Plasma Peptide Concentrations and Peptide-Reactive Immunoglobulins in Patients with Eating Disorders at Inclusion in the French EDILS Cohort (Eating Disorders Inventory and Longitudinal Survey)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design: EDILS Cohort
2.2. Peptide Concentrations
2.3. IgG Concentrations
2.4. Affinity Measurements
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
3.2. Peptide Concentrations
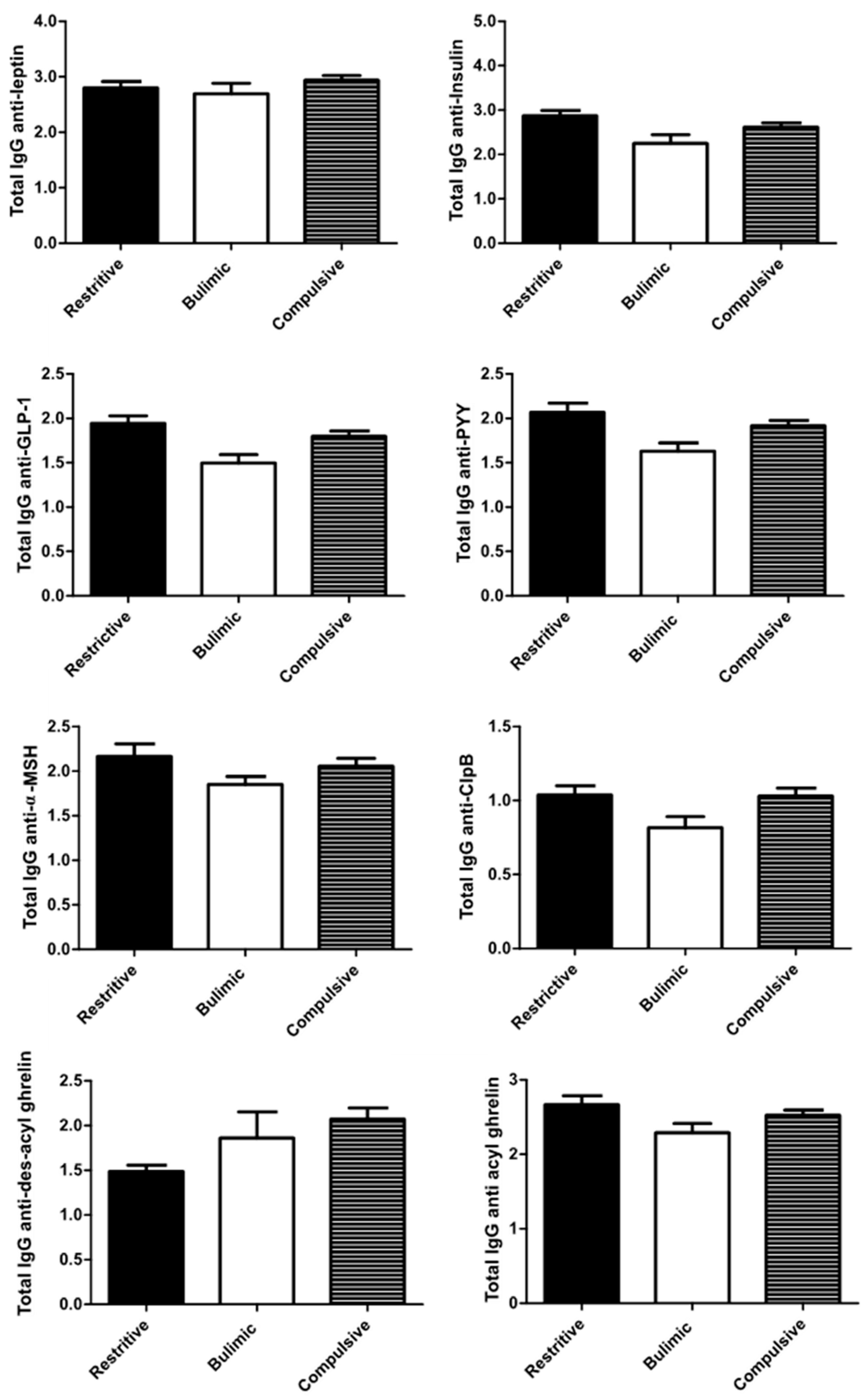
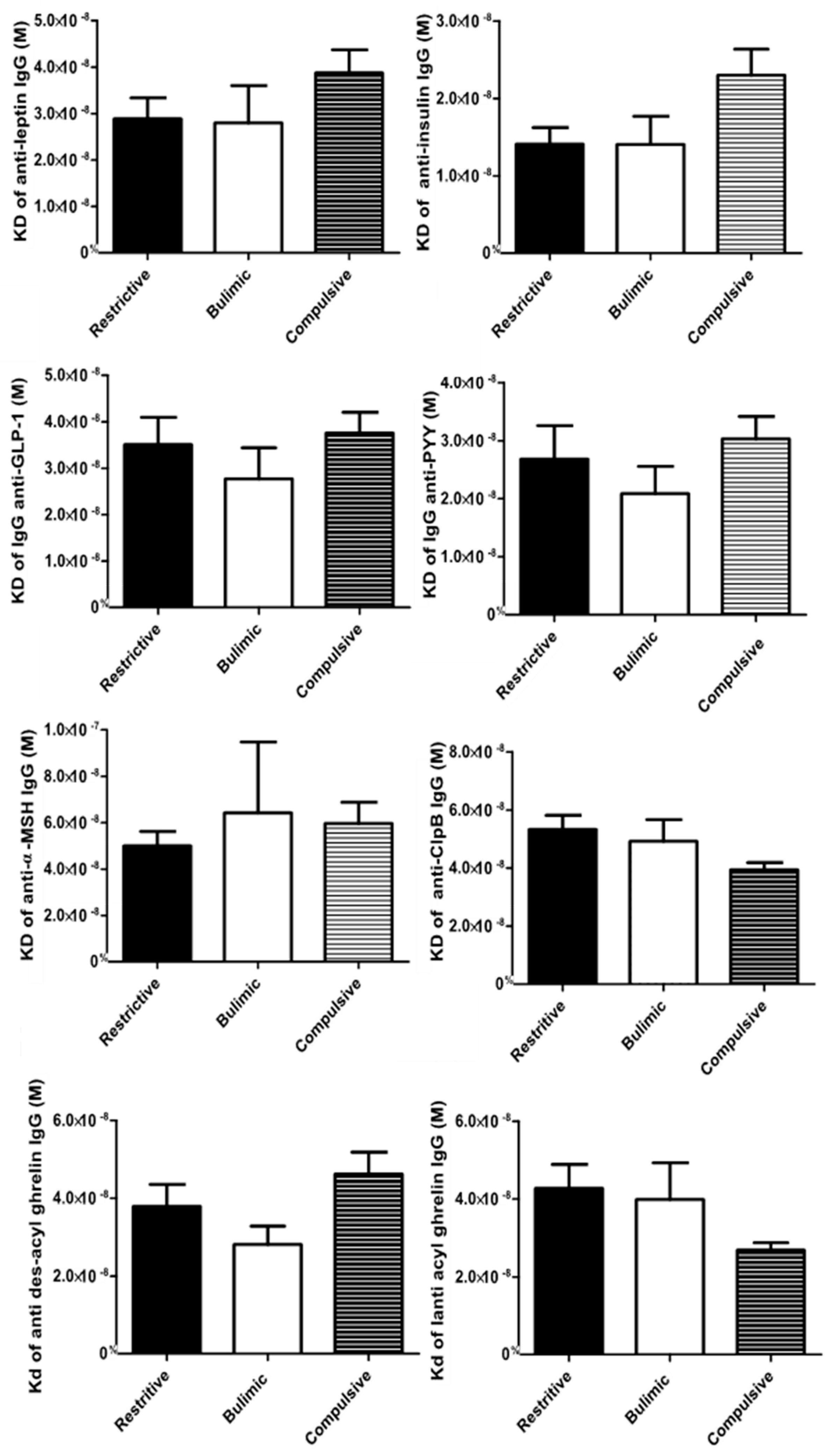
3.3. Immunoglobulin Concentrations and Affinity
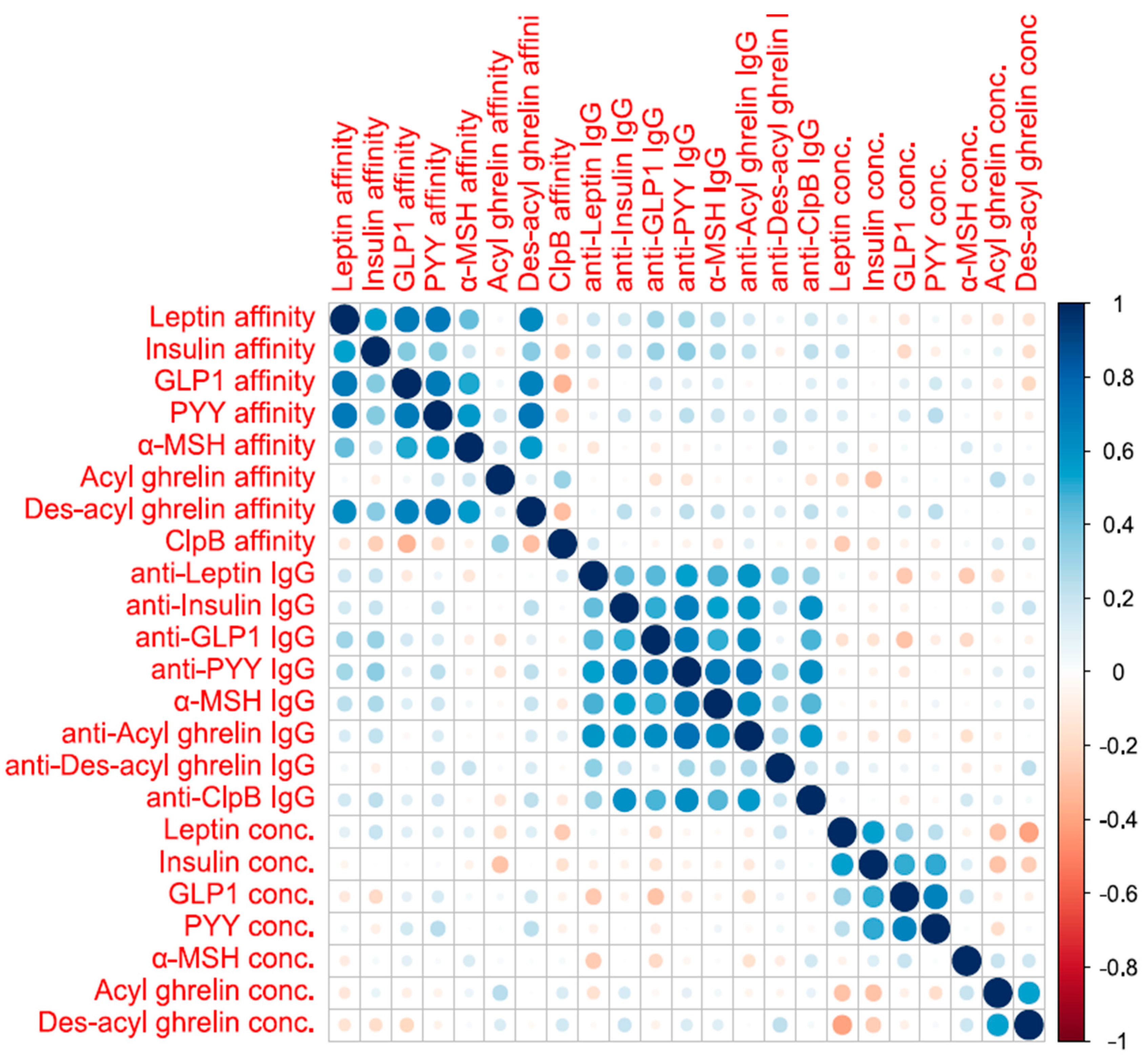
3.4. Association between Biological Data/Principal Component Analyses (PCAs)
3.5. Association between Clinical and Biological Data
4. Discussions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-MSH | alpha-Melanocyte-stimulating hormone |
| AN | Anorexia nervosa |
| BN | Bulimia nervosa |
| BED | Binge eating disorder |
| BSQ | Body Shape Questionnaire |
| ED | Eating Disorder |
| EDI-2 | Eating Disorder Inventory |
| HAD | Hospital Anxiety and Depression |
| IgG | Immunoglobulin G |
| OSFED | Other Specified Feeding or Eating Disorders |
| UFED | Unspecified Feeding or Eating Disorders |
References
- Schmidt, U.; Adan, R.; Böhm, I.; Campbell, I.C.; Dingemans, A.; Ehrlich, S.; Elzakkers, I.; Favaro, A.; Giel, K.; Harrison, A.; et al. Eating disorders: The big issue. Lancet Psychiatry 2016, 3, 313–315. [Google Scholar] [CrossRef]
- Galmiche, M.; Déchelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000–2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, D.; Morin, A.; Mond, J.; Slewa-Younan, S.; Hay, P. The Bidirectional Relationship between Quality of Life and Eating Disorder Symptoms: A 9-Year Community-Based Study of Australian Women. PLoS ONE 2015, 10, e0120591. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Moore, J.; Ashimi, S.S.; Mason, B.L.; Drawbridge, J.N.; Han, S.; Hing, B.; Matthews, A.; McAdams, C.J.; Darbro, B.W.; et al. Eating disorder predisposition is associated with ESRRA and HDAC4 mutations. J. Clin. Investig. 2013, 123, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- Fairburn, C.G.; Cooper, Z.; Shafran, R. Cognitive behaviour therapy for eating disorders: A “transdiagnostic” theory and treatment. Behav. Res. Ther. 2003, 41, 509–528. [Google Scholar] [CrossRef]
- Hardaway, J.A.; Crowley, N.A.; Bulik, C.M.; Kash, T.L. Integrated circuits and molecular components for stress and feeding: Implications for eating disorders: Integrated circuits and molecular components. Genes Brain Behav. 2015, 14, 85–97. [Google Scholar] [CrossRef]
