The Effect of Birch Pollen Immunotherapy on Apple and rMal d 1 Challenges in Adults with Apple Allergy
Abstract
:1. Introduction
2. Material and Methods
2.1. Subject Selection and Study Design
2.2. Immunotherapy With Birch Pollen
2.3. Questionnaires
2.3.1. Dietary History Questionnaire
2.3.2. Global Assessment Questionnaire
2.4. Skin-Prick Tests
2.5. Measurement of Allergen-Specific Antibodies
2.6. Nasal Challenge Tests
2.7. Oral Challenge Tests
2.7.1. Open Food Challenge With Apple (Golden Delicious)
2.7.2. Double-Blind, Placebo-Controlled Challenge Test With rMal d 1
2.8. Statistical Analyses
3. Results
3.1. Study Population
3.2. Effect of the Birch Pollen Immunotherapy
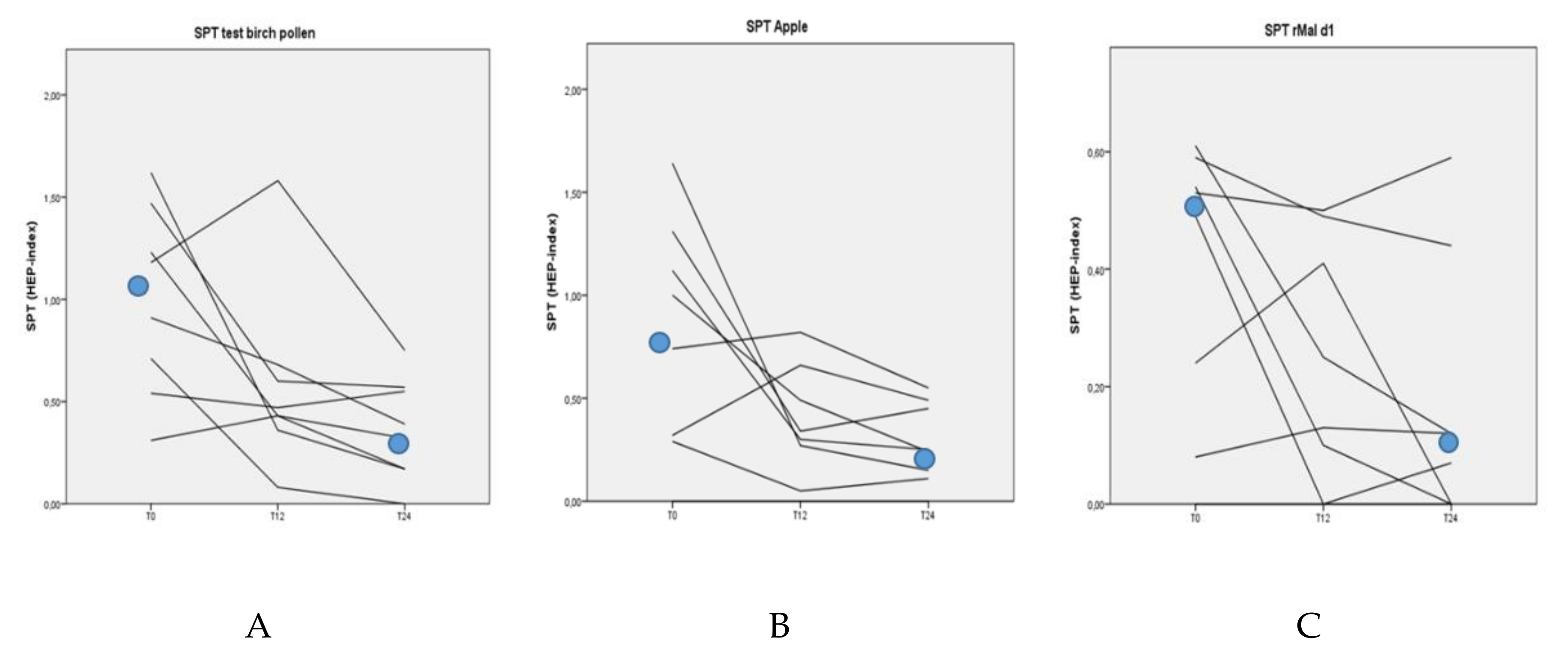
3.3. Skin-Prick Tests
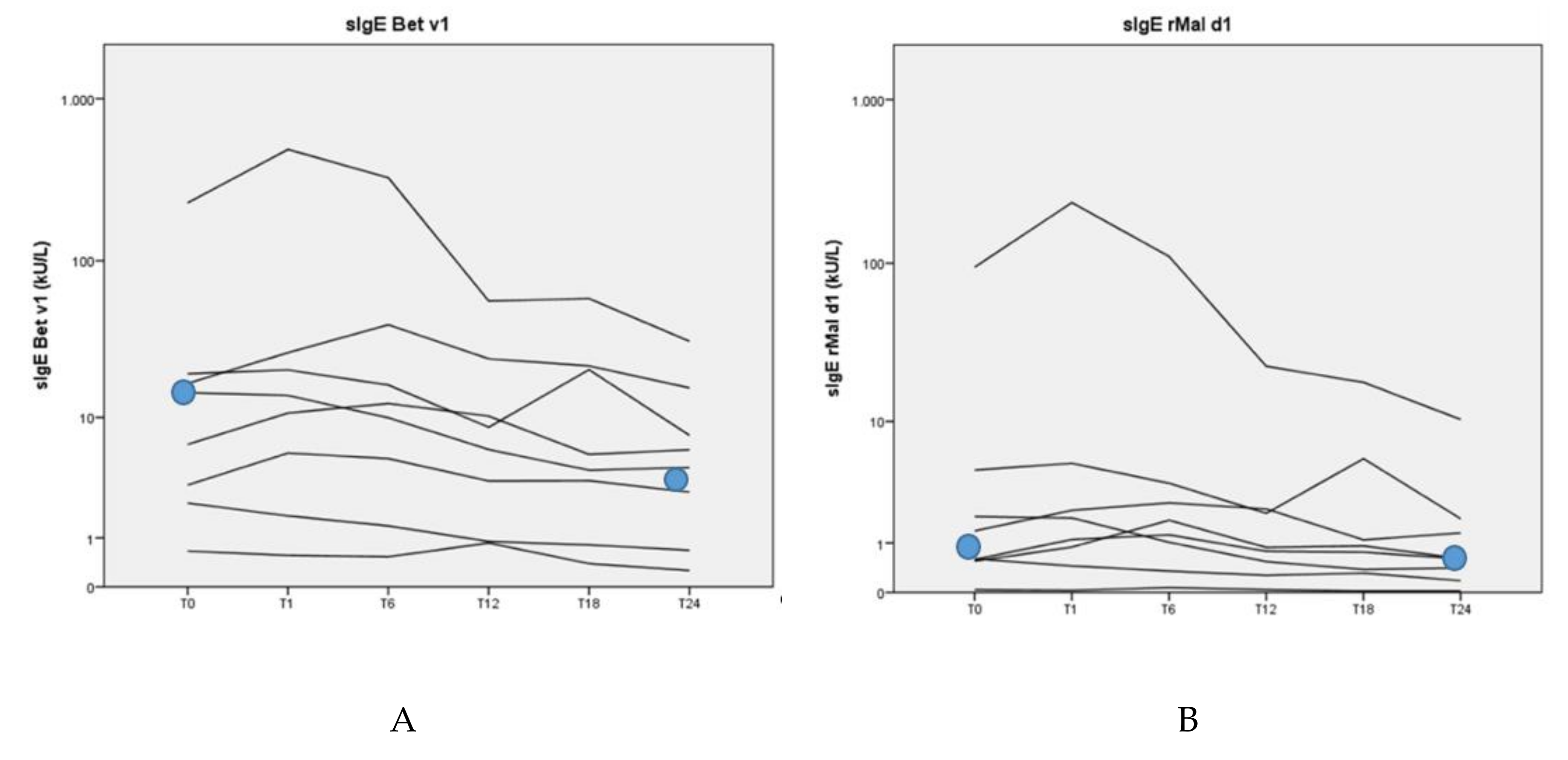
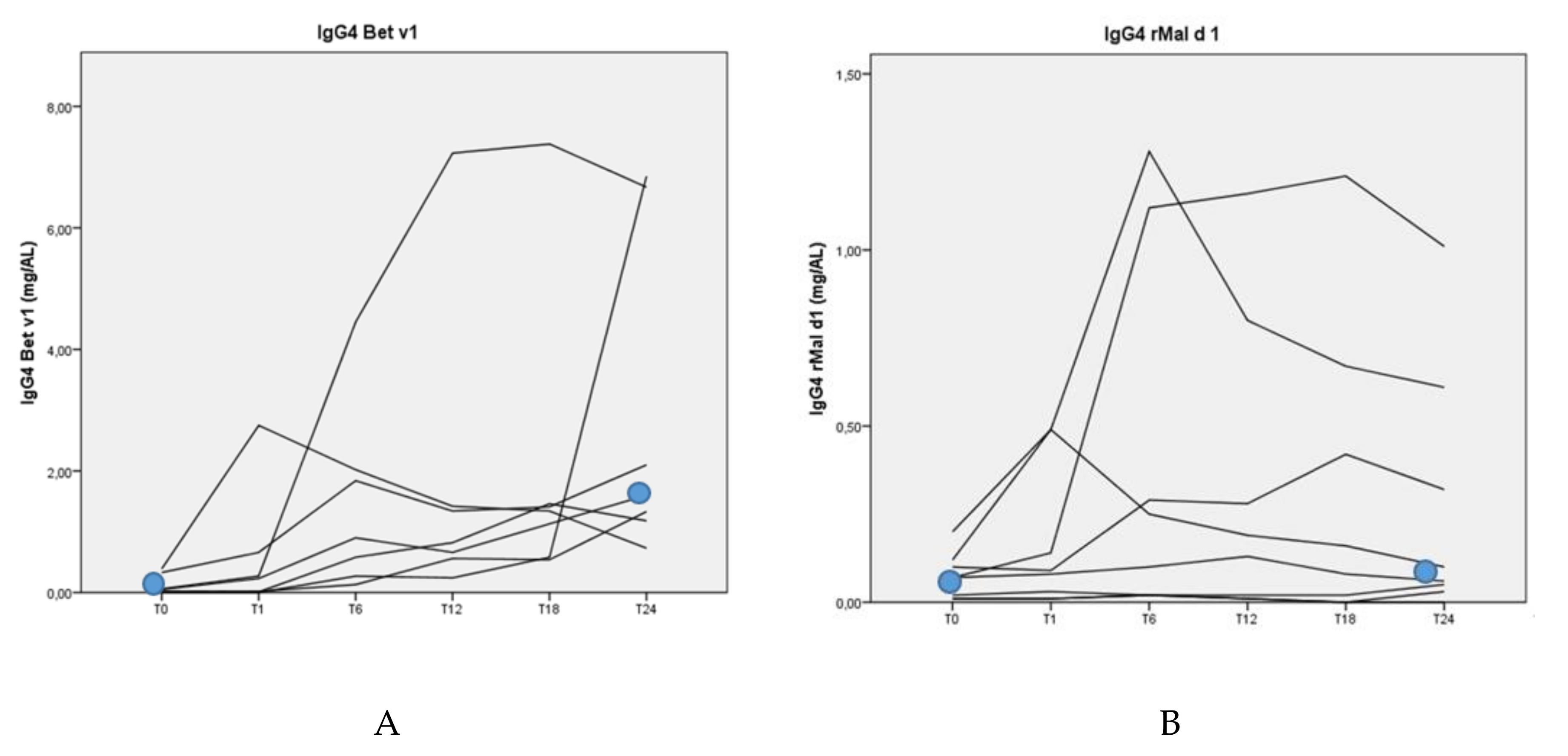
3.4. Allergen-Specific Antibody Levels
3.5. Oral Challenge Tests
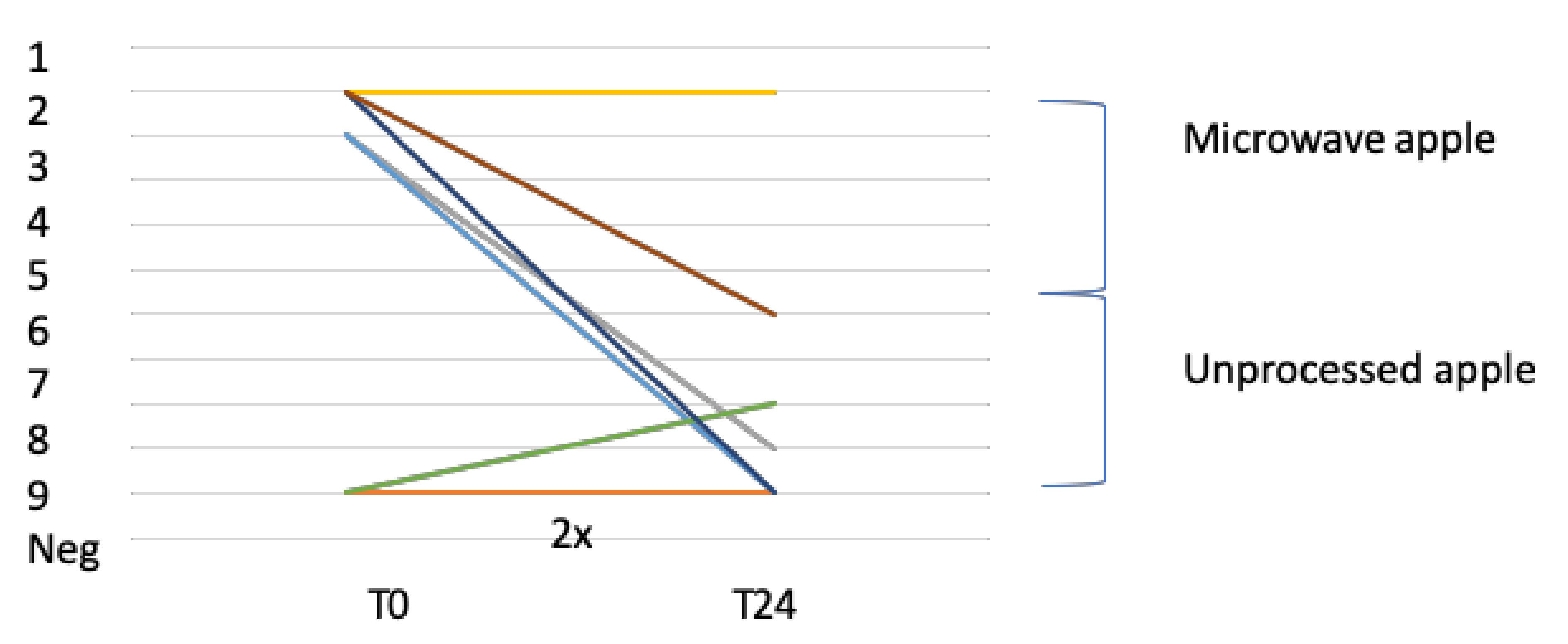
3.5.1. Open Food Challenge With Apple (Golden Delicious)
3.5.2. Double-Blind, Placebo-Controlled Challenge Test With rMal d 1
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kleine-Tebbe, J.; Zuberbier, T.; Werfel, T.; Krüll, M.; Wagenmann, M.; Johansen, N.; Würtzen, P.A.; Wolf, H.; Mücke, V.; Wüstenberg, E.; et al. Is allergy immunotherapy with birch sufficient to treat patients allergic to pollen of tree species of the birch homologous group? Allergy 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon, M.A.; Alves, B.; Jacobson, M.; Hurwitz, B.; Sheikh, A.; Durham, S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst. Rev. 2007, 24, CD001936. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Mewes, T.; Wolf, H.; Hansen, I.; Schnitker, J.; Mann, W.J. The effects of short-term immunotherapy using molecular standardized grass and rye allergens compared with symptomatic drug treatment on rhino conjunctivitis symptoms, skin sensitivity, and specific nasal reactivity. Otolaryngol. Head Neck Surg. 2005, 133, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Subiza, J.L.; Feliú, A.; Subiza, J.L.; Uhlig, J.; Fernández-Caldas, E. Cluster immunotherapy with a glutaraldehyde-modified mixture of grasses results in an improvement in specific nasal provocation tests in less than 2.5 months of treatment. Clin. Exp. Allergy 2008, 38, 987–994. [Google Scholar] [CrossRef]
- Geroldinger-Simic, M.; Zelniker, T.; Aberer, W.; Ebner, C.; Egger, C.; Greiderer, A.; Prem, N.; Lidholm, J.; Ballmer-Weber, B.K.; Vieths, S.; et al. Birch pollen–related food allergy: Clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J. Allergy Clin. Immunol. 2011, 127, 616–622. [Google Scholar] [CrossRef]
- Hansen, K.S.; Vestergaard, H.; Skov, P.S.; Khinchi, M.S.; Vieths, S.; Poulsen, L.K.; Bindslev-Jensen, C. Double-blind, placebo-controlled food challenge with apple. Allergy 2001, 56, 109–117. [Google Scholar] [CrossRef]
- De Jong, N.W.; Van Maaren, M.S.; Vlieg-Boersta, B.J.; Dubois, A.E.; De Groot, H.; Van Wijk, R.G. Sensitization to lupine flour: Is it clinically relevant? Clin. Exp. Allergy 2010, 40, 1571–1577. [Google Scholar] [CrossRef]
