Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life
Abstract
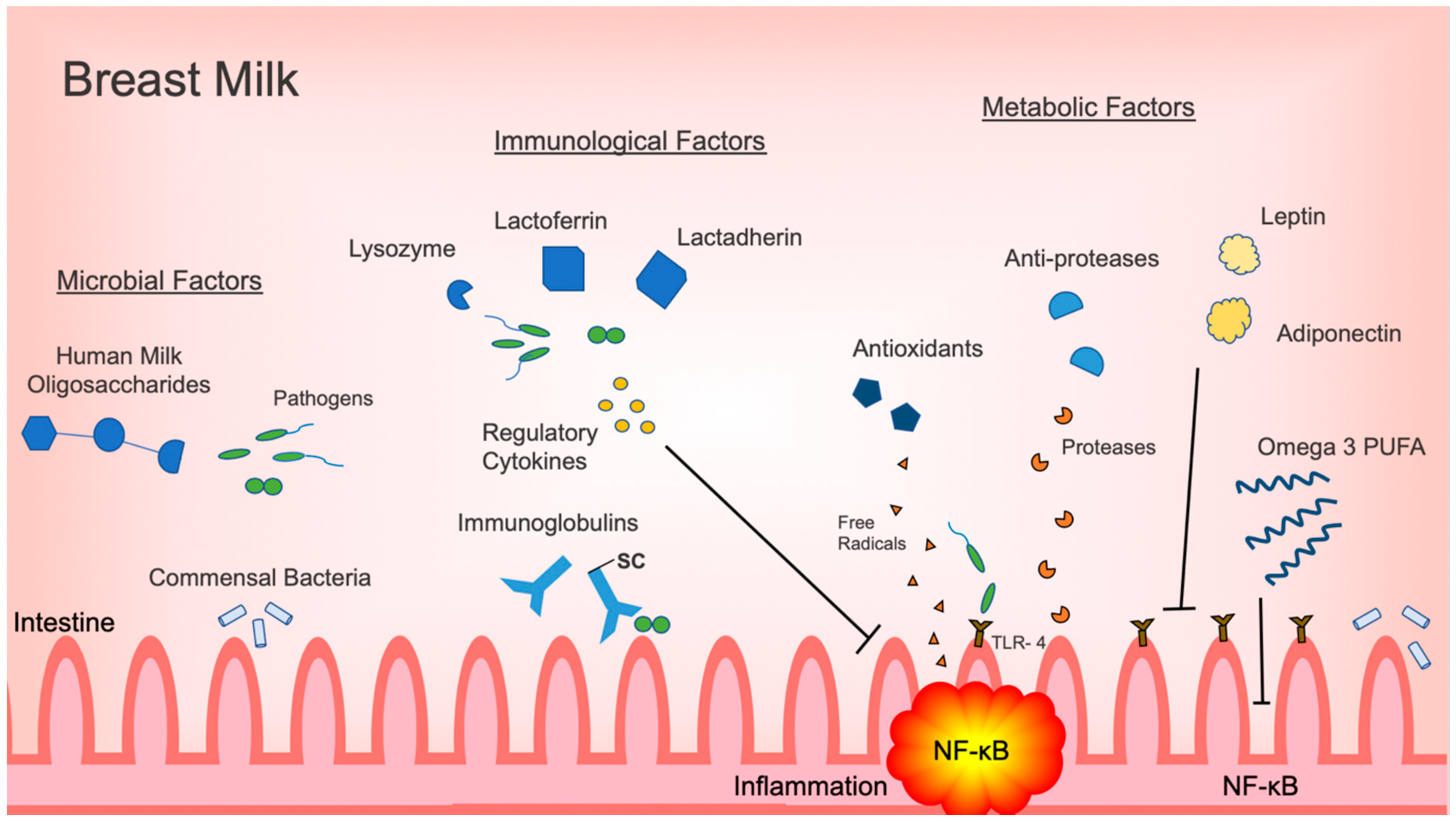
:1. Introduction
2. Methods
Literature Search
3. Microbiome and Microbial Factors
3.1. Microbiome and Probiotics
3.2. Human Milk Oligosaccharides and Glycans
4. Immunological Factors: Immunoglobulins and Immunological Proteins
4.1. Immunoglobulins
4.2. Cytokines and Growth Factors
4.3. Lactoferrin, Lactadherin, and Lysozyme
5. Metabolic Factors
5.1. Adipokines
5.2. Antioxidants and Anti-Proteases
5.3. Dietary Fatty Acids
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eldelman, A.I.; Schandler, R.J. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.A. Human milk for the premature infant. Pediatr. Clin. North Am. 2013, 60, 189–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schanler, R.J.; Shulman, R.J.; Lau, C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999, 103, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Cortez, J.; Makker, K.; Kraemer, D.F.; Neu, J.; Sharma, R.; Hudak, M.L. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J. Perinatol. 2018, 38, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Sisk, P.M.; A Lovelady, C.; Dillard, R.G.; Gruber, K.; O’Shea, T.M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. 2007, 27, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Meinzen-Derr, J.; Poindexter, B.; Wrage, L.; Morrow, A.L.; Stoll, B.; Donovan, E.F. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J. Perinatol. 2009, 29, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast Milk Microbiota Is Shaped by Mode of Delivery and Intrapartum Antibiotic Exposure. Front. Nutr. 2019, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature. 2009, 457, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; LeVan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef] [PubMed]
- Quin, C.; Gibson, D. Dietary Fatty Acids and Host–Microbial Crosstalk in Neonatal Enteric Infection. Nutr. 2019, 11, 2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Kuban, K.C.; O’Shea, T.M.; Allred, E.N.; Fichorova, R.N.; Heeren, T.; Paneth, N.; Hirtz, D.; Dammann, O.; Leviton, A.; ELGAN Study Investigators. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr. Neurol. 2015, 52, 42–48. [Google Scholar] [CrossRef] [Green Version]
- McElroy, S.J.; Frey, M.R.; Torres, B.A.; Maheshwari, A. Innate and Mucosal Immunity in the Developing Gastrointestinal Tract. In Avery’s Disease of the Newborn, 10th ed.; Elsevier: Philadelphia, PA, USA, 2017; pp. 1054–1067. [Google Scholar]
- McElroy, S.J.; Weitkamp, J.-H. Innate Immunity in the Small Intestine of the Preterm Infant. NeoReviews 2011, 12, e517–e526. [Google Scholar] [CrossRef] [Green Version]
- Palmeira, P.; Carneiro-Sampaio, M. Immunology of breast milk. Revista da Associação Médica Brasileira 2016, 62, 584–593. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing Enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Hackam, D.J.; Good, M.; Sodhi, C.P.; Sodhi, C.P. