Iron Status of Burkinabé Adolescent Girls Predicts Malaria Risk in the Following Rainy Season
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
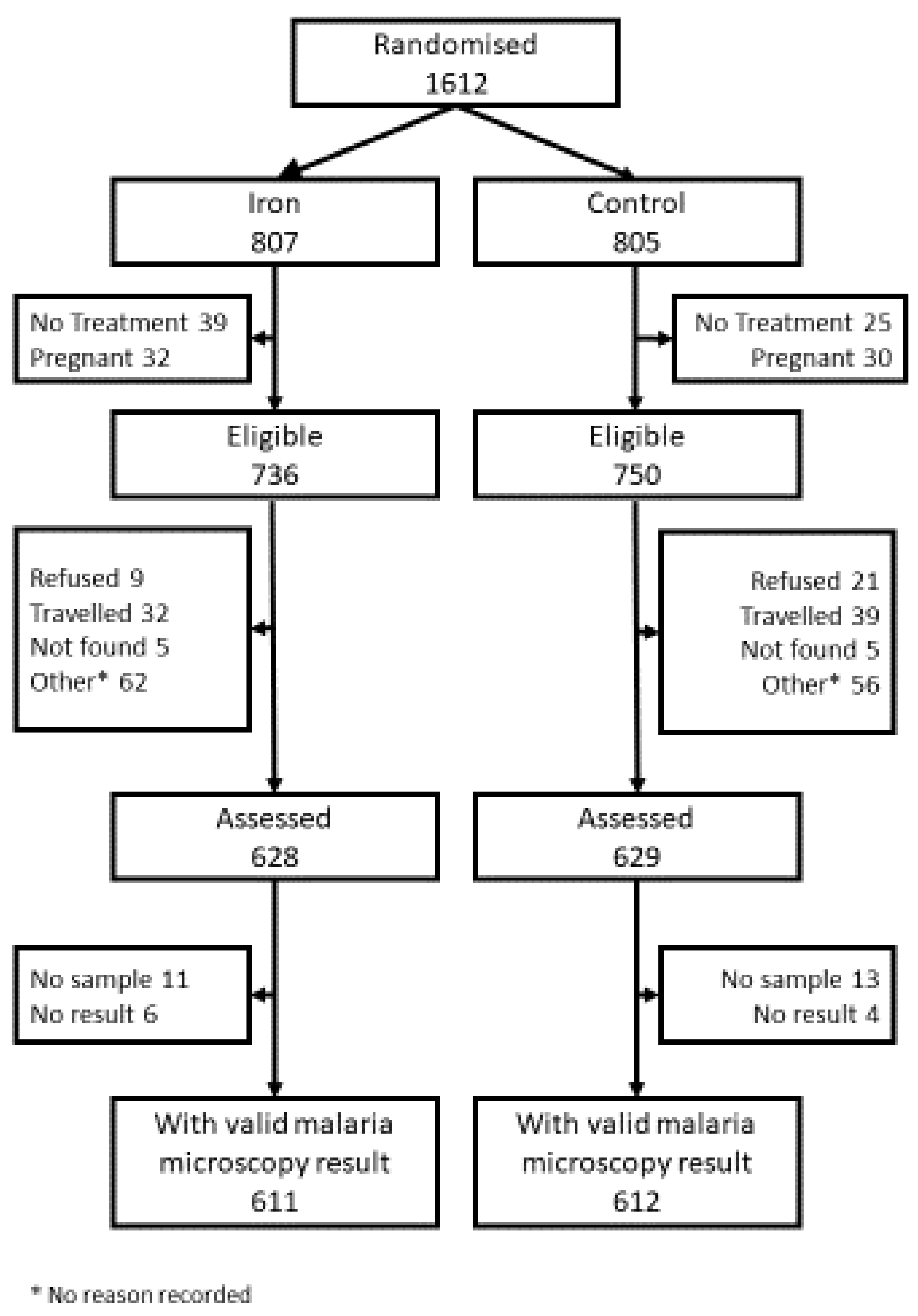
2.2. Background to the Trial
2.3. Baseline Assessment
2.4. Interim Safety Survey
2.5. Laboratory Tests
2.5.1. Assessment of Iron Status
- A non-pregnant internal regression slope log (ferritin) against log (CRP) estimate was used for ferritin correction, adjusting ferritin levels where CRP exceeded a reference level of the tenth centile to that reference level [15].