- Van Der Valk, J.; Van Wijk, R.G.; Hoorn, E.; Groenendijk, L.; Groenendijk, I.; De Jong, N.W. Measurement and interpretation of skin prick test results. Clin. Transl. Allergy 2016, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Patiwael, J.A.; Vullings, L.; De Jong, N.; Van Toorenenbergen, A.; Van Wijk, R.G.; De Groot, H. Occupational Allergy in Strawberry Greenhouse Workers. Int. Arch. Allergy Immunol. 2010, 152, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Giséle, C.M.; Groenewoud, M.D.; De G raaf in′ t Veld, C.; Van Oorschot–van Nes, A.J.; De J ong, N.W.; Vermeulen, A.M.; Van Wijk, R.G. Prevalence of sensitization to the predatory mite Amblyseius cucumeris as a new occupational allergen in horticulture. Allergy 2002, 57, 614–619. [Google Scholar]
- Veld, C.D.G.-I.; Garrelds, I.M.; Van Toorenenbergen, A.W.; Van Wijk, R.G. Nasal responsiveness to allergen and histamine in patients with perennial rhinitis with and without a late phase response. Thorax 1997, 52, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Graaf-in’t Veld, C.; Garrelds, I.M.; Jansen, A.P.; Van Toorenenbergen, A.W.; Mulder, P.G.; Meeuwis, J.; Van Wijk, R.G. Effect of intranasal fluticasone propionate on the immediate and late allergic reaction and nasal hyper reactivity in patients with a house dust mite allergy. Clin. Exp. Allergy 1995, 25, 966–973. [Google Scholar] [CrossRef]
- Bousquet, J.; Lebel, B.; Dhivert, H.; Bataille, Y.; Martinot, B.; Michel, F.B. Nasal challenge with pollen grains, skin-prick tests and specific IgE in patients with grass pollen allergy. Clin. Exp. Allergy 1987, 17, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Lebel, B.; Bousquet, J.; Morel, A.; Chanal, I.; Godard, P.; Michel, F.