- Stice, E.; Marti, C.N.; Durant, S. Risk Factors for Onset of Eating Disorders: Evidence of Multiple Risk Pathways from an 8-Year Prospective Study. Behav. Res. 2011, 49, 622–627. [Google Scholar] [CrossRef]
- Ford, E.S.; Zhao, G.; Tsai, J.; Li, C. Low-Risk Lifestyle Behaviors and All-Cause Mortality: Findings From the National Health and Nutrition Examination Survey III Mortality Study. Am. J. Public Health 2011, 101, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Ferguson, A.V. Neurophysiology of hunger and satiety. Dev. Disabil. Res. Rev. 2008, 14, 96–104. [Google Scholar] [CrossRef]
- Williams, K.W.; Elmquist, J.K. From neuroanatomy to behavior: Central integration of peripheral signals regulating feeding behavior. Nat. Neurosci. 2012, 15, 1350–1355. [Google Scholar] [CrossRef]
- Delhanty, P.P.J.D.; Neggers, B.S.J.C.M.M.; van der Lely, A.-J.A.-J. Ghrelin: The differences between acyl- and des-acyl ghrelin. Eur. J. Endocrinol. 2012, 167, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Littman, D.R.; Pamer, E.G. Role of the Commensal Microbiota in Normal and Pathogenic Host Immune Responses. Cell Host Microbe 2011, 10, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, W. Food elimination based on IgG antibodies in irritable bowel syndrome: A randomised controlled trial. Gut 2004, 53, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Uhr, T. Compartmentalization of the Mucosal Immune Responses to Commensal Intestinal Bacteria. Ann. N. Y. Acad. Sci. 2004, 1029, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, S.; Irwin, J.; Mackay, I.R.; Marsh, S.; Cowling, D.C. Autoantibodies in Healthy Subjects. Australas. Ann. Med. 1969, 18, 130–134. [Google Scholar] [CrossRef]
- Thompson, G.R.; Trexler, P.C. Gastrointestinal structure and function in germ-free or gnotobiotic animals. Gut 1971, 12, 230–235. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Hallman, J.; Oreland, L.; af Klinteberg, B.; Grenback, E.; Hulting, A.-L.; Hokfelt, T. Autoantibodies against -MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients. Proc. Natl. Acad. Sci. USA 2002, 99, 17155–17160. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Hamze Sinno, M.; Coquerel, Q.; Do Rego, J.C.; Coëffier, M.; Gilbert, D.; Hökfelt, T.; Déchelotte, P. Emerging role of autoantibodies against appetite-regulating neuropeptides in eating disorders. Nutrition 2008, 24, 854–859. [Google Scholar] [CrossRef]
- Tennoune, N.; Chan, P.; Breton, J.; Legrand, R.; Chabane, Y.N.; Akkermann, K.; Järv, A.; Ouelaa, W.; Takagi, K.; Ghouzali, I.; et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Transl. Psychiatry 2014, 4, e458. [Google Scholar] [CrossRef]