- sTfR concentration of >8.3 µg/mL [33].
- Ratio of sTfR (mg/L) to log10 adjusted ferritin (μg/L) >5.6, which assesses both stored and functional iron and is possibly less affected by inflammation. The ratio derives from the cut-offs sTfR > 8.3 μg/mL and ferritin < 30 μg/L. RAMCO was the assay used for sTfR, as its cut-off (>5.6) best predicted iron deficient bone marrow stores (sensitivity 74%, specificity 73%, accuracy 73%), in another area of high malaria transmission [34].
- The median hepcidin reference level of serum/plasma based on a healthy non-malarious Dutch population [35]. The hepcidin 95% reference range for women 18–24 years of age from that population was: median 2.6 nM; 2.5th percentile 0.7 nm; and 97.5th percentile 10.5 nM.
- BIS was calculated using the regression-adjusted ferritin estimate. Low body iron was defined as zero iron stores <0 mg/kg.
2.5.2. Assessment of Malaria
2.6. Statistical Analysis
3. Results
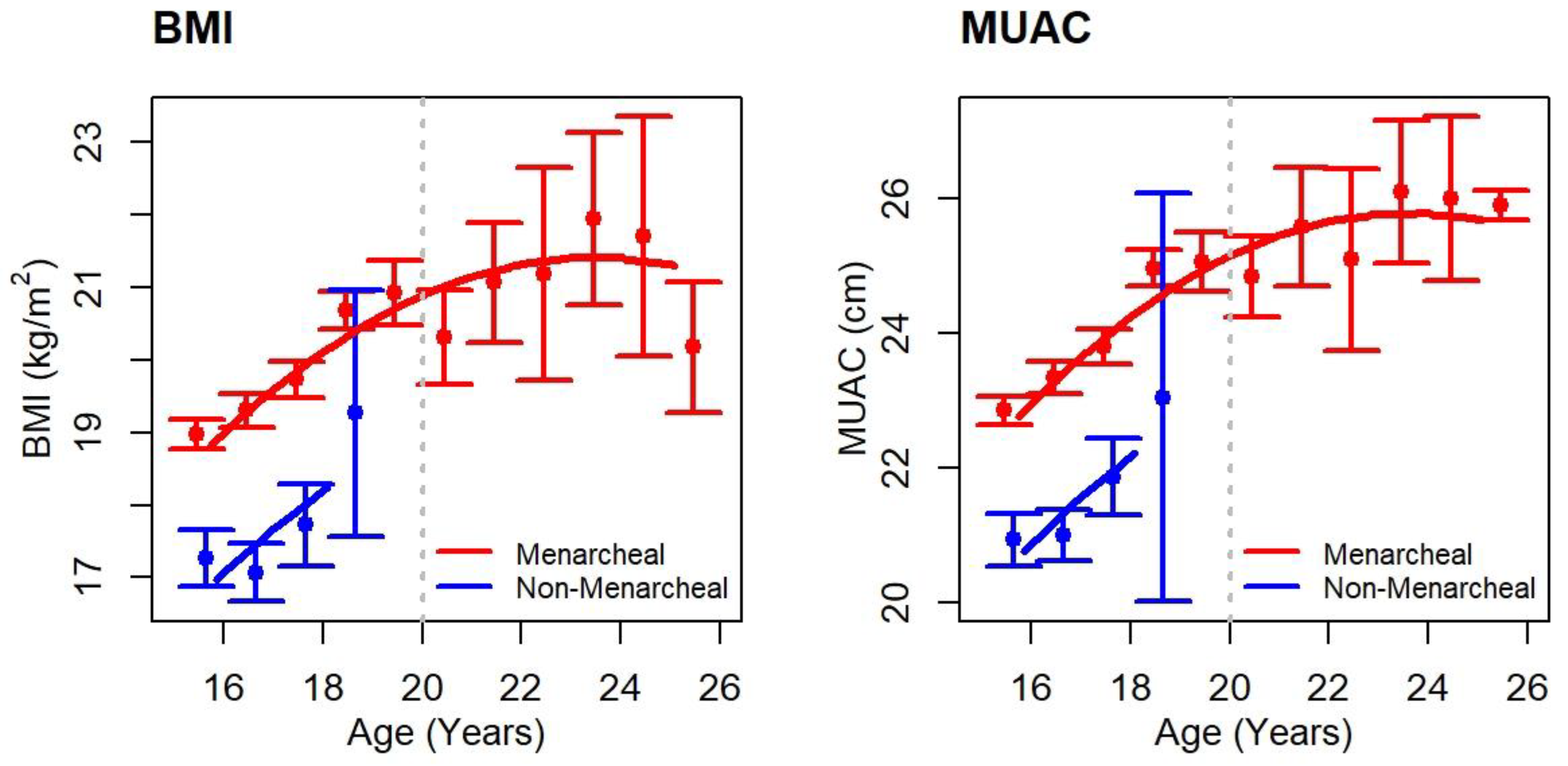
3.1. Sample Description
3.2. Iron Biomarker Profiles at the Baseline Survey
3.3. Malaria Indices at the Interim Safety Survey
3.4. Baseline Iron Status and Malaria Prevalence at the Interim Survey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNICEF; UNU; WHO. Iron Deficiency Anaemia. Assessment, Prevention, and Control. In A Guide for Programme Managers; WHO; NHD: Geneva, Switzerland, 2001; pp. 1–144. [Google Scholar]
- World Health Organization. Mainstreaming nutrition through the Life-course. In Essential Nutrition Actions; WHO: Geneva, Switzerland, 2019; ISBN 978-92-4-151585-6. [Google Scholar]
- World Health Organization. Evidence-Based Nutrition Interventions Included in the WHO e-Library of Evidence for Nutrition Actions (eLENA) that may Contribute to the Achievement of the WHO Global Nutrition and Diet-Related NCD Targets. Available online: https://www.who.int/elena/titles/summary_eLENA_interventions_global_targets.pdf?ua=1 (accessed on 15 May 2020).
- Fernández-Gaxiola, A.C.; De-Regil, L.M. Intermittent iron supplementation for reducing anaemia and its associated impairments in adolescent and adult menstruating women. Syst. Rev. 2019, 1, CD009218. [Google Scholar] [CrossRef] [PubMed]
- Peña Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily oral iron supplementation during pregnancy. Syst. Rev. 2015, CD004736. [Google Scholar] [CrossRef] [Green Version]
- Lassi, Z.S.; Moin, A.; Das, J.K.; Salam, R.A.; Bhutta, Z.A. Systematic review on evidence-based adolescent nutrition interventions. Ann. N. Y. Acad. Sci. 2017, 1393, 34–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gies, S.; Diallo, S.; Roberts, S.A.; Kazienga, A.; Powney, M.; Brabin, L.; Ouedraogo, S.; Swinkels, D.W.; Geurts-Moespot, H.J.; Claeys, Y.; et al. Effects of weekly iron and folic acid supplements on malaria risk in nulliparous women in Burkina Faso: A periconceptional double-blind randomized controlled non-inferiority trial. J. Inf. Dis. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, N. Anaemia and malaria. Mal. J. 2018, 17, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petry, N.; Olofin, I.; Hurrell, R.F.; Boy, E.; Wirth, J.P.; Moursi, M.; Angel, M.D.; Rohner, F. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: A systematic analysis of national surveys. Nutrients 2016, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M.; Ghattas, H.; Doherty, C.; Cox, S.E. Iron metabolism and malaria. Food. Nutr. Bull. 2007, 28, S524–S539. [Google Scholar] [CrossRef] [Green Version]
- Suchdev, P.S.; Leeds, I.L.; McFarlane, D.A.; Flores, R. Is it time to change guidelines for iron supplementation in malarial areas? J. Nutr. 2010, 4, 875–876. [Google Scholar] [CrossRef]
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.k.; Bhutta, Z.A. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013, 382, 29–40. [Google Scholar] [CrossRef]
- Brabin, L.; Roberts, S.A.; Gies, S.; Diallo, S.; Stewart, C.L.; Kazienga, A.; Birtles, J.; Ouedraogo, S.; Claeys, Y.; Tinto, H.; et al. Effects of long-term weekly iron and folic acid supplementation on lower genital tract infection—A double blind, randomized controlled trial in Burkina Faso. BMC Med. 2017, 15, 206. [Google Scholar] [CrossRef] [Green Version]
- Barffour, M.A.; Schulze, K.J.; Coles, C.L.; Chileshe, J.; Kalungwana, N.; Arguello, M.; Siamusantu, W.; Moss, W.J.; West, K.P., Jr.; Palmer, A.C. High iron stores in the low malaria season increase malaria risk in the high transmission season in a prospective cohort of rural Zambian children. J. Nutr. 2017, 147, 1531–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diallo, S.; Roberts, S.A.; Gies, S.; Rouamba, T.; Swinkels, D.; Geurts-Moespot, A.J.; Ouedraogo, S.; Ouedraogo, G.A.; Tinto, H.; Brabin, B.J. Malaria early in the first pregnancy: Potential impact of iron status. Clin. Nutr. 2020, 39, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojukwu, J.U.; Okebe, J.U.; Yahav, D.; Paul, M. Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic regions. Syst. Rev. 2009, 3, CD006589. [Google Scholar]
- Nutrition International. Weekly Iron Folic Acid Supplementation (WIFAS). Frequently Asked Questions. Available online: https://www.nutritionintl.org/content/user_files/2019/04/WIFAS_for_Adolescents_FAQs_2019.pdf (accessed on 15 May 2020).
