Chronic Stress Contributes to Osteosarcopenic Adiposity via Inflammation and Immune Modulation: The Case for More Precise Nutritional Investigation
Abstract
:1. Introduction
2. Methods
3. Hypothalamic–Pituitary–Adrenal (HPA) Axis
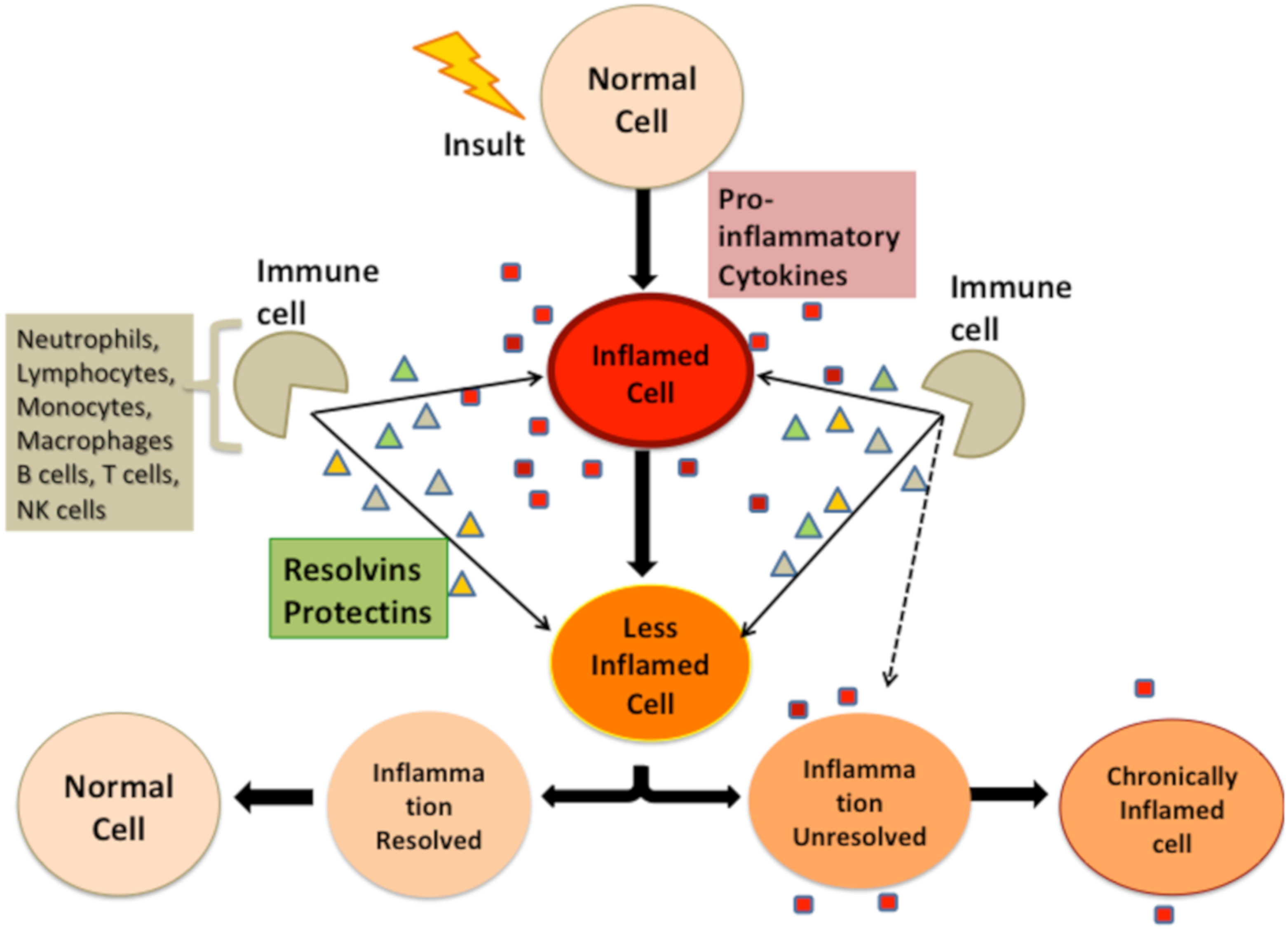
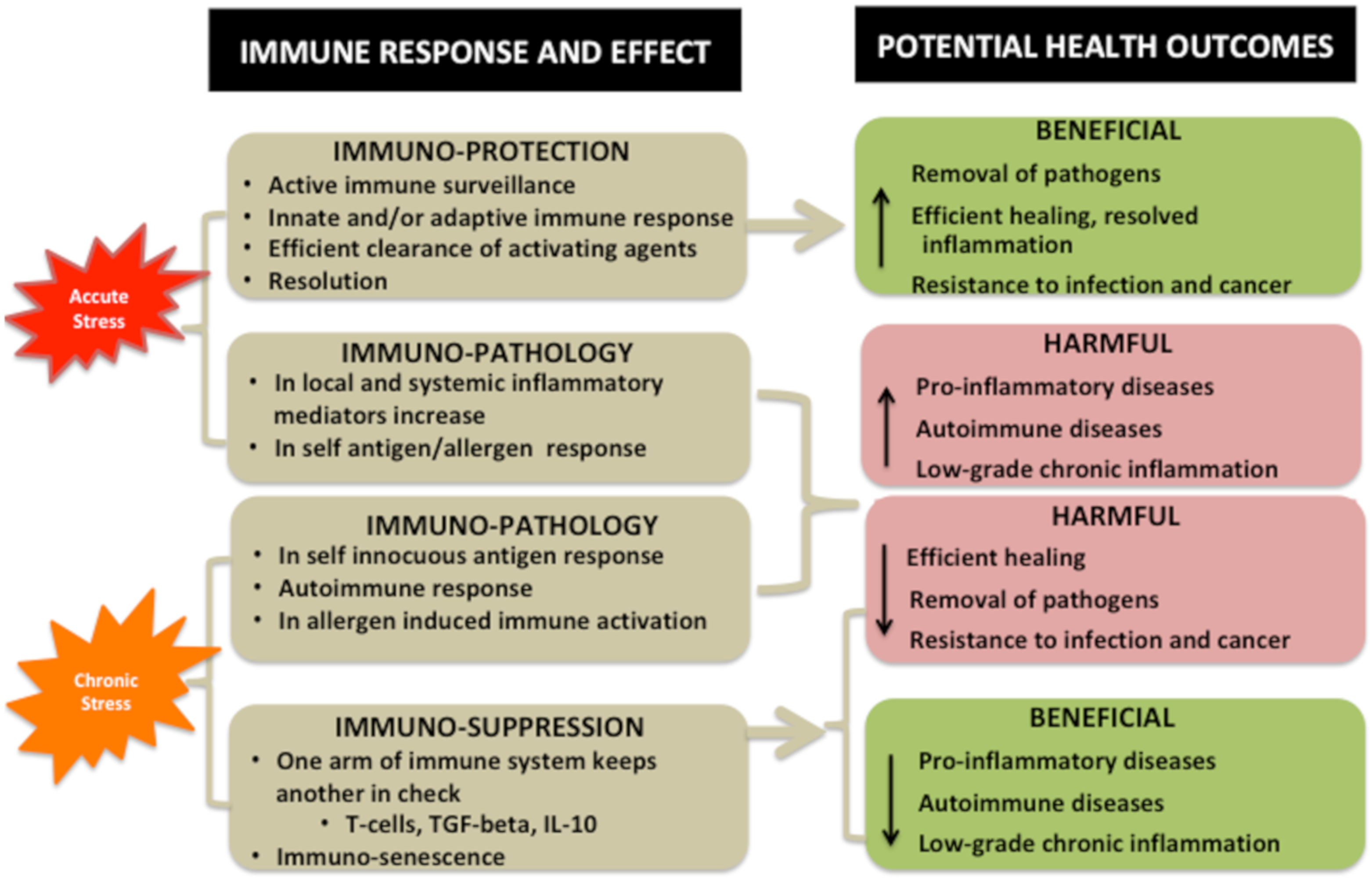
4. Bidirectional Relationship between Stressors and Products of Immune Response
Interaction of Stressors and Immune System and Potential Health Outcomes
5. Osteosarcopenic Adiposity (OSA) as a Model for Studying and Monitoring Changes in Body Composition
Key Cellular and Endocrine Interactions among Bone, Muscle, and Fat Tissues
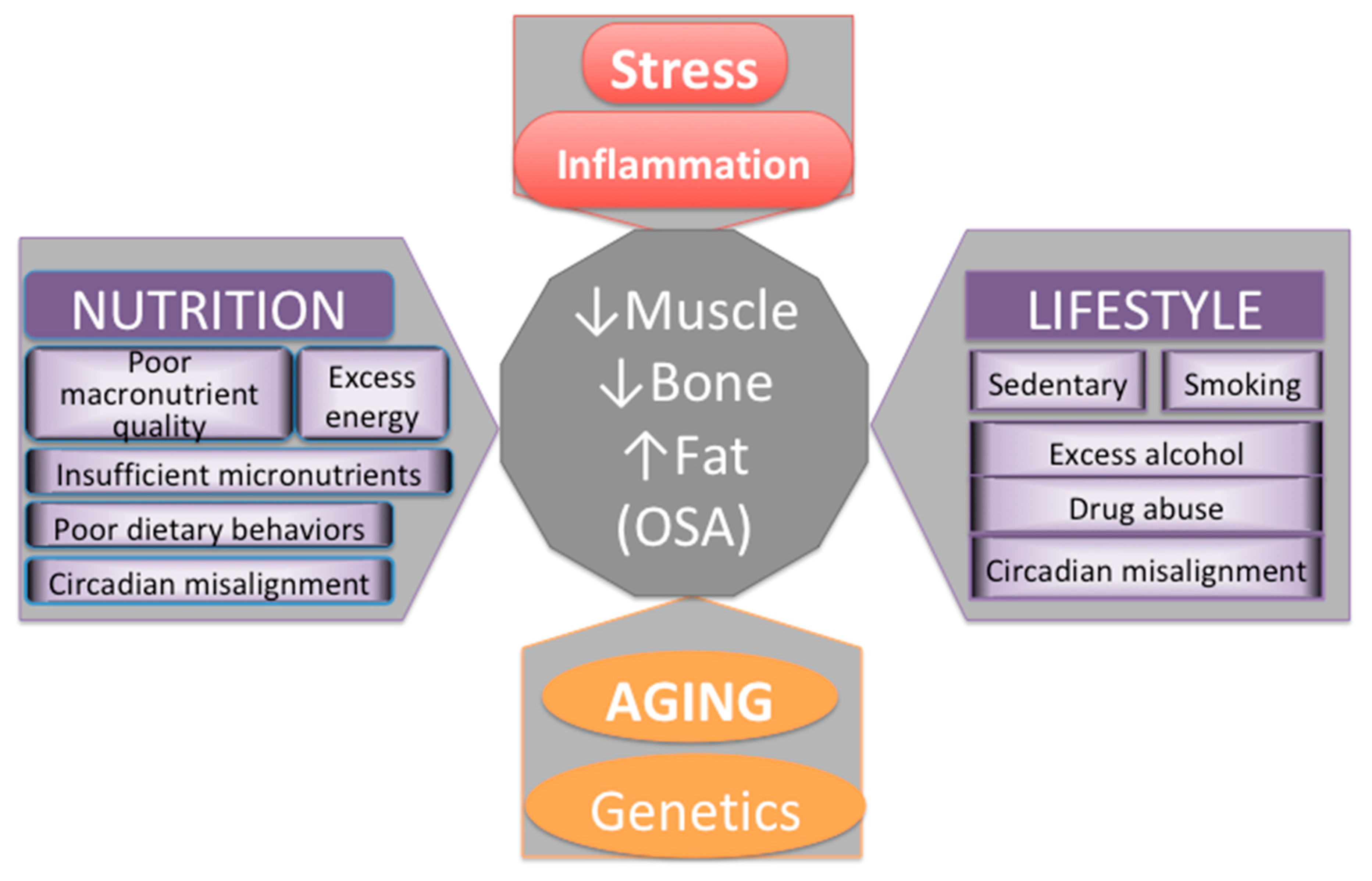
6. Evolutionary Changes in Lifestyle and Nutrition Contributing to Stress, Inflammation, and Unfavorable Changes in Body Composition
6.1. Misalignments of Circadian Rhythm
6.2. Diet and Lifestyle of Modern Humans, in Comparison to Our Prehistoric Ancestors, Adversely Affect Metabolic Health and Body Composition
6.2.1. Energy (Calorie) Intake
6.2.2. Transformation of Meat and Fat Composition and Changes in Their Intake
6.2.3. Changes in Carbohydrate Intake
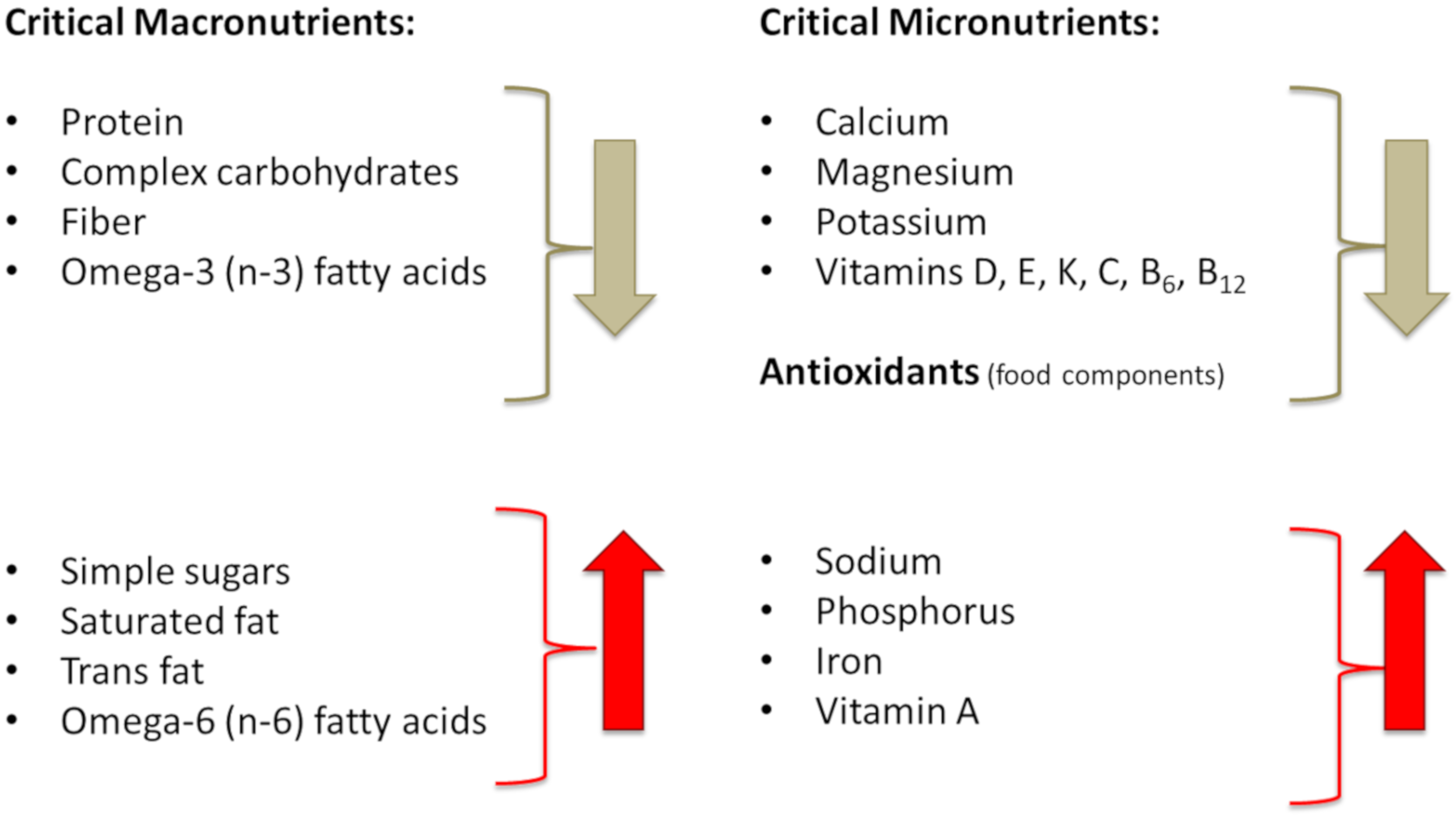
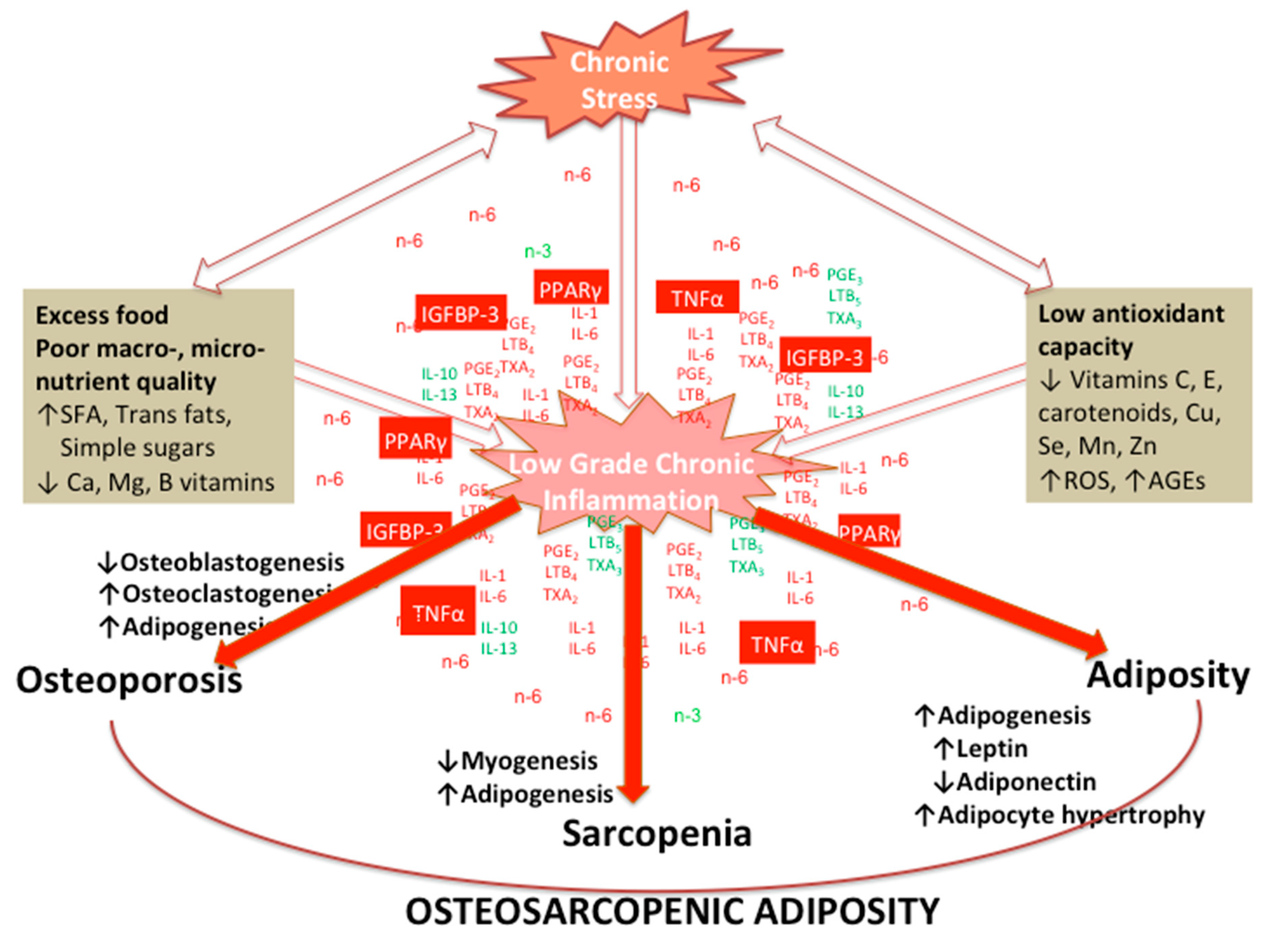
7. Effects of Chronic Stress and LGCI Magnified by Proinflammatory and/or Inadequate Diet on Osteosarcopenic Adiposity
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Straub, R.H. The origin of chronic inflammatory systemic diseases and their sequelae. In The Origin of Chronic Inflammatory Systemic Diseases and Their Sequelae; Straub, R.H., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 173–235. [Google Scholar]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [PubMed]
- McEwen, B.S. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues Clin. Neurosci. 2006, 8, 367–381. [Google Scholar] [PubMed]
- Tamashiro, K.L.; Hegeman, M.A.; Nguyen, M.M.; Melhorn, S.J.; Ma, L.Y.; Woods, S.C.; Sakai, R.R. Dynamic body weight and body composition changes in response to subordination stress. Physiol. Behav. 2007, 91, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salleh, M.R. Life event, stress and illness. Malays. J. Med. Sci. 2008, 15, 9–18. [Google Scholar] [PubMed]
