1.5) | −0.8 (−2.7, 1) | −0.6 (−2.6, 1.4) | −1 (−3.5, 1.5) | −0.4 (−3.1, 2.4) | 0.3 | 0.9 |
| Energy from carbohydrate (%) | 0.9 (−4, 5.9) | 1.4 (−3.2, 6) | −1 (−5.6, 3.6) | −1.1 (−5.8, 3.5) | −2.7 (−8.4, 3) | −2.5 (−7.7, 2.7) | 0.4 | 0.5 |
| Energy from protein (%) | 2.3 (−1.9, 6.4) | 0.2 (−3.8, 4.1) | 2.4 (−1.4, 6.2) | 1.2 (−2.1, 4.6) | 5.8 (1.1, 10.6)1 | 3.6 (−0.6, 7.8) | 0.1 | 0.06 |
| Body weight (kg) | −8 (−19.4, 3.6) | −7.6 (−19.4, 4.2) | −8.3 (−23.6, 7) | −9.5(−24.2, 5.3) | −9.5 (−24, 5) | −10 (−24.6, 4.7) | 0.3 | 0.23 |
| Body Mass Index (kg/m2) | −2.8 (−5.8, 0.3) | −2.6 (−5.8, 0.6) | −3 (−8, 2.1) | −3.3 (−8.3, 1.6) | −3.1 (−6.7, 0.5) | −3.3 (−7, 0.5) | 0.4 | 0.32 |
| HbA1c (%) | −1.32 (−2.2, −0.5) 2 | −1.13 (−2, −0.3) 1 | −1.12 (−1.84, −0.4) 2 | −0.64 (−1.65, 0.37) | −1.48 (−2.3, −0.6) 2 | −1.18 (−2, −0.34) 2 | 0.8 | 0.6 |
| Lipid profile | ||||||||
| Total Cholesterol (mg/dL) | −13 (−38, 12) | −10 (−31, 12) | −16 (−39, 6) | −5 (−30, 19) | −20 (−50, 9) | −11 (−40, 18) | 0.5 | 0.6 |
| HDL−C (mg/dL) | 2 (−8, 12) | 6 (−4, 15) | 3 (−8, 14) | 6 (−5, 17) | −2 (−10, 6) | 2 (−7, 11) | 0.03 | 0.09 |
| LDL−C (mg/dL) | −6 (−24, 11) | −4 (−19, 10) | −11 (−30, 8) | −6 (−26, 14) | −15 (−41, 12) | −7 (−31, 18) | 0.6 | 0.9 |
| VLDL−C (mg/dL) | −16 (−34, 1) | −18 (−35, −1) 1 | −5 (−15, 5) | −2 (−14, 10) | −1 (−10, 8) | −3 (−11, 5) | 0.06 | 0.04 |
| Triglycerides (mg/dL) | −48 (−106, 11) | −58 (−111, −4) 1 | −52 (−115, 10) | −37 (−108, 33) | −58 (−182, 66) | −69 (−192, 54) | 0.2 | 0.15 |
| Waist circumference (cm) | −7.8 (−15, −0.4) 1 | −7.5 (−15, −0.3) 1 | −9 (−19, 1) | −9.6 (−20, 0.5) | −6.5 (−18, 5) | −8 (−21, 5) | 0.5 | 0.9 |
| Hip circumference (cm) | −7 (−13, −0.6) 1 | −5 (−11, 2) | −7 (−19, 5) | −8 (−20, 3.6) | −9 (−20, 1,7) | −6.5 (−19, 6) | 0.7 | 0.7 |
| Waist/hip ratio | −0.01 (−0.07, 0.05) | −0.02 (−0.09, 0.04) | −0.02 (−0.07, 0.03) | −0.01 (−0.07, 0.04) | 0.02 (−0.03, 0.07) | −0.02 (−0.08, 0.04) | 0.2 | 0.9 |
| Body fat (%) | −4.3 (−11, 2.5) | −4.5 (−11, 1.8) | −4.1 (−10.4, 2.1) | −4.2 (−10.6, 2.1) | −2.6 (−8.7, 3.6) | −2.2 (−8.4, 3.4) | 0.4 | 0.2 |
| Fat mass (kg) | −5 (−14.6, 4.6) | −3.7 (−12.5, 5.1) | −1.5 (−11.8, 8.7) | −1.9 (−12.2, 8.3) | −0.73 (−12.7, 11.2) | −0.4 (−12.5, 11.8) | 0.3 | 0.3 |
| Fat free mass (kg) | −2 (−15, 10.8) | 3.2 (−10.5, 17) | 4.8 (−7.5, 17) | 4.1 (−8, 16.2) | 5.5 (−9.5, 20.5) | 4.8 (−10.2, 19.8) | 0.5 | 0.4 |
| Total body water (kg) | −1.5 (−10.9, 8) | 2.4 (−7.7, 12.4) | 3.5 (−5.5, 12.5) | 3 (−5.9, 12) | −4 (−7, 15) | 3.5 (−7.5, 14.5) | 0.5 | 0.4 |