-B. Correlation between symptoms and the threshold for release of mediators in nasal secretions during nasal challenge with grass-pollen grains. J. Allergy Clin. Immunol. 1988, 82, 869–877. [Google Scholar] [CrossRef]
- Skypala, I.J.; Calderón, M.A.; Leeds, A.R.; Emery, P.; Till, S.; Durham, S.R. Development and validation of a structured questionnaire for the diagnosis of oral allergy syndrome in subjects with seasonal allergic rhinitis during the UK birch pollen season. Clin. Exp. Allergy 2011, 41, 1001–1011. [Google Scholar] [CrossRef]
- Geroldinger-Simic, M.; Kinaciyan, T.; Nagl, B.; Baumgartner-Durchschlag, U.; Huber, H.; Ebner, C.; Lidholm, J.; Bartel, D.; Vieths, S.; Jahn-Schmida, B.; et al. Oral exposure to Mal d 1 affects the immune response in patients with birch pollen allergy. J. Allergy Clin. Immunol. 2013, 131, 94–102. [Google Scholar] [CrossRef]
- Canonica, G.W.; Cox, L.; Pawankar, R.; Bena-Cagnani, C.E.; Blaiss, M.; Bonini, S.; Bousquet, J.; Calderón, M.; Compalati, E.; Durham, S.R.; et al. Sublingual immunothrapy: World Allergy Organization position paper 2013 update. World Allergy Organ. J. 2014, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Malling, H.-J.; Bousquet, J. Subcutaneous immunotherapy for allergic rhinoconjunctivitis, allergic asthma, and prevention of allergic diseases. Clin. Allergy Immunol. 2008, 21, 343–358. [Google Scholar]