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin. Pediatr. Surg. 2013, 22, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Maheshwari, A.; Kelly, D.R.; Nicola, T.; Ambalavanan, N.; Jain, S.K.; Murphy–Ullrich, J.; Athar, M.; Shimamura, M.; Bhandari, V.; Aprahamian, C.; et al. TGF-β2 Suppresses Macrophage Cytokine Production and Mucosal Inflammatory Responses in the Developing Intestine. Gastroenterology 2011, 140, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.M.; Kandefer, S.; Walsh, M.C.; Bell, E.F.; Carlo, W.A.; Laptook, A.R.; Sanchez, P.J.; Shankaran, S.; Van Meurs, K.P.; Ball, M.B.; et al. Causes and timing of death in extremely premature infants from 2000 through 2011. New Engl. J. Med. 2015, 372, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, A.; Cole, T. Breast milk and neonatal necrotising enterocolitis. Lancet 1990, 336, 1519–1523. [Google Scholar] [CrossRef]
- Furman, L.; Taylor, G.; Minich, N.; Hack, M. The effect of maternal milk on neonatal morbidity of very low-birth-weight infants. Arch. Pediatr. Adolesc. Med. 2003, 157, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.A. Probiotics and the prevention of necrotizing enterocolitis. J. Pediatr. Surg. 2019, 54, 405–412. [Google Scholar] [CrossRef]
- Dunne-Castagna, V.P.; Taft, D.H. Mother’s Touch: Milk IgA and Protection from Necrotizing Enterocolitis. Cell Host Microbe 2019, 26, 147–148. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides in the Prevention of Necrotizing Enterocolitis: A Journey From in vitro and in vivo Models to Mother-Infant Cohort Studies. Front. Pediatr. 2018, 6, 385. [Google Scholar] [CrossRef]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.P.; Ma, C.; Good, M.; et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; MacPherson, A.J. Interactions Between the Microbiota and the Immune System. Sci. 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Boil. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [Green Version]
- Newburg, D.; He, Y. Neonatal Gut Microbiota and Human Milk Glycans Cooperate to Attenuate Infection and Inflammation. Clin. Obstet. Gynecol. 2015, 58, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Mshvildadze, M.; Neu, J.; Shuster, J.; Theriaque, U.; Li, N.; Mai, V. Intestinal Microbial Ecology in Premature Infants Assessed with Non–Culture-Based Techniques. J. Pediatr. 2010, 156, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.E.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.; McGuire, M.A. Characterization of the Diversity and Temporal Stability of Bacterial Communities in Human Milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, L.; Langa, S.; Martin, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic review of the human milk microbiota. Nutr. Clin. Pract. 2017, 32, 354–364. [Google Scholar] [CrossRef]
- Schwab, C.; Voney, E.; Garcia, A.R.; Vischer, M.; Lacroix, C. Characterization of the Cultivable Microbiota in Fresh and Stored Mature Human Breast Milk. Front. Microbiol. 2019, 10, 2666. [Google Scholar] [CrossRef] [Green Version]
- Urbaniak, C.; Angelini, M.; Gloor, G.B.; Reid, G. Human milk microbiota profiles in relation to birthing method, gestation and infant gender. Microbiome 2016, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Gallego, C.G.; Garcia-Mantrana, I.; Salminen, S.; Collado, M.C. The human milk microbiome and factors influencing its composition and activity. Semin. Fetal Neonatal Med. 2016, 21, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A.; Miras, A.; Jackson, R.N.; Goldstone, A.P.; Olbers, T.; et al. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Sci. 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nat. 