- Beasley, N.M.R.; Tomkins, A.M.; Hall, A.; Lorri, W.; Kihamia, C.M.; Bundy, D.A.P. The impact of weekly iron supplementation on the iron status and growth of adolescent girls in Tanzania. Trop. Med. Hyg. 2000, 5, 794–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muro, S.G.; Gross, U.; Gross, R.; Wahyuniar, L. Increase in compliance with weekly iron supplementation of adolescent girls by an accompanying communication programme in secondary schools in Dar-Es-Salaam, Tanzania. Nutr. Bull. 1999, 20, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.; Roschnik, N.; Ouattara, F.; Touré I Maiga, F.; Sacko, S.; Moestue, H.; Bendech, M.A. A randomised trial of the effectiveness of weekly iron supplementation given by teachers on the haemoglobin concentration of schoolchildren. Public Health Nutr. 2001, 5, 413–418. [Google Scholar]
- Leenstra, T.; Kariuki, S.K.; Kurtis, J.D.; Oloo, A.J.; Kager, P.A.; ter Kuile, F.O. The effect of weekly iron and vitamin A supplementation on hemoglobin levels and iron status in adolescent schoolgirls in western Kenya. Eur. J. Clin. Nutr. 2009, 63, 173–182. [Google Scholar] [CrossRef]
- World Health Organization. Global Accelerated Action for the Health of Adolescents (AA-HA). In Guidance to Support Country Implementation—Summary; WHO; FWC; MCA: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Weekly Iron and Folic Acid Supplementation as an Anaemia-Prevention Strategy in Women and Adolescent Girls: Lessons Learnt from Implementation of Programmes among Non-Pregnant Women of Reproductive Age; WHO/NMH/NHD/18.8; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-156572-1. [Google Scholar]
- Institut National de la Statistique et de la Démographie (INSD) et ICF International. Enquête Démographique et de Santé et Indicateurs Multiples du Burkina Faso 2010; INSD et ICF International: Calverton, MD, USA, 2012. [Google Scholar]
- World Health Organization. Guideline: Daily Iron Supplementation in Adult Women and Adolescent Girls; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-1510196. [Google Scholar]
- World Health Organization. Malaria Rapid Diagnostic Test Performance: Results of WHO Product Testing of Malaria RDTs: Round 1. (2008); World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- de Onis, M.; Onyango, A.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. WHO 2007, 85, 661–668. [Google Scholar] [CrossRef]
- Kroot, J.C.C.; Laarakkers, C.M.; Geurts-Moespot, A.; Grebenchtchikov, N.; Pickkers, P.; van Ede, A.E.; Peters, H.P.; van Dongen-Lases, E.; Wetzels, J.F.; Sweep, F.C.; et al. Immunochemical and mass spectrometry-based serum hepcidin assays for a variety of iron metabolism disorders. Clin. Chem. 2010, 56, 1570–1579. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.; Flowers, C.H.; Skine, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3364. [Google Scholar] [CrossRef] [Green Version]
- Namaste, S.M.; Rohner, F.; Huang, J.J.; Bhushan, N.L.; Flores-Ayala, R.; Kupka, R.; Mei, Z.; Rawat, R.; Williams, A.M.; Raiten, D.J.; et al. Adjusting ferritin concentrations for inflammation: Biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 359S–371S. [Google Scholar] [PubMed]