- Breton, J.; Legrand, R.; Akkermann, K.; Järv, A.; Harro, J.; Déchelotte, P.; Fetissov, S.O. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int. J. Eat. Disord. 2016, 49, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Sinno, M.H.; Rego, J.C.D.; Coëffier, M.; Bole-Feysot, C.; Ducrotté, P.; Gilbert, D.; Tron, F.; Costentin, J.; Hökfelt, T.; Déchelotte, P.; et al. Regulation of feeding and anxiety by α-MSH reactive autoantibodies. Psychoneuroendocrinology 2009, 34, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Jésus, P.; Ouelaa, W.; François, M.; Riachy, L.; Guérin, C.; Aziz, M.; Do Rego, J.-C.; Déchelotte, P.; Fetissov, S.O.; Coëffier, M. Alteration of intestinal barrier function during activity-based anorexia in mice. Clin. Nutr. 2014, 33, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.M.; Olmstead, M.P.; Polivy, J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int. J. Eat. Disord. 1983, 2, 15–34. [Google Scholar] [CrossRef]
- Rousseau, A.; Knotter, R.-M.; Barbe, R.-M.; Raich, R.-M.; Chabrol, H. Étude de validation de la version française du Body Shape Questionnaire. L’Encéphale 2005, 31, 162–173. [Google Scholar] [CrossRef]
- Mykletun, A.; Stordal, E.; Dahl, A.A. Hospital Anxiety and Depression (HAD) scale: Factor structure, item analyses and internal consistency in a large population. Br. J. Psychiatry 2001, 179, 540–544. [Google Scholar] [CrossRef]
- Walsh, B.T.; Sysko, R. Broad Categories for the Diagnosis of Eating Disorders (BCD-ED): An Alternative System for Classification. Int. J. Eat. Disord. 2009, 42, 754–764. [Google Scholar] [CrossRef]
- Chelikani, P.K.; Haver, A.C.; Reidelberger, R.D. Comparison of the inhibitory effects of PYY(3-36) and PYY(1-36) on gastric emptying in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R1064–R1070. [Google Scholar] [CrossRef]
- Dailey, M.J.; Moran, T.H. Glucagon-like peptide 1 and appetite. Trends Endocrinol. Metab. 2013, 24, 85–91. [Google Scholar] [CrossRef]
- Fetissov, S.O. Neuropeptide Autoantibodies Assay. In Neuropeptides; Merighi, A., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 789, pp. 295–302. ISBN 978-1-61779-309-7. [Google Scholar]
- Andreeva, V.A.; Tavolacci, M.-P.; Galan, P.; Ladner, J.; Buscail, C.; Péneau, S.; Galmiche, M.; Hercberg, S.; Déchelotte, P.; Julia, C. Sociodemographic correlates of eating disorder subtypes among men and women in France, with a focus on age. J. Epidemiol. Community Health 2019, 73, 56–64. [Google Scholar] [CrossRef]
- Volpe, U.; Tortorella, A.; Manchia, M.; Monteleone, A.M.; Albert, U.; Monteleone, P. Eating disorders: What age at onset? Psychiatry Res. 2016, 238, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Blood-Brain Barrier as a Regulatory Interface. Front. Eat. Weight Regul. 2010, 63, 102–110. [Google Scholar]