- Arun, C.P. Fight or flight, forbearance and fortitude: The spectrum of actions of the catecholamines and their cousins. Ann. N. Y. Acad. Sci. 2004, 1018, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Kelly, O.J.; Kim, Y.; Spicer, M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh. Hig. Rada. Toxicol. 2014, 65, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E.; Panton, L.B.; Duque, G.; Ormsbee, M.J. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Res. Rev. 2014, 15, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr. Res. 2013, 33, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.B.; Cordain, L.; Sparling, P.B. Evolution, body composition, insulin receptor competition, and insulin resistance. Prev. Med. 2009, 49, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.B.; Eaton, S.B. Physical inactivity, obesity, and type 2 diabetes: An evolutionary perspective. Res. Q. Exerc. Sport 2017, 88, 1–8. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Freese, J.; Klement, R.; Ruiz-Núñez, B.; Schwarz, S.; Lötzerich, H. The sedentary (r)evolution: Have we lost our metabolic flexibility? [version 2; peer review: 2 approved, 1 approved with reservations]. F1000Research 2017, 6, 1787. [Google Scholar] [CrossRef]
- De Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef]
- Li, L.; Messina, J.L. Acute insulin resistance following injury. Trends Endocrinol. Metab. 2009, 20, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Eaks, G.A.; Tiszka, R. Chronic complications of diabetes: A creative management approach. Nurse Pract. Forum 1998, 9, 74–86. [Google Scholar]
- Kelly, O.J.; Gilman, J.C.; Boschiero, D.; Ilich, J.Z. Osteosarcopenic obesity: Current knowledge, revised identification criteria and treatment principles. Nutrients 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhabhar, F.S. The short-term stress response—Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, K.E.; Bishop, M.D. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [Green Version]
- Anacker, C.; Zunszain, P.A.; Carvalho, L.A.; Pariante, C.M. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 2011, 36, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.M.; Monsalves-Alvarez, M.; Henriquez, S.; Llanos, M.N.; Troncoso, R. Glucocorticoid resistance in chronic diseases. Steroids 2016, 115, 182–192. [Google Scholar] [CrossRef]
- Chrousos, G. Q&a: Primary generalized glucocorticoid resistance. BMC Med. 2011, 9, 27. [Google Scholar]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Chrousos, G.P. The hpa axis and the stress response. Endocr. Res. 2000, 26, 513–514. [Google Scholar] [CrossRef]