- Mei, Z.; Namaste, S.M.; Serdula, M.; Suchdev, P.S.; Rohner, F.; Flores-Ayala, R.; Add, O.Y.; Raiten, D.J. Adjusting total body iron for inflammation: Biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 383S–389S. [Google Scholar]
- Rohner, F.; Namaste, S.M.; Larson, L.M.; Addo, O.Y.; Mei, Z.; Suchdev, P.S.; Williams, A.M.; Sakr Ahour, F.A.; Rawat, R.; Raiten, D.J.; et al. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 372S–382S. [Google Scholar] [PubMed]
- Phiri, K.S.; Calis, J.C.; Siyasiya, A.; Bates, I.; Brabin, B.; van Hensbroek, M.B. New cut-off values for ferritin and soluble transferrin receptor for the assessment of iron deficiency in children in a high infection pressure area. J. Clin. Pathol. 2009, 62, 1103–1106. [Google Scholar] [CrossRef] [Green Version]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; van Tienoven, D.; Wetzels, J.F.; Kiemeney, L.A.; Sweep, F.C.; den Heijer, M.; et al. Serum hepcidin: Reference ranges and biochemical correlates in the general population. Blood 2011, 117, e218–e225. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.J.I.; Davidson, E.; Agius, P.A.; Beeson, J.G. Understanding the interactions between iron supplementation, infectious diseases and adverse birth outcomes is essential to guide public health recommendations. BMC Med. 2019, 17, 153. [Google Scholar] [CrossRef] [Green Version]
- Lalloo, D.G.; Olukoya, P.; Olliaro, P. Malaria in adolescence: Burden of disease, consequences, and opportunities for intervention. Lancet Infect. Dis. 2006, 6, 780–793. [Google Scholar] [CrossRef]
- Krezanoski, P.J.; Bangsberg, D.R.; Tsai, A.C. Quantifying bias in measuring insecticide-treated bed net use: Meta-analysis of self-reported vs objectively measured adherence. J. Glob. Health 2018, 8, 010411. [Google Scholar] [CrossRef]
- Brabin, B.; Tinto, H.; Roberts, S.A. Testing an infection model to explain excess risk of preterm birth with long-term iron supplementation in a malaria endemic are. Mal. J. 2019, 18, 374. [Google Scholar] [CrossRef] [Green Version]
- Halliday, K.E.; Witek-McManus, S.S.; Opondo, C.; Mtali, A.; Allen, E.; Bauleni, A.; Ndau, S.; Phondiwa, E.; Ali, D.; Kachigunda, V.; et al. Impact of school-based malaria case management on school attendance, health and education outcomes: A cluster randomised trial in southern Malawi. BMJ Glob. Health 2020, 5, e001666. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S.E.; Rouhani, S.; Diarra, S.; Saye, R.; Bamadi, M.; Jones, R.; Traore, K.; Jukes, M.C.H.; Thuilliez, J.; Brooker, S.; et al. Impact of a malaria intervention package in schools on Plasmodium infection, anaemia and cognitive function in schoolchildren in Mali: A pragmatic cluster-randomised trial. BMJ Glob. Health 2017, 2, e000182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compaoré, A.; Gies, S.; Brabin, B.; Tinto, H.; Brabin, L. Community