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.; Berrington, J.E.; Dorling, J.; Ewer, A.K.; Juszczak, E.; Kirby, J.A.; Lamb, C.A.; Lanyon, C.; McGuire, W.; Probert, C.S.; et al. Mechanisms Affecting the gut of preterm infants in enteral feeding trials. Front. Nutr. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, M.A.; Arriola, J.; Gerber, C.W.; Kaveti, A.; Kalanetra, K.M.; Kananurak, A.; Bevins, C.L.; Mills, D.A.; Dvořák, B. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr. Res. 2014, 76, 326–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Pang, B.; Liu, G.; Zhao, X.; Xu, X.; Jiang, C.; Yang, B.; Liu, Y.; Li, N. Lactobacillus rhamnosus from human breast milk shows therapeutic function against foodborne infection by multi-drug resistant Escherichia coli in mice. Food Funct. 2020, 11, 435–447. [Google Scholar] [CrossRef]
- Guo, S.; Guo, Y.; Ergun, A.; Lü, L.; Walker, W.A.; Ganguli, K. Secreted Metabolites of Bifidobacterium infantis and Lactobacillus acidophilus Protect Immature Human Enterocytes from IL-1β-Induced Inflammation: A Transcription Profiling Analysis. PLoS ONE 2015, 10, e0124549. [Google Scholar] [CrossRef]
- Pasparakis, M. Role of NF-κB in epithelial biology. Immunol. Rev. 2012, 246, 346–358. [Google Scholar] [CrossRef]
- Ganguli, K.; Meng, D.; Rautava, S.; Lu, L.; Walker, W.A.; Nanthakumar, N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G132–G141. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutr. 2018, 10, 1038. [Google Scholar] [CrossRef] [Green Version]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. HUMAN MILK GLYCANS PROTECT INFANTS AGAINST ENTERIC PATHOGENS. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Jiang, X.; Newburg, D.S. Human-Milk Glycans That Inhibit Pathogen Binding Protect Breast-feeding Infants against Infectious Diarrhea. J. Nutr. 2005, 135, 1304–1307. [Google Scholar] [CrossRef] [Green Version]
- Ballard, O.; Morrow, A.L. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef] [PubMed]
- Rogier, E.; Frantz, A.L.; Bruno, M.E.C.; Kaetzel, C. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens 2014, 3, 390–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianga, P.; Medesan, C.; Richardson, J.A.; Ghetie, V.; Ward, E.S. Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur. J. Immunol. 1999, 29, 2515–2523. [Google Scholar] [CrossRef]
- Gross, S.J.; Buckley, R.H.; Wakil, S.S.; McAllister, D.C.; David, R.J.; Faix, R.G. Elevated IgA concentration in milk produced by mothers delivered of preterm infants. J. Pediatr. 1981, 99, 389–393. [Google Scholar] [CrossRef]
- Mathur, N.B.; Dwarkadas, A.M.; Sharma, V.K.; Saha, K.; Jain, N. Anti-Infective Factors in Preterm Human Colostrum. Acta Paediatr. 1990, 79, 1039–1044. [Google Scholar] [CrossRef]
- Garofalo, R.; Chheda, S.; Mei, F.; Palkowetz, K.H.; E Rudloff, H.; Schmalstieg, F.C.; Rassin, D.K.; Goldman, A.S. Interleukin-10 in Human Milk. Pediatr. Res. 1995, 37, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Sydora, B.C.; Tavernini, M.M.; Wessler, A.; Jewell, L.D.; Fedorak, R. Lack of interleukin-10 leads to intestinal inflammation, independent of the time at which luminal microbial colonization occurs. Inflamm. Bowel Dis. 2003, 9, 87–97. [Google Scholar] [CrossRef]