- Bottaccioli, A.G.; Bottaccioli, F.; Minelli, A. Stress and the psyche-brain-immune network in psychiatric diseases based on psychoneuroendocrineimmunology: A concise review. Ann. N. Y. Acad. Sci. 2019, 1437, 31–42. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Translat. 2018, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- McKim, D.B.; Niraula, A.; Tarr, A.J.; Wohleb, E.S.; Sheridan, J.F.; Godbout, J.P. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J. Neurosci. 2016, 36, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccirillo, C.A. Regulatory t cells in health and disease. Cytokine 2008, 43, 395–401. [Google Scholar] [CrossRef]
- Wing, K.; Sakaguchi, S. Regulatory t cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010, 11, 7–13. [Google Scholar] [CrossRef]
- Whiteside, T.L. Regulatory t cell subsets in human cancer: Are they regulating for or against tumor progression? Cancer Immunol. Immunother. 2014, 63, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Ilich, J.Z. Another impairment in older age: What does osteosarcopenic obesity syndrome mean for middle-aged and older women? J. Am. Med. Dir. Assoc. 2017, 18, 648–650. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E. Osteosarcopenic obesity syndrome: What is it and how can it be identified and diagnosed? Curr. Gerontol. Geriatr. Res. 2016, 2016, 7325973. [Google Scholar] [CrossRef] [Green Version]
- Ilich, J.Z.; Inglis, J.E.; Kelly, O.J.; McGee, D.L. Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporos. Int. 2015, 26, 2587–2595. [Google Scholar] [CrossRef]
- Bae, Y.-J. Association of dietary diversity with health-related quality of life and osteosarcopenic obesity in korean female adults (p18-059-19). Curr. Dev. Nutr. 2019, 3, nzz039.P018-059-019. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Kim, S.; Won, Y.J.; Kim, S.H. Clinical manifestations and factors associated with osteosarcopenic obesity syndrome: A cross-sectional study in koreans with obesity. Calcif. Tissue Int. 2019, 105, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Na, W.; Sohn, C. Relationship between osteosarcopenic obesity and dietary inflammatory index in postmenopausal korean women: 2009 to 2011 korea national health and nutrition examination surveys. J. Clin. Biochem. Nutr. 2018, 63, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Lee, O. Association of serum vitamin d with osteosarcopenic obesity: Korea national health and nutrition examination survey 2008-2010. J. Cachexia Sarcopenia Muscle 2017, 8, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Kong, C.; Yu, H.; Gong, J.; Lan, L.; Zhou, L.; Gong, J.; Liu, P.; Xu, L.; Deng, Q. Association between osteosarcopenic obesity and hypertension among four minority populations in china: A cross-sectional study. BMJ Open 2019, 9, e026818. [Google Scholar] [CrossRef] [Green Version]