approval required for periconceptional adolescent adherence to weekly iron and/or folic acid supplementation: A qualitative study in rural Burkina Faso. Rep. Health 2018, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Ankomah, Y.; Chenoweth, M.S.; Tocker, A.M.; Doumbia, S.; Konate, D.; Doumbouya, M.; Keita, A.S.; Anderson, J.M.; Fairhurst, R.M.; Diakite, M.; et al. Host age and Plasmodium falciparum multiclonality are associated with gametocyte prevalence: A 1-year prospective cohort study. Mal. J. 2017, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, A.L.; Bousema, T.; de Vlas, S.J.; Cuzin-Ouattara, N.; Verhave, J.-P.; Drakeley, C.; Luty, A.J.F.; Sauerwein, R. The plasticity of Plasmodium falciparum gametocytaemia in relation to age in Burkina Faso. Mal. J. 2010, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- Geiser, D.L.; Conley, Z.R.; Elliott, J.L.; Mayo, J.J.; Winzerling, J.J. Characterization of Anopheles gambiae (African Malaria Mosquito) ferritin and the effect of iron on intracellular localization in mosquito cells. J. Insect. Sci. 2015, 15, 68. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Guidelines on Use of Ferritin Concentrations to Assess. Iron Status in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Stoltzfus, R.J. Iron and malaria interactions: Programmatic ways forward. Adv. Nutr. 2012, 3, 570–582. [Google Scholar] [CrossRef] [Green Version]



| Iron | Controls | ||
|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) |
| 222/611 | 36 (32.0–39.9) | 226/612 | 36.9 (33.2–40.8) |
| Clinical Parameters | All Women (15–24 Years) | Adolescents (<20 Years) |
|---|---|---|
| At Baseline | ||
| Median age, years (IQR)(N) | 16 (16–18) (1223) | 16 (16–18) (1084) |
| Menarcheal, n/N (%) | 978/1223 (80) | 842/1084 (78) |
| Median reproductive age, years (IQR)(N) | 2 (1–3) (978) | 1 (1–2) (842) |
| Became pregnant during trial, n/N(%) | 291/1223 (24) | 251/1084 (23) |
| Median BMI, kg/m2 (IQR)(N) | 19 (18–21) (1223) | 19 (18–21) (1084) |
| Median BMI Z score (IQR)(N) | −0.6 (−1.1–−0.1) (1223) | −0.6 (−1.1–−0.1) (1084) |
| Median MUAC, cm (IQR)(N) | 24 (22–25) (1223) | 23 (22–25) (1084) |
| Bed net use a n/N(%) | 392/1223 (32) | 336/1084 (31) |
| At Interim Assessment | ||
| Median age, years (IQR)(N) | 17 (16–19) (1223) | 17 (16–18) (1084) |
| Menarcheal, n/N (%) | 1073/1223 (88) | 934/1084 (86) |
| Median Reproductive age, years (IQR)(N) | 2 (1–3) (1073) | 1 (1–2) (934) |
| Bed net use a n/N (%) | 914/1198 (76) | 813/1061 (77) |
| Median weeks on supplementation (IQR)(N) | 22 (17–26) (1223) | 22 (18–26) (1084) |
| Median % adherence b (IQR)(N) | 69 (56–79) (1223) | 69 (56–79) (1084) |
| Iron Biomarkers at Baseline | ||
| Median CRP mg/L (IQR)(N) | 0.653 (0.262–1.571) (1216) | 0.663 (0.261–1.588) (1078) |