- Lepage, P.; Van De Perre, P. The Immune System of Breast Milk: Antimicrobial and Anti-inflammatory Properties. Adv. Exp. Med. Biol. 2012, 743, 121–137. [Google Scholar]
- Emami, C.N.; Chokshi, N.; Wang, J.; Hunter, C.J.; Guner, Y.; Goth, K.; Wang, L.; Grishin, A.; Ford, H.R. Role of interleukin-10 in the pathogenesis of necrotizing enterocolitis. Am. J. Surg. 2012, 203, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Fell, J.M.; Paintin, M.; Arnaud-Battandier, F.; Beattie, R.M.; Hollis, A.; Kitching, P.; Donnet-Hughes, A.; MacDonald, T.T.; Walker-Smith, J.A. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Ailm. Pharmacol. Ther. 2000, 14, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Dawod, B.; Marshall, J. Cytokines and soluble receptors in breast milk as enhancers of oral tolerance development. Front. Immunol. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwari, A.; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network; Schelonka, R.L.; Dimmitt, R.A.; Carlo, W.A.; Munoz-Hernandez, B.; Das, A.; McDonald, S.A.; Thorsen, P.; Skogstrand, K.; et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr. Res. 2014, 76, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, À. Premature Delivery Influences the Immunological Composition of Colostrum and Transitional and Mature Human Milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef]
- Liew, F.Y.; Xu, D.; Brint, E.; O’Neill, L.A. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef]
- Henrick, B.M.; Nag, K.; Yao, X.-D.; Drannik, A.G.; Aldrovandi, G.M.; Rosenthal, K.L. Milk Matters: Soluble Toll-Like Receptor 2 (sTLR2) in Breast Milk Significantly Inhibits HIV-1 Infection and Inflammation. PLoS ONE 2012, 7, e40138. [Google Scholar] [CrossRef]
- Coursodon, C.F.; Dvořák, B. Epidermal growth factor and necrotizing enterocolitis. Curr. Opin. Pediatr. 2012, 24, 160–164. [Google Scholar] [CrossRef]
- Halpern, M.D.; Holubec, H.; Dominguez, J.A.; Williams, C.S.; Meza, Y.G.; McWilliam, D.L.; Payne, C.M.; McCuskey, R.S.; Besselsen, D.G.; Dvorak, B. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr. Res. 2002, 51, 733–739. [Google Scholar] [CrossRef]
- Dvorak, B. Milk Epidermal Growth Factor and Gut Protection. J. Pediatr. 2010, 156, S31–S35. [Google Scholar] [CrossRef] [Green Version]
- Chatterton, D.E.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell. Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef]
- Rocourt, R.V.; Mehta, V.B.; Besner, G.E. Heparin-Binding EGF-like Growth Factor Decreases Inflammatory Cytokine Expression After Intestinal Ischemia/Reperfusion Injury. J. Surg. Res. 2007, 139, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozgurtas, T.; Aydin, I.; Turan, O.; Koç, E.; Hirfanoglu, I.M.; Acikel, C.H.; Akyol, M.; Erbil, M.K. Vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor-I and platelet-derived growth factor levels in human milk of mothers with term and preterm neonates. Cytokine 2010, 50, 192–194. [Google Scholar] [CrossRef]
- Karatepe, H.Ö.; Kilincaslan, H.; Berber, M.; Ozen, A.; Saricoban, H.E.; Ustek, D.; Kemik, A.S.; Adas, M.; Bakar, F. The effect of vascular endothelial growth factor overexpression in experimental necrotizing enterocolitis. Pediatr. Surg. Int. 2014, 30, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Buescher, E.S. Anti-inflammatory characteristics of human milk: how, where, why. Single Mol. Single Cell Seq. 2001, 501, 207–222. [Google Scholar]