- Meier, U.; Gressner, A.M. Endocrine Regulation of Energy Metabolism: Review of Pathobiochemical and Clinical Chemical Aspects of Leptin, Ghrelin, Adiponectin, and Resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Caudwell, P.; Finlayson, G.; Webb, D.-L.; Hellström, P.M.; Näslund, E.; Blundell, J.E. Comparison of Postprandial Profiles of Ghrelin, Active GLP-1, and Total PYY to Meals Varying in Fat and Carbohydrate and Their Association With Hunger and the Phases of Satiety. J. Clin. Endocrinol. Metab. 2013, 98, E847–E855. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef]
- Williams, D.L.; Baskin, D.G.; Schwartz, M.W. Evidence that Intestinal Glucagon-Like Peptide-1 Plays a Physiological Role in Satiety. Endocrinology 2009, 150, 1680–1687. [Google Scholar] [CrossRef]
- Steinert, R.E.; Beglinger, C.; Langhans, W. Intestinal GLP-1 and satiation: From man to rodents and back. Int. J. Obes. 2016, 40, 198–205. [Google Scholar] [CrossRef]
- Culbert, K.M.; Racine, S.E.; Klump, K.L. Hormonal Factors and Disturbances in Eating Disorders. Curr. Psychiatry Rep. 2016, 18, 65. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Bruning, J.C. Role of Brain Insulin Receptor in Control of Body Weight and Reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef]
- Obici, S.; Feng, Z.; Karkanias, G.; Baskin, D.G.; Rossetti, L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002, 5, 566–572. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Veit, R.; Scheffler, K.; Machann, J.; Häring, H.-U.; Fritsche, A.; Preissl, H. Selective Insulin Resistance in Homeostatic and Cognitive Control Brain Areas in Overweight and Obese Adults. Diabetes Care 2015, 38, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Galusca, B.; Prévost, G.; Germain, N.; Dubuc, I.; Ling, Y.; Anouar, Y.; Estour, B.; Chartrel, N. Neuropeptide Y and α-MSH Circadian Levels in Two Populations with Low Body Weight: Anorexia Nervosa and Constitutional Thinness. PLoS ONE 2015, 10, e0122040. [Google Scholar] [CrossRef] [PubMed]
- Moriya, J.; Takimoto, Y.; Yoshiuchi, K.; Shimosawa, T.; Akabayashi, A. Plasma agouti-related protein levels in women with anorexia nervosa. Psychoneuroendocrinology 2006, 31, 1057–1061. [Google Scholar] [CrossRef]
- Nam, S.-Y.; Kratzsch, J.; Wook Kim, K.; Rae Kim, K.; Lim, S.-K.; Marcus, C. Cerebrospinal Fluid and Plasma Concentrations of Leptin, NPY, andα -MSH in Obese Women and Their Relationship to Negative Energy Balance. J. Clin. Endocrinol. Metab. 2001, 86, 4849–4853. [Google Scholar] [CrossRef] [PubMed]