- Burks, A.W.; Calderon, M.A.; Casala, T.; Cox, L.; Demoly, P.; Jutel, M.; Nelson, H.; Akdis, C.A. Update on allergic Immunology/ European Academy of Allergy and Clinical Immunology/ PRACTALL consensus report. J. Allergy Clin. Immunol. 2013, 131, 1288–1296. [Google Scholar] [CrossRef]
- Asero, R.; Fernandez-Rivas, M.; Knulst, A.C.; Bruijnzeel-Koomen, C.A. Double-blind, placebo-controlled food challenge in adults in everyday clinical practice: A reappraisal of their limitations and real indications. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 379–385. [Google Scholar] [CrossRef]
- Kinaciyan, T.; Jahn-Schmid, B.; Radakovics, A.; Zwölfer, B.; Schreiber, C.; Francis, J.N.; Ebner, C.; Bohle, B. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J. Allergy Clin. Immunol. 2007, 119, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Kinaciyan, T.; Nagl, B.; Faustmann, S.; Kopp, S.; Wolkersdorfer, M.; Bohle, B. Recombinant Mal d 1 facilitates sublingual challenge tests of birch pollen-allergic patients with apple allergy. Allergy 2016, 71, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Russello, M.; Incorvaia, C.; Gazzola, G.; Frati, F.; Moingeon, P.; Passalacqua, G. Birch-Apple Syndrome Treated with Birch Pollen Immunotherapy. Int. Arch. Allergy Immunol. 2011, 156, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Bolhaar, S.T.H.P.; Tiemessen, M.M.; Zuidmeer, L.; Van Leeuwen, A.; Hoffmann-Sommergruber, K.; Bruijnzeel-Koomen, C.A.F.M.; Taams, L.S.; Knol, E.F.; Van Hoffen, E.; Van Ree, R.; et al. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin. Exp. Allergy 2004, 34, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Asero, R. Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin. Exp. Allergy 1998, 28, 1368–1373. [Google Scholar] [CrossRef]