- Mo, D.; Hsieh, P.; Yu, H.; Zhou, L.; Gong, J.; Xu, L.; Liu, P.; Chen, G.; Chen, Z.; Deng, Q. Osteosarcopenic obesity and its relationship with dyslipidemia in women from different ethnic groups of china. Arch. Osteoporos. 2018, 13, 65. [Google Scholar] [CrossRef]
- Hong, W.; Cheng, Q.; Zhu, X.; Zhu, H.; Li, H.; Zhang, X.; Zheng, S.; Du, Y.; Tang, W.; Xue, S.; et al. Prevalence of sarcopenia and its relationship with sites of fragility fractures in elderly chinese men and women. PLoS ONE 2015, 10, e0138102. [Google Scholar] [CrossRef] [Green Version]
- Szlejf, C.; Parra-Rodriguez, L.; Rosas-Carrasco, O. Osteosarcopenic obesity: Prevalence and relation with frailty and physical performance in middle-aged and older women. J. Am. Med. Dir. Assoc. 2017, 18, 733.e731–733.e735. [Google Scholar] [CrossRef]
- Dos Santos, V.R.; Gobbo, L.A. Physical activity is associated with functional capacity of older women with osteosarcopenic obesity: 24-month prospective study. Eur. J. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Cunha, P.M.; Ribeiro, A.S.; Tomeleri, C.M.; Schoenfeld, B.J.; Silva, A.M.; Souza, M.F.; Nascimento, M.A.; Sardinha, L.B.; Cyrino, E.S. The effects of resistance training volume on osteosarcopenic obesity in older women. J. Sports Sci. 2018, 36, 1564–1571. [Google Scholar] [CrossRef]
- Perna, S.; Spadaccini, D.; Nichetti, M.; Avanzato, I.; Faliva, M.A.; Rondanelli, M. Osteosarcopenic visceral obesity and osteosarcopenic subcutaneous obesity, two new phenotypes of sarcopenia: Prevalence, metabolic profile, and risk factors. J. Aging Res. 2018, 2018, 6147426. [Google Scholar] [CrossRef] [Green Version]
- Stefanaki, C.; Peppa, M.; Boschiero, D.; Chrousos, G.P. Healthy overweight/obese youth: Early osteosarcopenic obesity features. Eur. J. Clin. Investig. 2016, 46, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Alalwan, T.A. Phenotypes of Sarcopenic Obesity: Exploring the Effects on Peri-Muscular Fat, the Obesity Paradox, Hormone-Related Responses and the Clinical Implications. Geriatrics 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perna, S.; Peroni, G.; Milena Anna Faliva, M.; Bartolo, A.; Naso, M.; Miccono, A.; Rondanelli, M. Sarcopenia and sarcopenic obesity in comparison: Prevalence, metabolic profile, and key differences. A cross-sectional study in Italian hospitalized elderl. Aging Clin. Exp. Res. 2017, 29, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Fassio, A.; Idolazzi, L.; Rossini, M.; Gatti, D.; Adami, G.; Giollo, A.; Viapiana, O. The obesity paradox and osteoporosis. Eat. Weight Disord. 2018, 23, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Peterson, C.M.; Thomas, D.M.; Heo, M.; Schuna, J.M., Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes. Rev. 2016, 17, 262–275. [Google Scholar] [CrossRef] [Green Version]