- Albenzio, M.; Santillo, A.; Stolfi, I.; Manzoni, P.; Iliceto, A.; Rinaldi, M.; Magaldi, R. Lactoferrin Levels in Human Milk after Preterm and Term Delivery. Am. J. Perinatol. 2016, 33, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Jenkins, P.; Vargova, M.; Bowler, U.; Juszczak, E.; King, A.; Linsell, L.; Murray, D.; Partlett, C.; Patel, M.; et al. Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet 2019, 393, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Bu, H.-F.; Zuo, X.-L.; Wang, X.; Ensslin, M.A.; Koti, V.; Hsueh, W.; Raymond, A.S.; Shur, B.D.; Tan, X.-D. Milk fat globule–EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Investig. 2007, 117, 3673–3683. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Lawlor, N.; Newburg, D. Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. Adv. Nutr. 2016, 7, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Lee-Huang, S.; Huang, P.L.; Sun, Y.; Huang, P.L.; Kung, H.F.; Blithe, D.L.; Chen, H.C. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proc. Natl. Acad. Sci. USA 1999, 96, 2678–2681. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, C.; Ziegler, J.F.; Letizia, M.; Schmidt, F.; Siegmund, B. Adipokines and their role in intestinal inflammation. Front. Immunol. 2018, 9, 1974. [Google Scholar] [CrossRef] [Green Version]
- Zulian, A.; Cancello, R.; Micheletto, G.; Gentilini, D.; Gilardini, L.; Danelli, P.; Invitti, C. Visceral adipocytes: old actors in obesity and new protagonists in Crohn’s disease? Gut 2012, 61, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kredel, L.I.; Batra, A.; Stroh, T.; Kühl, A.A.; Zeitz, M.; Erben, U.; Siegmund, B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn’s disease. Gut. 2013, 62, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, B.; Lehr, H.A.; Fantuzzi, G. Leptin: A pivotal mediator of intestinal inflammation in mice. Gastroenterol. 2002, 122, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition: Current Knowledge and Future Opportunities. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valledor, A.F.; Ricote, M. Nuclear receptor signaling in macrophages. Biochem. Pharmacol. 2004, 67, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Gottrand, F. Long-Chain Polyunsaturated Fatty Acids Influence the Immune System of Infants. J. Nutr. 2008, 138, 1807S–1812S. [Google Scholar] [CrossRef]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D. Fish Oil Attenuates Omega-6 Polyunsaturated Fatty Acid-Induced Dysbiosis and Infectious Colitis but Impairs LPS Dephosphorylation Activity Causing Sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef]
- Hughes, D.; Pinder, A.C.; Piper, Z.; Johnson, I.; Lund, E.K. Fish oil supplementation inhibits the expression of major histocompatibility complex class II molecules and adhesion molecules on human monocytes. Am. J. Clin. Nutr. 1996, 63, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.; Pedersen, J.; Ekelund, S.; Grunnet, N.; Jersild, C.; Dyerberg, J. Cod liver oil inhibits neutrophil and monocyte chemotaxis in healthy males. Atherosclerosis 1989, 77, 53–57. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [Green Version]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.C.; Wedlund, L.; Cohen, N.A.; Stromberg, A.J.; Kaetzel, C.S. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3074–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, E.D.; Gonçalves, A.K.; Cornetta, M.D.C.; Cunha, H.; Cardoso, M.L.; Morais, S.S.; Giraldo, P.C. Evaluation of the secretory immunoglobulin A levels in the colostrum and milk of mothers of term and pre-term newborns. Braz. J. Infect. Dis. 2005, 9, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballabio, C.; Bertino, E.; Coscia, A.; Fabris, C.; Fuggetta, D.; Molfino, S.; Testa, T.; Sgarrella, M.; Sabatino, G.; Restani, P. Immunoglobulin-A Profile in Breast Milk from Mothers Delivering Full Term and Preterm Infants. Int. J. Immunopathol. Pharmacol. 2007, 20, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.S.; Goldblum, R.M.; Hanson, L.Å. Anti-Inflammatory Systems in Human Milk. Adv. Exp. Med. Biol. 1990, 262, 69–76. [Google Scholar] [PubMed]
- Lewis, E.D.; Richard, C.; Larsen, B.; Field, C. The Importance of Human Milk for Immunity in Preterm Infants. Clin. Perinatol. 2017, 44, 23–47. [Google Scholar] [CrossRef] [PubMed]
- Khaleva, E.; Gridneva, Z.; Geddes, D.T.; Oddy, W.H.; Colicino, S.; Blyuss, O.; Boyle, R.J.; Warner, J.O.; Munblit, D. Transforming growth factor beta in human milk and allergic outcomes in children: A systematic review. Clin. Exp. Allergy 2019, 49, 1201–1213. [Google Scholar] [CrossRef] [Green Version]
- Ustundag, B. Levels of cytokines (IL-1beta, IL-2, IL-6, IL-8, TNF-alpha) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediat. Inflamm. 2005, 2005, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2017, 2017, CD007137. [Google Scholar] [CrossRef]
- Obeid, S.; Wankell, M.; Charrez, B.; Sternberg, J.; Kreuter, R.; Esmaili, S.; Ramezani-Moghadam, M.; Devine, C.; Read, S.; Bhathal, P.; et al. Adiponectin confers protection from acute colitis and restricts a B cell immune response. J. Boil. Chem. 2017, 292, 6569–6582. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef] [Green Version]
- Caplan, M.S.; Russell, T.; Xiao, Y.; Amer, M.; Kaup, S.; Jilling, T. Effect of Polyunsaturated Fatty Acid (PUFA) Supplementation on Intestinal Inflammation and Necrotizing Enterocolitis (NEC) in a Neonatal Rat Model. Pediatr. Res. 2001, 49, 647–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Jilling, T.; Li, D.; Caplan, M.S. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr. Res. 2007, 61, 427–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Bioactive Components in Breast Milk | Role in Intestinal Inflammation Regulation or Prevention | Effect | References |
|---|---|---|---|
| Microbial or microbial modulating factors | |||
| Lactobacillus spp, | -Inhibit NF-κB pathway -decrease pro-inflammatory cytokines, TNF-α, IL-6 -reverse intestinal dysbiosis in bacterial intestinal infection | -decrease inflammatory response -Restore intestinal microbiome homeostasis | [41,42,43,44] |
| Bifidobacterium spp | -increase SCFA production -Decrease pro-inflammatory CK release (IL-6, CXCL-1, TNF-α, IL-23) and iNOS | -promote anti-inflammatory commensal bacteria proliferation -decrease inflammatory response | [45,46,47,48] |
| Human Milk Oligosaccharides | -regulate commensal bacteria -act as decoy receptors for pathogens -modulate immune signaling pathways, TLR3, TLR5, PAMP | -promote healthy intestinal microbiota with anti-inflammatory properties -prevent and decrease inflammatory response | [32,49,50,51,52,53] |