| Median GM adjusted ferritin, µg/L, (IQR)(N) c | 40.4 (22.5–64.2) (1214) | 41.2 (24.0–65.3) (1075) |
| Adjusted ferritin < 15 µg/L, n/N (%) c | 178/1214 (15) | 137/1075 (13) |
| Median GM sTfR µg/mL, (IQR)(N) | 6.46 (5.34–8.01) (1216) | 6.44 (5.34–7.94) (1078) |
| sTfR > 8.3 µg/mL, n/N (%) | 276/1216 (23) | 236/1078 (22) |
| Median sTfR/log10 ferritin c, (IQR)(N) | 4.07 (3.17–5.75) (1213) | 4.02 (3.17–5.50) (1075) |
| sTfR/log10 ferritin c > 5.6, n/N (%) | 320/1213 (26) | 265/1075 (25) |
| Median hepcidin nmol/L (IQR)(N) | 4.8 (2.0–10.4) (1213) | 5.0 (2.2–10.7) (1076) |
| Hepcidin < 0.7 nmol/L, n/N (%) d | 112/1213 (9) | 84/1076 (8) |
| Median adjusted BIS c, mg/kg (IQR)(N) | 5.2 (2.7–7.1) (1213) | 5.3 (2.8–7.1) (1075) |
| Low adjusted BIS < 0 mg/kg, n/N (%) | 118/1213 (10) | 88/1075 (8) |
| Parameter | All Women (15–24 Years) | Adolescents (<20 Years) |
|---|---|---|
| Microscopy positive, n/N (%) a | 446/1223 (36) | 414/1084 (38) |
| Fever, n/N (%) b | 132/1202 (11) | 117/1065 (11) |
| Clinical malaria, n/N (%) c | 53/1218 (4) | 47/1079 (4) |
| RDT positive, n/N (%) d | 605/1177 (51) | 551/1044 (53) |
| Parasite density, parasites/mm3, median (IQR)[N] | 227 (105–614) (446) | 231 (110–656) (414) |
| Parameter | Adjusted Odds Ratio (95% CI) | |||
|---|---|---|---|---|
| Rapid Test Positivity | p-Value | Microscopy Positive | p-Value | |
| Bed nets a | 1.50 (1.14–1.97) | 0.003 | 1.01 (0.77–1.34) | 0.930 |
| Age | 0.91 (0.85–0.97) | 0.007 | 0.88 (0.82–0.95) | 0.002 |
| Menarche | 1.44 (0.97–2.13) | 0.069 | 0.80 (0.54–1.18) | 0.260 |
| BMI Z score | 0.88 (0.75–1.04) | 0.130 | 0.98 (0.83–1.16) | 0.850 |
| Log10 (Adjusted Ferritin) | 1.15 (1.02–1.30) | 0.021 | 1.16 (1.03–1.32) | 0.016 |
| sTfR | 0.87 (0.77–0.98) | 0.024 | 0.87 (0.76–0.99) | 0.035 |
| Log10 (sTfR/Log10 ferritin ratio) | 0.85 (0.75–0.96) | 0.007 | 0.84 (0.74–0.96) | 0.007 |
| Adjusted body iron stores b | 1.16 (1.03–1.31) | 0.014 | 1.18 (1.05–1.34) | 0.007 |
| Log10 (Hepcidin) | 1.13 (1.00–1.27) | 0.043 | 1.10 (0.97–1.24) | 0.140 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brabin, L.; Roberts, S.A.; Tinto, H.; Gies, S.; Diallo, S.; Brabin, B. Iron Status of Burkinabé Adolescent Girls Predicts Malaria Risk in the Following Rainy Season. Nutrients 2020, 12, 1446. https://doi.org/10.3390/nu12051446
Brabin L, Roberts SA, Tinto H, Gies S, Diallo S, Brabin B. Iron Status of Burkinabé Adolescent Girls Predicts Malaria Risk in the Following Rainy Season. Nutrients. 2020; 12(5):1446. https://doi.org/10.3390/nu12051446
Chicago/Turabian StyleBrabin, Loretta, Stephen A. Roberts, Halidou Tinto, Sabine Gies, Salou Diallo, and Bernard Brabin. 2020. "Iron Status of Burkinabé Adolescent Girls Predicts Malaria Risk in the Following Rainy Season" Nutrients 12, no. 5: 1446. https://doi.org/10.3390/nu12051446