- Pantalone, K.M.; Hobbs, T.M.; Wells, B.J.; Kong, S.X.; Kattan, M.W.; Bouchard, J.; Yu, C.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabet. Res. Care 2015, 3, e000093. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, K.; Nakashima, T.; Takeda, S.; Isogai, M.; Hamada, M.; Kimura, A.; Kodama, T.; Yamaguchi, A.; Owen, M.J.; Takahashi, S.; et al. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J. Clin. Investig. 2010, 120, 3455–3465. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.K.; Pande, S.; Pratap, J.; Gaur, T.; Grigoriu, S.; Ali, S.A.; Stein, J.L.; Lian, J.B.; van Wijnen, A.J.; Stein, G.S. Runx2 deficiency and defective subnuclear targeting bypass senescence to promote immortalization and tumorigenic potential. Proc. Natl. Acad. Sci. USA 2007, 104, 19861–19866. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.E.; Jin, B.; Li, Y.P. Tnf-alpha regulates myogenesis and muscle regeneration by activating p38 mapk. Am. J. Physiol. Cell Physiol. 2007, 292, C1660–C1671. [Google Scholar] [CrossRef]
- Langen, R.C.; Van Der Velden, J.L.; Schols, A.M.; Kelders, M.C.; Wouters, E.F.; Janssen-Heininger, Y.M. Tumor necrosis factor-alpha inhibits myogenic differentiation through myod protein destabilization. FASEB J. 2004, 18, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ackert-Bicknell, C.; Rosen, C. The genetics of pparg and the skeleton. PPAR Res. 2006, 2006, 93258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecka-Czernik, B.; Suva, L.J. Resolving the two “bony” faces of ppar-gamma. PPAR Res. 2006, 2006, 27489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, J.; Yamane, T.; Oishi, Y.; Kobayashi-Hattori, K. Perfluorooctanoic acid binds to peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation in 3t3-l1 adipocytes. Biosci. Biotechnol. Biochem. 2015, 79, 636–639. [Google Scholar] [CrossRef]
- Clarke, D.C.; Liu, X. Decoding the quantitative nature of tgf-beta/smad signaling. Trends Cell Biol. 2008, 18, 430–442. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, M.S.; Gibson, J.M.; Heald, A.H.; Dunger, D.B.; Wareham, N.J. Association between insulin-like growth factor-i: Insulin-like growth factor-binding protein-1 ratio and metabolic and anthropometric factors in men and women. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Cawthorn, W.P.; Sethi, J.K. Tnf-alpha and adipocyte biology. FEBS Lett. 2008, 582, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Karsenty, G.; Ferron, M. The contribution of bone to whole-organism physiology. Nature 2012, 481, 314–320. [Google Scholar] [CrossRef]