| Immunological factors | |||
| Secretory IgA | -bind to pathogens and commensal bacteria | -prevention of typical inflammatory response, or immune exclusion -influence intestinal microbiome | [29,54] |
| IgG | -opsonization, agglutination of bacteria | -prevention of typical acute inflammatory response | [52,55,56,57] |
| IL-10 | -inhibit Th1, NK cell, macrophages | -provide immunoregulation and prevent inflammation | [18,58,59,60,61] |
| TGF- β | -inhibit differentiation of naïve T cells into Th1, Th2 cells -Stabilize FOXP3 expression | -decrease pro-inflammatory cytokine expression and inflammation -inhibit immune response and decrease inflammation | [18,60,62,63,64] |
| ILRA-1 TNFR I and II soluble TLR2 | -compete with IL-1 receptor for IL-1 -directly bind, inhibit TNF- α -decoy receptor to inhibit IL-8, TNF | -prevent pro-inflammatory cytokine expression and inflammation | [52,60,65,66,67] |
| EGF HB-EGF VEGF | -upregulate IL-10 expression -bind to bacteria -stimulate angiogenesis- | -decrease pro-inflammatory cytokine expression -prevent intestinal edema | [68,69,70,71,72,73,74] |
| Lactoferrin | -direct cytotoxicity on pathogens by forming lactoferricin -inhibit IL-1, IL-6, TNF-α, IL-8 -promote growth of probiotics | -eliminate trigger for acute inflammatory response -decrease pro-inflammatory cytokine expression and inflammation -regulate intestinal microbiome | [18,75,76,77] |
| Lactadherin | -enhance phagocytosis of apoptotic cells -blocks NF-κB pathway via TLR4 inhibition -promote healing during intestinal inflammation | -eliminate trigger for acute inflammatory response -prevent pro-inflammatory signaling and decreasing inflammatory response -limit degree of intestinal inflammation | [78,79] |
| Lysozyme | -degrades GP bacteria outer wall -kill GN bacteria with lactoferrin | -eliminate trigger for acute inflammatory response | [18,80] |
| Metabolic factors | |||
| Adiponectin | -suppress mature macrophage function | -decrease inflammatory response | [52,81] |
| Leptin | -stimulates T cells -influence polarization of macrophages to anti-inflammatory phenotype | -regulate immune response and prevent inflammation | [81,82,83,84] |
| Omega 3 PUFA | -decrease NF- κB, bind to PPAR-γ -increase proliferation of Lactobacillus and Bifidobacterium -change membrane PL concentration -inhibit leukocyte migration | -downregulate pro-inflammatory genes --promote anti-inflammatory commensal bacteria proliferation -decrease degree of inflammatory response | [13,85,86,87,88,89,90] |
| Antioxidants | -scavenge free radicals | -prevent injury and inflammation | [60] |
| Anti-proteases | -metabolize proteases produced by inflammatory cells | -prevent excessive inflammatory response | [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thai, J.D.; Gregory, K.E. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients 2020, 12, 581. https://doi.org/10.3390/nu12020581
Thai JD, Gregory KE. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients. 2020; 12(2):581. https://doi.org/10.3390/nu12020581
Chicago/Turabian StyleThai, Julie D., and Katherine E. Gregory. 2020. "Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life" Nutrients 12, no. 2: 581. https://doi.org/10.3390/nu12020581