- Coquerel, Q.; Sinno, M.H.; Boukhettala, N.; Coëffier, M.; Terashi, M.; Bole-Feysot, C.; Breuillé, D.; Déchelotte, P.; Fetissov, S.O. Intestinal inflammation influences α-MSH reactive autoantibodies: Relevance to food intake and body weight. Psychoneuroendocrinology 2012, 37, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Lucas, N.; Legrand, R.; Bôle-Feysot, C.; Breton, J.; Coëffier, M.; Akkermann, K.; Järv, A.; Harro, J.; Déchelotte, P.; Fetissov, S.O. Immunoglobulin G modulation of the melanocortin 4 receptor signaling in obesity and eating disorders. Transl. Psychiatry 2019, 9, 87. [Google Scholar] [CrossRef]
- Al Massadi, O.; Tschöp, M.H.; Tong, J. Ghrelin acylation and metabolic control. Peptides 2011, 32, 2301–2308. [Google Scholar] [CrossRef]
- Kirchner, H.; Gutierrez, J.A.; Solenberg, P.J.; Pfluger, P.T.; Czyzyk, T.A.; Willency, J.A.; Schürmann, A.; Joost, H.-G.; Jandacek, R.J.; Hale, J.E.; et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat. Med. 2009, 15, 741–745. [Google Scholar] [CrossRef]
- Nishi, Y.; Hiejima, H.; Hosoda, H.; Kaiya, H.; Mori, K.; Fukue, Y.; Yanase, T.; Nawata, H.; Kangawa, K.; Kojima, M. Ingested Medium-Chain Fatty Acids Are Directly Utilized for the Acyl Modification of Ghrelin. Endocrinology 2005, 146, 2255–2264. [Google Scholar] [CrossRef]
- Terashi, M.; Asakawa, A.; Harada, T.; Ushikai, M.; Coquerel, Q.; Sinno, M.H.; Déchelotte, P.; Inui, A.; Fetissov, S.O. Ghrelin reactive autoantibodies in restrictive anorexia nervosa. Nutrition 2011, 27, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Legrand, R.; Asakawa, A.; Amitani, H.; François, M.; Tennoune, N.; Coëffier, M.; Claeyssens, S.; do Rego, J.-C.; Déchelotte, P.; et al. Anti-ghrelin immunoglobulins modulate ghrelin stability and its orexigenic effect in obese mice and humans. Nat. Commun. 2013, 4, 2685. [Google Scholar] [CrossRef] [PubMed]
- Lucas, N.; Legrand, R.; Breton, J.; Déchelotte, P.; Fetissov, S.O. Increased affinity of ghrelin-reactive immunoglobulins in obese Zucker rats. Nutrition 2017, 39–40, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Spinhoven, P.; Ormel, J.; Sloekers, P.P.A.; Kempen, G.I.J.M.; Speckens, A.E.M.; Hemert, A.M.V. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol. Med. 1997, 27, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Nevonen, L.; Clinton, D.; Norring, C. Validating the EDI-2 in three Swedish female samples: Eating disorders patients, psychiatric outpatients and normal controls. Nord. J. Psychiatry 2006, 60, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, A.; Scott, B. Prevalence and psychological correlates of eating disorders among females aged 18–30 years in the general population. Acta Psychiatr. Scand. 1999, 99, 261–266. [Google Scholar] [CrossRef]