- Liu, J.M.; Zhao, H.Y.; Zhao, L.; Chen, Y.; Zhang, L.Z.; Tao, B.; Sun, L.H.; Zhao, Y.J.; Wang, W.Q.; Xu, M.Y.; et al. An independent positive relationship between the serum total osteocalcin level and fat-free mass in healthy premenopausal women. J. Clin. Endocrinol. Metab. 2013, 98, 2146–2152. [Google Scholar] [CrossRef] [Green Version]
- Buday, B.; Pach, F.P.; Literati-Nagy, B.; Vitai, M.; Vecsei, Z.; Koranyi, L. Serum osteocalcin is associated with improved metabolic state via adiponectin in females versus testosterone in males. Gender specific nature of the bone-energy homeostasis axis. Bone 2013, 57, 98–104. [Google Scholar] [CrossRef]
- Raschke, S.; Eckel, J. Adipo-myokines: Two sides of the same coin—Mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardi, M.; Munoz-Canoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washington, T.A.; White, J.P.; Davis, J.M.; Wilson, L.B.; Lowe, L.L.; Sato, S.; Carson, J.A. Skeletal muscle mass recovery from atrophy in il-6 knockout mice. Acta Physiol. 2011, 202, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Reid, I.R. Relationships between fat and bone. Osteoporos. Int. 2008, 19, 595–606. [Google Scholar] [CrossRef]
- Lecke, S.B.; Morsch, D.M.; Spritzer, P.M. Leptin and adiponectin in the female life course. Braz. J. Med. Biol. Res. 2011, 44, 381–387. [Google Scholar] [CrossRef]
- Bae, S.A.; Fang, M.Z.; Rustgi, V.; Zarbl, H.; Androulakis, I.P. At the interface of lifestyle, behavior, and circadian rhythms: Metabolic implications. Front. Nutr. 2019, 6, 132. [Google Scholar] [CrossRef]
- Cao, R. Mtor signaling, translational control, and the circadian clock. Front. Genet. 2018, 9, 367. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Rifas-Shiman, S.L.; Quante, M.; Redline, S.; Oken, E.; Taveras, E.M. Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatr. 2019. [Google Scholar] [CrossRef]
- Cordain, L.; Miller, J.B.; Eaton, S.B.; Mann, N.; Holt, S.H.; Speth, J.D. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am. J. Clin. Nutr. 2000, 71, 682–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the anthropocene: The eat—Lancet commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 2019, 178, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Hung, J.H.; Su, I.J.; Lei, H.Y.; Wang, H.C.; Lin, W.C.; Chang, W.T.; Huang, W.; Chang, W.C.; Chang, Y.S.; Chen, C.C.; et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of nf-kappab and pp38 mitogen-activated protein kinase. J. Biol. Chem. 2004, 279, 46384–46392. [Google Scholar] [CrossRef] [Green Version]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, C.; Lu, M.; Dong, Q.; Wang, Z.; Wang, Z.; Xiong, W.; Zhang, N.; Zhou, J.; Liu, Q.; et al. Calorie restriction is the most reasonable anti-ageing intervention: A meta-analysis of survival curves. Sci. Rep. 2018, 8, 5779. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Eaton, S.B.; Konner, M. Paleolithic nutrition. A consideration of its nature and current implications. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef]
- Wihitaker, J.W. Feedlot empire: Beef cattle feeding in illinois and iowa, 1840–1900. Ann. Iowa 1976, 43, 233–234. [Google Scholar]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in dietary carbohydrate, protein, and fat intake and diet quality among us adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the lyon diet heart study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kelly, O.J.; Ilich, J.Z. Synergism of alpha-linolenic acid, conjugated linoleic acid and calcium in decreasing adipocyte and increasing osteoblast cell growth. Lipids 2013, 48, 787–802. [Google Scholar] [CrossRef]
- Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Colditz, G.A.; Speizer, F.E.; Rosner, B.A.; Hennekens, C.H.; Hennekens, C.H.; Willett, W.C.; Stampfer, M.J.; et al. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet 1993, 341, 581–585. [Google Scholar] [CrossRef]
- Sloop, G.D.; Weidman, J.J.; St Cyr, J.A. Perspective: Interesterified triglycerides, the recent increase in deaths from heart disease, and elevated blood viscosity. Ther. Adv. Cardiovasc. Dis. 2018, 12, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferre, M.; Hu, F.B. Are fruit juices just as unhealthy as sugar-sweetened beverages? JAMA Netw. Open 2019, 2, e193109. [Google Scholar] [CrossRef]
- Newens, K.J.; Walton, J. A review of sugar consumption from nationally representative dietary surveys across the world. J. Hum. Nutr. Diet. 2016, 29, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the united states. J. Nutr. 2009, 139, 1228S–1235S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, J.A.; Sharma, A.J.; Grellinger, L.; Vos, M.B. Consumption of added sugars is decreasing in the united states. Am. J. Clin. Nutr. 2011, 94, 726–734. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications-detail/9789241549028 (accessed on 25 February 2020).