- Son, D.; Scheurer, S.; Hoffmann, A.; Haustein, D.; Vieths, S. Pollen-related food allergy: Cloning and immunological analysis of isoforms and mutants of Mal d 1, the major apple allergen, and Bet v 1, the major birch pollen allergen. Eur. J. Nutr. 1999, 38, 201–215. [Google Scholar] [CrossRef]
- Van Neerven, R.J.; Wikborg, T.; Lund, G.; Jacobsen, B.; Brinch-Nielsen, A.; Arnved, J.; Ipsen, H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J. Immunol. 1999, 163, 2944–2952. [Google Scholar]
- Wurtzen, P.; Lund, G.; Lund, K.; Arvidsson, M.; Rak, S.; Ipsen, H. A double-blind placebo-controlled birch allergy vaccination study II: Correlation between inhibition of IgE binding, histamine release and facilitated allergen presentation. Clin. Exp. Allergy 2008, 38, 1290–1301. [Google Scholar] [CrossRef]

| Nasal Challenge Score on the Highest Concentration BP Allergen (3000 BU/mL) | ||||
|---|---|---|---|---|
| Months AIT | 0 | 12 | 24 | |
| Patient | Score | Score | Score | Change |
| 1 | 5 | 1 | 4 | −1 |
| 2 | 8 | 5 | 2 | −6 |
| 3 | 6 | 9 | 8 | 2 |
| 4 | 11 | 2 | 4 | −7 |
| 5 | 6 | 2 | 0 | −6 |
| 6 | 3 | 4 | 1 | −2 |
| 7 | 11 | 6 | 1 | −10 |
| 8 | 4 | 6 | 5 | +1 |
| Median | ||||
| 6 | 3 | −3 (p = 0.07) | ||
| Open Food Challenge (OFC) with Apple | ||||||
|---|---|---|---|---|---|---|
| Negative or Positive with Eliciting-Dose Reaction | ||||||
| Months | Before Start AIT | Dose Reaction | After 12 Months AIT | Dose Reaction | After 24 Months AIT | Dose Reaction |
| Patient | ||||||