- Furnham, A.; Badmin, N.; Sneade, I. Body Image Dissatisfaction: Gender Differences in Eating Attitudes, Self-Esteem, and Reasons for Exercise. J. Psychol. 2002, 136, 581–596. [Google Scholar] [CrossRef]
- Paxton, S.J.; Neumark-Sztainer, D.; Hannan, P.J.; Eisenberg, M.E. Body Dissatisfaction Prospectively Predicts Depressive Mood and Low Self-Esteem in Adolescent Girls and Boys. J. Clin. Child Adolesc. Psychol. 2006, 35, 539–549. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut–brain axis in 2016: Brain–gut–microbiota axis—Mood, metabolism and behaviour. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 69–70. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Cella, S.G.; Bonomo, S.M.; Mancia, G.; Grassi, G.; Perotti, M.; Agosti, F.; Sartorio, A.; Müller, E.E.; Pincelli, A.I. Effect of somatostatin infusion on peptide YY secretion: Studies in the acute and recovery phase of anorexia nervosa and in obesity. Eur. J. Endocrinol. 2011, 165, 421–427. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Sartorio, A.; Scognamiglio, P.; Bini, S.; Monteleone, A.M.; Mastromo, D.; Marazzi, N.; Cella, S.G.; Monteleone, P. Different Effects of Cholestyramine on Postprandial Secretions of Cholecystokinin and Peptide YY in Women with Bulimia Nervosa. NPS 2014, 70, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Martiadis, V.; Rigamonti, A.E.; Fabrazzo, M.; Giordani, C.; Muller, E.E.; Maj, M. Investigation of peptide YY and ghrelin responses to a test meal in bulimia nervosa. Biol. Psychiatry 2005, 57, 926–931. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Restrictive | Bulimic | Compulsive | p-Value |
|---|---|---|---|---|
| Men/Women | 2/33 | 1/11 | 14/59 | 0.98 |
| BMI (kg/m2) | 16.4 ± 2 | 23.2 ± 6 | 38.1 ± 6.8 | <0.001 |
| Age (Years) | 29.4 ± 11.2 | 36.0 ± 16.5 | 39.4 ± 12.3 | <0.001 |
| EDI-2 | 77.5 ± 41.8 | 85.3 ± 29.2 | 90.4 ± 34.1 | 0.18 |
| Drive for thinness | 9.1 ± 6.9 | 12.1 ± 5.2 | 10.2 ± 5.1 | 0.28 |
| Bulimia | 2.1 ± 4.4 | 8.8 ± 5.2 | 5.6 ± 5.9 | <0.001 |
| Body dissatisfaction | 11.1 ± 7.0 | 13.9 ± 7.0 | 20.3 ± 7.4 | <0.001 |
| Ineffectiveness | 9.8 ± 7.3 | 8.5 ± 6.8 | 9.6 ± 7.4 | 0.92 |
| Perfectionism | 6.7 ± 4.6 | 7.0 ± 4.7 | 5.3 ± 4.1 | 0.23 |
| Interpersonal distrust | 5.7 ± 4.3 | 4.8 ± 3.5 | 5.0 ± 3.7 | 0.83 |
| Interoceptive awareness | 8.2 ± 6.8 | 9.3 ± 4.5 | 8.3 ± 6.6 | 0.59 |
| Maturity fears | 6.3 ± 5.1 | 4.0 ± 3.8 | 5.8 ± 4.3 | 0.26 |
| Asceticism | 5.3 ± 3.3 | 5.9 ± 2.9 | 5.5 ± 3.3 | 0.65 |
| Impulse Regulation | 5.5 ± 6.2 | 5.8 ± 4.6 | 7.2 ± 4.6 | 0.04 |
| Social Insecurity | 7.8 ± 3.4 | 5.1 ± 3.1 | 7.0 ± 4.0 | 0.10 |
| BSQ | 74.6 ± 34.0 | 96.2 ± 28.9 | 101.4 ± 24.3 | <0.01 |
| Using laxatives and vomiting in order to reduce body dissatisfaction | 5.9 ± 3.9 | 7.7 ± 3.1 | 5.3 ± 2.0 | 0.03 |