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of dietary sugar intake on biomarkers of subclinical inflammation: A systematic review and meta-analysis of intervention studies. Nutrients 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarantino, G.; Citro, V.; Finelli, C. Hype or reality: Should patients with metabolic syndrome-related nafld be on the hunter-gatherer (paleo) diet to decrease morbidity? J. Gastrointestin. Liver Dis. 2015, 24, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, M.B.; Lavine, J.E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology 2013, 57, 2525–2531. [Google Scholar] [CrossRef]
- Gersch, M.S.; Mu, W.; Cirillo, P.; Reungjui, S.; Zhang, L.; Roncal, C.; Sautin, Y.Y.; Johnson, R.J.; Nakagawa, T. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am. J. Physiol. Renal Physiol. 2007, 293, F1256–F1261. [Google Scholar] [CrossRef] [Green Version]
- Angelopoulos, T.J.; Lowndes, J.; Sinnett, S.; Rippe, J.M. Fructose containing sugars at normal levels of consumption do not effect adversely components of the metabolic syndrome and risk factors for cardiovascular disease. Nutrients 2016, 8, 179. [Google Scholar] [CrossRef] [Green Version]
- Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Prevention of metabolic diseases: Fruits (including fruit sugars) vs. Vegetables. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 286–293. [Google Scholar] [CrossRef]
- Rippe, J.M.; Angelopoulos, T.J. Relationship between added sugars consumption and chronic disease risk factors: Current understanding. Nutrients 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Macronutrient intake and distribution in the etiology, prevention and treatment of osteosarcopenic obesity. Curr. Aging Sci. 2017, 10, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, H.M.; Ilich, J.Z.; Kim, J.-S.; Levenson, C.W.; Arjmandi, B.H.; Spicer, M.T. Dietary advanced glycation end-products exacerbate oxidative stress in patients with diabetic foot ulcers. J. Diabet. Res. Clin. Metab. 2014. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Gao, F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J. Diabet. 2014, 5, 89–96. [Google Scholar] [CrossRef]
- Du, J.; Zhu, M.; Bao, H.; Li, B.; Dong, Y.; Xiao, C.; Zhang, G.Y.; Henter, I.; Rudorfer, M.; Vitiello, B. The role of nutrients in protecting mitochondrial function and neurotransmitter signaling: Implications for the treatment of depression, ptsd, and suicidal behaviors. Crit. Rev. Food Sci. Nutr. 2016, 56, 2560–2578. [Google Scholar] [CrossRef] [Green Version]
- Czysz, A.H.; Rasenick, M.M. G-protein signaling, lipid rafts and the possible sites of action for the antidepressant effects of n-3 polyunsaturated fatty acids. CNS Neurol. Disord. Drug Targets 2013, 12, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Bourre, J.M. The role of nutritional factors on the structure and function of the brain: An update on dietary requirements. Revue Neurol. 2004, 160, 767–792. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Apostolopoulos, V. The effects of vitamin B in depression. Curr. Med. Chem. 2016, 23, 4317–4337. [Google Scholar] [CrossRef] [Green Version]
- Perna, S.; Alalwan, T.A.; Alaali, Z.; Alnashaba, T.; Gasparri, C.; Infantino, V.; Hammad, L.; Riva, A.; Petrangolini, G.; Allegrini, P.; et al. The Role of Glutamine in the Complex Interaction between Gut Microbiota and Health—A Narrative Review. Int. J. Mol. Sci. 2019, 20, 5232. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.Z.; Zambom, A.Z.; Abboud, K.Y.; Reis, S.K.; Tannihão, F.; Guadagnini, D.; Saad, M.J.; Prada, P.O. Oral supplementation with l-glutamine alters gut microbiota of obese and overweight adults: A pilot study. Nutrition 2015, 31, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yang, Y.; Chen, Q.; Xie, L. Glutamine Metabolism is essential for stemness of bone marrow mesenchymal stem cells and bone homeostasis. Stem Cells Int. 2019, 2019, 8928934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, T.G.; Gray, J.D.; McEwen, B.S. Experience and the ever-changing brain: What the transcriptome can reveal. Bioessays 2014, 36, 1072–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilich, J.Z.; Gilman, J.C.; Cvijetic, S.; Boschiero, D. Chronic Stress Contributes to Osteosarcopenic Adiposity via Inflammation and Immune Modulation: The Case for More Precise Nutritional Investigation. Nutrients 2020, 12, 989. https://doi.org/10.3390/nu12040989
Ilich JZ, Gilman JC, Cvijetic S, Boschiero D. Chronic Stress Contributes to Osteosarcopenic Adiposity via Inflammation and Immune Modulation: The Case for More Precise Nutritional Investigation. Nutrients. 2020; 12(4):989. https://doi.org/10.3390/nu12040989
Chicago/Turabian StyleIlich, Jasminka Z., Jennifer C. Gilman, Selma Cvijetic, and Dario Boschiero. 2020. "Chronic Stress Contributes to Osteosarcopenic Adiposity via Inflammation and Immune Modulation: The Case for More Precise Nutritional Investigation" Nutrients 12, no. 4: 989. https://doi.org/10.3390/nu12040989
APA StyleIlich, J. Z., Gilman, J. C., Cvijetic, S., & Boschiero, D. (2020). Chronic Stress Contributes to Osteosarcopenic Adiposity via Inflammation and Immune Modulation: The Case for More Precise Nutritional Investigation. Nutrients, 12(4), 989. https://doi.org/10.3390/nu12040989