| 1 | Neg | Pos | 2 | Neg | ||
| 2 | Neg | Neg | Neg | |||
| 3 | Pos | 2 | Pos | 1 | Pos | 9 |
| 4 | Pos | 1 | Pos | 1 | Pos | 1 |
| 5 | Pos | 2 | Neg | Neg | ||
| 6 | Neg | Pos | 8 | Pos | 8 | |
| 7 | Pos | 1 | Pos | 9 | Neg | |
| 8 | Pos | 1 | Pos | 9 | Pos | 6 |
| DBPCC with rMal d 1 | ||||||
|---|---|---|---|---|---|---|
| Negative or Positive with Eliciting-Dose Reaction | ||||||
| Months | Before Start AIT | Dose Reaction | After 12 Months AIT | Dose Reaction | After 24 Months AIT | Dose Reaction |
| Patient | ||||||
| 1 | Pos | 20 µg | Neg | Neg | ||
| 2 | Neg | Neg | Neg | |||
| 3 | Pos | 50 µg | Pos | 5 µg | Pos | 5 µg |
| 4 | Neg | Pos | 10 µg | UD | ||
| 5 | Pos | 20 µg | Neg | Neg | ||
| 6 | Neg | Neg | Neg | |||
| 7 | Pos | 10 µg | Neg | Neg | ||
| 8 | Neg | Pos | 5 µg | Neg | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Valk, J.; Nagl, B.; Wljk, R.G.v.; Bohle, B.; de Jong, N. The Effect of Birch Pollen Immunotherapy on Apple and rMal d 1 Challenges in Adults with Apple Allergy. Nutrients 2020, 12, 519. https://doi.org/10.3390/nu12020519
van der Valk J, Nagl B, Wljk RGv, Bohle B, de Jong N. The Effect of Birch Pollen Immunotherapy on Apple and rMal d 1 Challenges in Adults with Apple Allergy. Nutrients. 2020; 12(2):519. https://doi.org/10.3390/nu12020519
Chicago/Turabian Stylevan der Valk, Johanna, Birgit Nagl, Roy Gerth van Wljk, Barbara Bohle, and Nicolette de Jong. 2020. "The Effect of Birch Pollen Immunotherapy on Apple and rMal d 1 Challenges in Adults with Apple Allergy" Nutrients 12, no. 2: 519. https://doi.org/10.3390/nu12020519