| Unsuited cognitions and behaviors in order to control the weight | 16.0 ± 7.1 | 21.5 ± 6.6 | 19.0 ± 4.7 | 0.01 |
| Body dissatisfaction compared to the lower parts of the body | 32.7 ± 16.9 | 43.1 ± 13.1 | 47.2 ± 12.2 | <0.01 |
| Social avoidance and shame of the exposure of the body | 19.9 ± 8.2 | 23.9 ± 10.4 | 30.0 ± 8.6 | <0.001 |
| HAD | ||||
| Anxiety | 11.3 ± 4.6 | 12.3 ± 4.3 | 10.5 ± 4.6 | 0.17 |
| Proven anxiety (Score > 11) | 68% | 81% | 49% | |
| Depression | 8.4 ± 4.2 | 7.9 ± 4.1 | 8.1 ± 4.3 | 0.79 |
| Proven depression (Score > 11) | 29% | 72% | 31% |
| Peptides Conc. | Restrictive vs. Compulsive * Unadjusted Models | p ** | Restrictive vs. Compulsive * Adjusted Models 1 † | p ** | Restrictive vs. Compulsive * Adjusted Models 2 ‡ | p ** |
|---|---|---|---|---|---|---|
| Leptin | −51.3 [−58 to −44.5] | <0.0001 | −51.8 [−59.1 to −44.5] | <0.0001 | −11.1 [−22.3 to 0] | 1.00 |
| Insulin | −39.1 [−48.5 to −29.8] | <0.0001 | −35.8 [−45.7 to −25.9] | <0.0001 | −19.8 [−38.9 to −0.8] | 0.96 |
| GLP-1 | −17.6 [−28.7 to −6.5] | 0.05 | −17.8 [−29.7 to −5.8] | 0.09 | −10.3 [−33.6 to 13] | 1.00 |
| PYY | −15.9 [−27.3 to −4.5] | 0.15 | −18.1 [−30.4 to −5.8] | 0.10 | −21.6 [−45.6 to 2.4] | 1.00 |
| α-MSH | 10.8 [−0.8 to 22.5] | 1.00 | 16.2 [3.9 to 28.5] | 0.23 | 24.2 [0.3 to 48.1] | 1.00 |
| Des-acyl ghrelin | 29.2 [18.8 to 39.6] | <0.0001 | 29 [18.2 to 39.8] | <0.0001 | 14.4 [−6.4 to 35.2] | 1.00 |
| Acyl ghrelin | 25.7 [14.9 to 36.5] | 0.0002 | 25.7 [14.3 to 37.1] | 0.0004 | 14.6 [−7.4 to 36.7] | 1.00 |
| Plasma Anti-Peptide IgG/ Kd | Restrictive vs. Compulsive * Unadjusted Models | p ** | Restrictive vs Compulsive Adjusted Models 1 † | p ** | Restrictive vs Compulsive * Adjusted Models 2 ‡ | p ** | |
|---|---|---|---|---|---|---|---|
| Plasma anti-peptide IgG | Anti-leptin | −6.5 [−18.2 to 5.2] | 1.00 | −8.5 [−21.1 to 4.2] | 1.00 | −17.6 [−42.2 to 7] | 1.00 |
| Anti-insulin | 8 [−3.5 to 19.5] | 1.00 | 7.8 [−4.4 to 19.9] | 1.00 | 0.2 [−23.4 to 23.9] | 1.00 | |
| Anti-GLP-1 | 10.1 [−1.3 to 21.5] | 1.00 | 10.1 [−2 to 22.2] | 1.00 | −0.7 [−24.2 to 22.9] | 1.00 | |
| Anti-PYY | 5.9 [−5.6 to 17.5] | 1.00 | 3.5 [−8.9 to 15.8] | 1.00 | −5.9 [−30 to 18.1] | 1.00 | |
| Anti-α-MSH | 4.3 [−7.3 to 15.9] | 1.00 | −0.7 [−12.8 to 11.5] | 1.00 | −1.1 [−24.9 to 22.7] | 1.00 | |
| Anti-Acyl ghrelin | 4.1 [−7.5 to 15.7] | 1.00 | −0.2 [−12.5 to 12.2] | 1.00 | −12.9 [−37 to 11.1] | 1.00 | |
| Anti-Des-acyl ghrelin | −9.3 [−21 to 2.3] | 1.00 | −10 [−22.6 to 2.6] | 1.00 | −5.2 [−29.7 to 19.3] | 1.00 | |
| Anti-ClpB | 4.9 [−6.6 to 16.4] | 1.00 | 6.8 [−5.6 to 19.1] | 1.00 | 5.1 [−19 to 29.1] | 1.00 | |
