Postural Balance Effects Associated with 400, 4000 or 10,000 IU Vitamin D3 Daily for Three Years: A Secondary Analysis of a Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Randomization and Intervention
2.3. Sample Size
2.4. Descriptive Variables
2.5. Balance
2.6. Statistical Analysis
3. Results
3.1. Descriptive Characteristics
3.2. Vitamin D Supplementation Adherence and Serum Levels
3.3. Sway Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tinetti, M.E. Preventing falls in elderly persons. N. Engl. J. Med. 2003, 348, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Staehelin, H.B.; Orav, J.E.; Stuck, A.E.; Theiler, R.; Wong, J.B.; Egli, A.; Kiel, D.P.; Henschkowski, J. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. BMJ 2009, 339, b3692. [Google Scholar] [CrossRef] [Green Version]
- Cangussu, L.M.; Nahas-Neto, J.; Orsatti, C.L.; Poloni, P.F.; Schmitt, E.B.; Almeida-Filho, B.; Nahas, E.A.P. Effect of isolated vitamin D supplementation on the rate of falls and postural balance in postmenopausal women fallers: A randomized, double-blind, placebo-controlled trial. Menopause 2016, 23, 267–274. [Google Scholar] [CrossRef]
- Dhesi, J.K.; Jackson, S.H.D.; Bearne, L.M.; Moniz, C.; Hurley, M.V.; Swift, C.G.; Allain, T.J. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing 2004, 33, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Suppan, K.; Fahrleitner-Pammer, A.; Dobnig, H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos. Int. 2009, 20, 315–322. [Google Scholar] [CrossRef]
- Uusi-Rasi, K.; Patil, R.; Karinkanta, S.; Kannus, P.; Tokola, K.; Lamberg-Allardt, C.; Sievänen, H. Exercise and vitamin D in fall prevention among older women: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Bislev, L.S.; Rødbro, L.L.; Rolighed, L.; Sikjaer, T.; Rejnmark, L. Effects of vitamin D3 supplementation on muscle strength, mass, and physical performance in women with vitamin D insufficiency: A randomized placebo-controlled trial. Calcif. Tissue Int. 2018, 103, 483–493. [Google Scholar] [CrossRef]
- Bunout, D.; Barrera, G.; Leiva, L.; Gattas, V.; la Maza, M.P.; Avendano, M.; Hirsch, S. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp. Gerontol. 2006, 41, 746–752. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Orav, E.J.; Staehelin, H.B.; Meyer, O.W.; Theiler, R.; Dick, W.; Willett, W.C.; Egli, A. Monthly high-dose vitamin D treatment for the prevention of functional decline: A randomized clinical trial. JAMA Intern. Med. 2016, 176, 175–183. [Google Scholar] [CrossRef]