| Trunk fat (%) | −3 (−8.4, 2.3) | −3.8 (−9.2, 1.5) | −8.3 (−17, 0.3) | −7.4 (−16.5, 1.6) | −5.4 (−12.2, 1.4) | −7.1 (−14.3, 0.2) | 0.4 | 0.6 |
| Visceral fat (arbitrary units) | −2.4 (−7.7, 2.9) | −2.1 (−7.7, 3.4) | −4.4 (−11, 2.1) | −3.6 (−10.7, 3.4) | −1.8 (−9.2, 5.6) | −2 (−9.5, 5.6) | 0.4 | 0.5 |
| CRP (mg/L) | −0.9 (−2.3, 0.6) | −0.2 (−1.9, 1.4) | −0.7 (−3.4, 2) | 0.1 (−3.1, 3.2) | 0.3 (−8.1, 8.7) | −0.3 (−10, 9.3) | 0.2 | 0.6 |
| Fasting glucose (mg/dL) | −24 (−60, 12) | −17 (−53, 17) | −12 (−38, 14) | 3 (−35, 41) | −36 (−72, −1) 1 | −38 (−72, −4) 1 | 0.2 | 0.4 |
| HOMA−IR | −3.6 (−7.6, 0.4) | −3.2 (−7.2, 0.9) | −1 (−5.3, 3.4) | −0.6 (−5.2, 3.9) | −1.4 (−4.5, 1.6) | −1.8 (−5.4, 1.7) | 0.3 | 0.6 |
| Systolic blood pressure (mmHg) | −3 (−14, 7) | 1 (−7, 10) | −7 (−18, 4) | −8 (−21, 5) | −5 (−20, 10) | −6 (−22, 10) | 0.9 | 0.4 |
| Diastolic blood pressure (mmHg) | −1 (−6, 6) | 2 (−4, 9) | −6 (−13, 3) | −7 (−15, 1) | −1 (−8, 5) | −1 (−8, 6) | 0.6 | 0.3 |
| HDL-C 1 | Crude | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | |
| Dairy (servings per week) | −1.93 (−4, 0.2) | 0.07 | −3 (−5.5, −0.5) | 0.022 | −3.4 (−6, −0.7) | 0.013 | −3.4 (−6.2, −0.7) | 0.015 |
| VLDL-C 2 | Crude | Model 1 | Model 2 | Model 3 | ||||
| β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | |
| Dairy (servings per week) | 6.1 (−0.2, 12.5) | 0.058 | 2.4 (−1.8, 6.7) | 0.25 | 2.8 (−1.7, 7.3) | 0.21 | 2.7 (−1.8, 7.3) | 0.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomah, S.; Eldib, A.H.; Tasabehji, M.W.; Mitri, J.; Salsberg, V.; Al-Badri, M.R.; Gardner, H.; Hamdy, O. Dairy Consumption and Cardiometabolic Risk Factors in Patients with Type 2 Diabetes and Overweight or Obesity during Intensive Multidisciplinary Weight Management: A Prospective Observational Study. Nutrients 2020, 12, 1643. https://doi.org/10.3390/nu12061643
Tomah S, Eldib AH, Tasabehji MW, Mitri J, Salsberg V, Al-Badri MR, Gardner H, Hamdy O. Dairy Consumption and Cardiometabolic Risk Factors in Patients with Type 2 Diabetes and Overweight or Obesity during Intensive Multidisciplinary Weight Management: A Prospective Observational Study. Nutrients. 2020; 12(6):1643. https://doi.org/10.3390/nu12061643
Chicago/Turabian StyleTomah, Shaheen, Ahmed H. Eldib, Mhd Wael Tasabehji, Joanna Mitri, Veronica Salsberg, Marwa R. Al-Badri, Hannah Gardner, and Osama Hamdy. 2020. "Dairy Consumption and Cardiometabolic Risk Factors in Patients with Type 2 Diabetes and Overweight or Obesity during Intensive Multidisciplinary Weight Management: A Prospective Observational Study" Nutrients 12, no. 6: 1643. https://doi.org/10.3390/nu12061643