| Kd of plasma IgG | Anti-leptin | −5.5 [−17.2 to 6.3] | 1.00 | −6.5 [−19.2 to 6.2] | 1.00 | −18.3 [−43 to 6.4] | 1.00 |
| Anti-insulin | −6 [−17.7 to 5.8] | 1.00 | −6.9 [−19.6 to 5.7] | 1.00 | −11.4 [−36.2 to 13.4] | 1.00 | |
| Anti-GLP1 | −5.4 [−17.1 to 6.3] | 1.00 | −4.2 [−16.9 to 8.5] | 1.00 | −9 [−33.8 to 15.7] | 1.00 | |
| Anti-PYY | −5.6 [−17.3 to 6.2] | 1.00 | −4.9 [−17.5 to 7.8] | 1.00 | −7.5 [−32.2 to 17.2] | 1.00 | |
| Anti-α-MSH | 0.5 [−11.2 to 12.3] | 1.00 | 0.9 [−11.7 to 13.5] | 1.00 | 9.3 [−15.2 to 33.9] | 1.00 | |
| Anti-Acyl ghrelin | 13.9 [2.4 to 25.5] | 0.42 | 13.1 [0.6 to 25.5] | 0.92 | 16.7 [−7.7 to 41] | 1.00 | |
| Anti-des-acyl ghrelin | −3.6 [−15.3 to 8.2] | 1.00 | −4.7 [−17.3 to 8] | 1.00 | −6.1 [−30.8 to 18.7] | 1.00 | |
| Anti-ClpB | 15.8 [4.3 to 27.2] | 0.17 | 16.4 [4 to 28.8] | 0.23 | 0.2 [−23.7 to 24.1] | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galmiche, M.; Lucas, N.; Déchelotte, P.; Deroissart, C.; Le Solliec, M.-A.; Rondeaux, J.; Azhar, S.; Grigioni, S.; Colange, G.; Delay, J.; et al. Plasma Peptide Concentrations and Peptide-Reactive Immunoglobulins in Patients with Eating Disorders at Inclusion in the French EDILS Cohort (Eating Disorders Inventory and Longitudinal Survey). Nutrients 2020, 12, 522. https://doi.org/10.3390/nu12020522
Galmiche M, Lucas N, Déchelotte P, Deroissart C, Le Solliec M-A, Rondeaux J, Azhar S, Grigioni S, Colange G, Delay J, et al. Plasma Peptide Concentrations and Peptide-Reactive Immunoglobulins in Patients with Eating Disorders at Inclusion in the French EDILS Cohort (Eating Disorders Inventory and Longitudinal Survey). Nutrients. 2020; 12(2):522. https://doi.org/10.3390/nu12020522
Chicago/Turabian StyleGalmiche, Marie, Nicolas Lucas, Pierre Déchelotte, Camille Deroissart, Marie-Anne Le Solliec, Julie Rondeaux, Saida Azhar, Sébastien Grigioni, Guillaume Colange, Julie Delay, and et al. 2020. "Plasma Peptide Concentrations and Peptide-Reactive Immunoglobulins in Patients with Eating Disorders at Inclusion in the French EDILS Cohort (Eating Disorders Inventory and Longitudinal Survey)" Nutrients 12, no. 2: 522. https://doi.org/10.3390/nu12020522
APA StyleGalmiche, M., Lucas, N., Déchelotte, P., Deroissart, C., Le Solliec, M.-A., Rondeaux, J., Azhar, S., Grigioni, S., Colange, G., Delay, J., Achamrah, N., Folope, V., Belmonte, L., Lamarre, A., Rimbert, A., Saillard, T., Petit, A., Quillard, M., Coeffier, M., ... Tavolacci, M.-P. (2020). Plasma Peptide Concentrations and Peptide-Reactive Immunoglobulins in Patients with Eating Disorders at Inclusion in the French EDILS Cohort (Eating Disorders Inventory and Longitudinal Survey). Nutrients, 12(2), 522. https://doi.org/10.3390/nu12020522