- Burt, L.A.; Gaudet, S.; Kan, M.; Rose, M.S.; Billington, E.O.; Boyd, S.K.; Hanley, D.A. Methods and procedures for: A randomized double-blind study investigating dose-dependent longitudinal effects of vitamin D supplementation on bone health. Contemp. Clin. Trials 2018, 67, 68–73. [Google Scholar] [CrossRef]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: A randomized clinical trial. JAMA 2019, 322, 736–745. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Wu, H.; Gozdzik, A.; Barta, J.L.; Wagner, D.; Cole, D.E.; Vieth, R.; Parra, E.J.; Whiting, S.J. The development and evaluation of a food frequency questionnaire used in assessing vitamin D intake in a sample of healthy young Canadian adults of diverse ancestry. Nutr. Res. 2009, 29, 255–261. [Google Scholar] [CrossRef]
- Biodex Medical Systems Inc. Biosway Portable Balance System; Biodex Medical Systems Inc.: Shirley, NY, USA, 2017. [Google Scholar]
- Shumway-Cook, A.; Horak, F.B. Assessing the influence of sensory interaction on balance. Suggestion from the field. Phys. Ther. 1986, 66, 1548–1550. [Google Scholar] [CrossRef]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef]
- Riemann, B.L.; Piersol, K. Intersession reliability of self-selected and narrow stance balance testing in older adults. Aging Clin. Exp. Res. 2017, 29, 1045–1048. [Google Scholar] [CrossRef]
- Grimnes, G.; Emaus, N.; Cashman, K.D.; Jorde, R. The effect of high--dose vitamin D supplementation on muscular function and quality of life in postmenopausal women—A randomized controlled trial. Clin. Endocrinol. 2017, 87, 20–28. [Google Scholar] [CrossRef]
- Cangussu, L.M.; Nahas-Neto, J.; Orsatti, C.L.; Bueloni-Dias, F.N.; Nahas, E.A.P. Effect of vitamin D supplementation alone on muscle function in postmenopausal women: A randomized, double-blind, placebo-controlled clinical trial. Osteoporos. Int. 2015, 26, 2413–2421. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Stel, V.S.; Smit, J.H.; Pluijm, S.M.F.; Lips, P. Balance and mobility performance as treatable risk factors for recurrent falling in older persons. J. Clin. Epidemiol. 2003, 56, 659–668. [Google Scholar] [CrossRef]
- Bergland, A.; Jarnlo, G.-B.; Laake, K. Predictors of falls in the elderly by location. Aging Clin. Exp. Res. 2003, 15, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bergland, A.; Wyller, T.B. Risk factors for serious fall related injury in elderly women living at home. Inj. Prev. 2004, 10, 308–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piirtola, M.; Era, P. Force platform measurements as predictors of falls among older people—A review. Gerontology 2006, 52, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pajala, S.; Era, P.; Koskenvuo, M.; Kaprio, J.; Törmäkangas, T.; Rantanen, T. Force platform balance measures as predictors of indoor and outdoor falls in community-dwelling women aged 63–76 years. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 171–178. [Google Scholar] [CrossRef]
- Lips, P.; Binkley, N.; Pfeifer, M.; Recker, R.; Samanta, S.; Cohn, D.A.; Chandler, J.; Rosenberg, E.; Papanicolaou, D.A. Once-weekly dose of 8400 IU vitamin D3 compared with placebo: Effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am. J. Clin. Nutr. 2010, 91, 985–991. [Google Scholar] [CrossRef] [Green Version]
- Murad, M.H.; Elamin, K.B.; Abu Elnour, N.O.; Elamin, M.B.; Alkatib, A.A.; Fatourechi, M.M.; Almandoz, J.P.; Mullan, R.J.; Lane, M.A.; Liu, H.; et al. The effect of vitamin D on falls: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2997–3006. [Google Scholar] [CrossRef] [Green Version]
- Bolland, M.J.; Grey, A.; Reid, I.R. Differences in overlapping meta-analyses of vitamin D supplements and falls. J. Clin. Endocrinol. Metab. 2014, 99, 4265–4272. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.M.; Gallagher, J.C.; Suiter, C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: A randomized clinical trial. J. Steroid Biochem. Mol. Biol. 2017, 173, 317–322. [Google Scholar] [CrossRef]
- Billington, E.O.; Burt, L.A.; Rose, M.S.; Davison, E.M.; Gaudet, S.; Kan, M.; Boyd, S.K.; Hanley, D.A. Safety of high-dose vitamin D supplementation: Secondary analysis of a randomized controlled trial. J. Clin. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef]
- IOM 2011 Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011.
| Descriptive Variable | 400 IU (n = 124) | 4000 IU (n = 125) | 10000 IU (n = 124) |
|---|---|---|---|
| Age (years) | 62.0 (4.2) | 62.7 (4.3) | 62.0 (4.1) |
| Height (cm) | 171.0 (9.0) | 168.4 (9.2) | 168.5 (9.5) |
| Weight (kg) | 81.1 (15.1) | 79.1 (15.7) | 77.5 (15.0) |
| Body mass index (kg/m2) | 27.7 (4.4) | 27.8 (5.0) | 27.2 (4.4) |
| Serum 25(OH)-vitamin D (nmol/L) | 76 (21) | 80 (20) | 78 (18) |
| Total hip T-score | 0.0 (1.1) | 0.1 (1.2) | 0.0 (1.1) |
| Falls a (%) | 27 (21.8%) | 22 (17.6%) | 19 (15.3%) |
| Fracture since 50 years (%) | 23 (18.5%) | 16 (12.8%) | 23 (18.5%) |
| History of cardiovascular condition (%) | 24 (19.4%) | 14 (11.2%) | 16 (12.9%) |
| Type 2 diabetes (%) | 3 (2.4%) | 4 (3.2%) | 5 (4.0%) |
| Rheumatoid arthritis (%) | 2 (1.6%) | 2 (1.6%) | 1 (0.8%) |
| Asthma (%) | 6 (4.8%) | 10 (8.0%) | 11 (8.9%) |
| Smoker (%) | 3 (2.4%) | 2 (1.6%) | 5 (4.0%) |
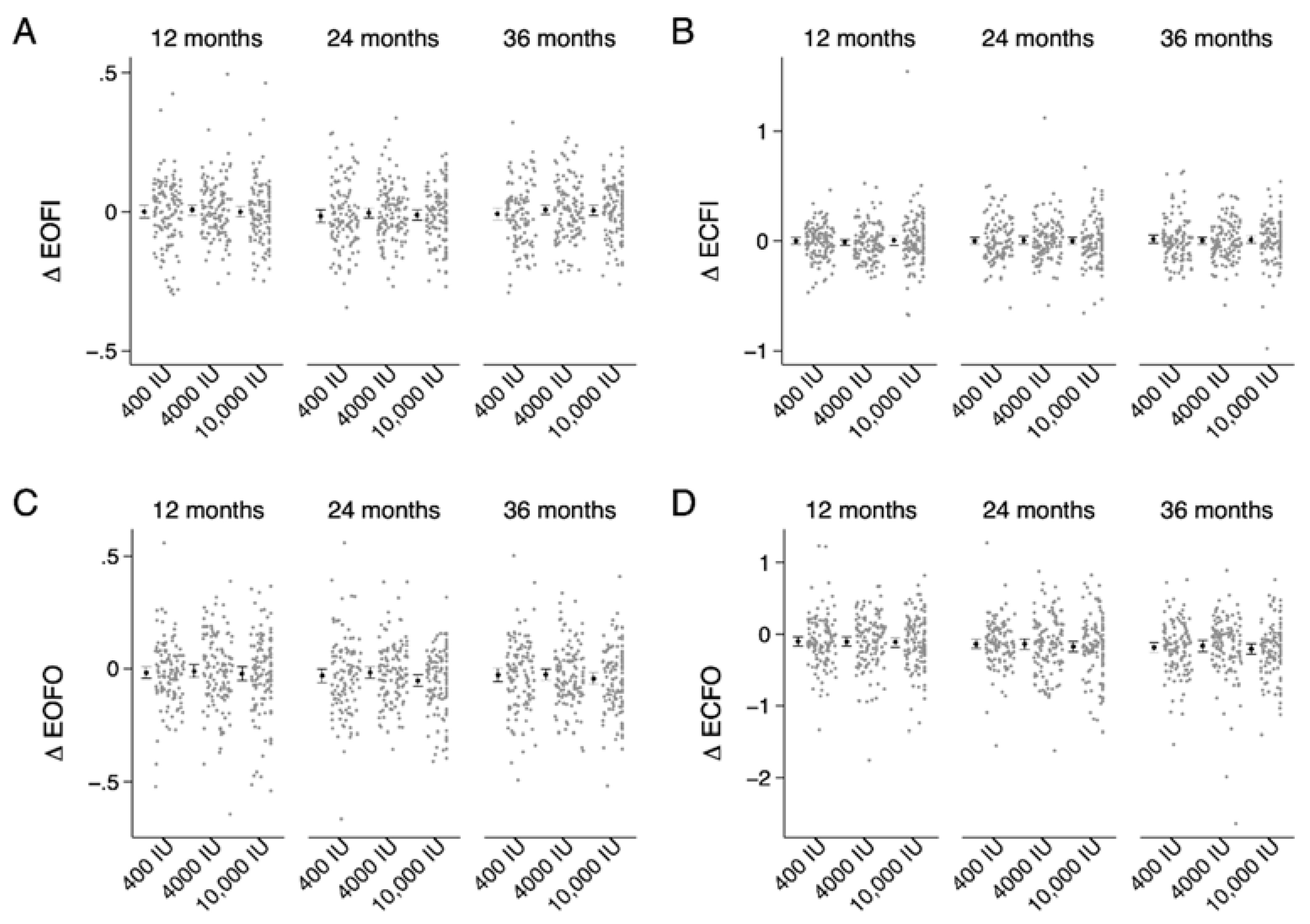
| SWAYEOFI | SWAYECFI | SWAYEOFO | SWAYECFO | ||
|---|---|---|---|---|---|
| Baseline | 400 IU | 0.4 (0.1) | 0.7 (0.2) | 0.7 (0.2) | 2.2 (0.4) |
| 4000 IU | 0.4 (0.1) | 0.7 (0.2) | 0.7 (0.2) | 2.2 (0.5) | |
| 10,000 IU | 0.4 (0.1) | 0.7 (0.2) | 0.7 (0.2) | 2.2 (0.5) | |
| Δ 12-month | 400 IU | 0.00 (0.1) | 0.00 (0.2) | −0.02 (0.1) | −0.10 (0.3) ad |
| 4000 IU | 0.01 (0.1) | −0.01 (0.2) | −0.01 (0.2) | −0.11 (0.4) ad | |
| 10,000 IU | 0.00 (0.1) | 0.01 (0.2) | −0.02 (0.2) | −0.11 (0.4) ad | |
| Δ 24-month | 400 IU | −0.02 (0.1) | 0.00 (0.2) | −0.03 (0.2) a | −0.14 (0.3) a |
| 4000 IU | 0.00 (0.1) | 0.01 (0.2) | −0.02 (0.1) a | −0.14 (0.4) a | |
| 10,000 IU | −0.01 (0.1) | 0.00 (0.2) | −0.05 (0.1) a | −0.18 (0.4) a | |
| Δ 36-month | 400 IU | −0.01 (0.1) | 0.02 (0.2) | −0.03 (0.2) a | −0.19 (0.4) ab |
| 4000 IU | 0.01 (0.1) | 0.00 (0.2) | −0.03 (0.1) a | −0.16 (0.4) ab | |
| 10,000 IU | 0.00 (0.1) | 0.01 (0.2) | −0.04 (0.1) a | −0.21 (0.4) ab |
| SWAYEOFI | SWAYECFI | SWAYEOFO | SWAYECFO | ||
|---|---|---|---|---|---|
| 12-month difference | 4000–400 | 0.004 (−0.03, 0.04) | 0.005 (−0.06, 0.07) | 0.015 (−0.03, 0.06) | 0.063 (−0.08, 0.21) |
| 10,000–400 | 0.000 (−0.03, 0.03) | 0.000 (−0.07, 0.07) | −0.008 (−0.06, 0.04) | 0.008 (−0.14, 0.15) | |
| 10,000–4000 | −0.003 (−0.04, 0.03) | −0.005 (−0.07, 0.06) | −0.023 (−0.07, 0.03) | −0.055 (−0.20, 0.09) | |
| 24-month difference | 4000–400 | 0.009 (−0.02, 0.04) | 0.023 (−0.05, 0.09) | 0.026 (−0.02, 0.08) | 0.068 (−0.07, 0.21) |
| 10,000–400 | 0.005 (−0.03, 0.04) | −0.008 (−0.08, 0.06) | −0.022 (−0.07, 0.03) | −0.015 (−0.16, 0.13) | |
| 10,000–4000 | −0.004 (−0.04, 0.03) | −0.031 (−0.10, 0.04) | −0.048 (−0.10, 0.00) | −0.082 (−0.22, 0.06) | |
| 36-month difference | 4000–400 | 0.012 (−0.02, 0.05) | 0.005 (−0.06, 0.07) | 0.013 (−0.04, 0.06) | 0.089 (−0.05, 0.23) |
| 10,000–400 | 0.014 (−0.02, 0.05) | −0.011 (−0.08, 0.06) | −0.015 (−0.07, 0.04) | 0.009 (−0.13, 0.15) | |
| 10,000–4000 | 0.001 (−0.03, 0.03) | −0.015 (−0.08, 0.05) | −0.028 (−0.08, 0.02) | −0.080 (−0.22, 0.06) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burt, L.A.; Gabel, L.; Billington, E.O.; Hanley, D.A.; Boyd, S.K. Postural Balance Effects Associated with 400, 4000 or 10,000 IU Vitamin D3 Daily for Three Years: A Secondary Analysis of a Randomized Clinical Trial. Nutrients 2020, 12, 527. https://doi.org/10.3390/nu12020527
Burt LA, Gabel L, Billington EO, Hanley DA, Boyd SK. Postural Balance Effects Associated with 400, 4000 or 10,000 IU Vitamin D3 Daily for Three Years: A Secondary Analysis of a Randomized Clinical Trial. Nutrients. 2020; 12(2):527. https://doi.org/10.3390/nu12020527
Chicago/Turabian StyleBurt, Lauren A., Leigh Gabel, Emma O. Billington, David A. Hanley, and Steven K. Boyd. 2020. "Postural Balance Effects Associated with 400, 4000 or 10,000 IU Vitamin D3 Daily for Three Years: A Secondary Analysis of a Randomized Clinical Trial" Nutrients 12, no. 2: 527. https://doi.org/10.3390/nu12020527





