Nutrition in Sepsis: A Bench-to-Bedside Review
Abstract
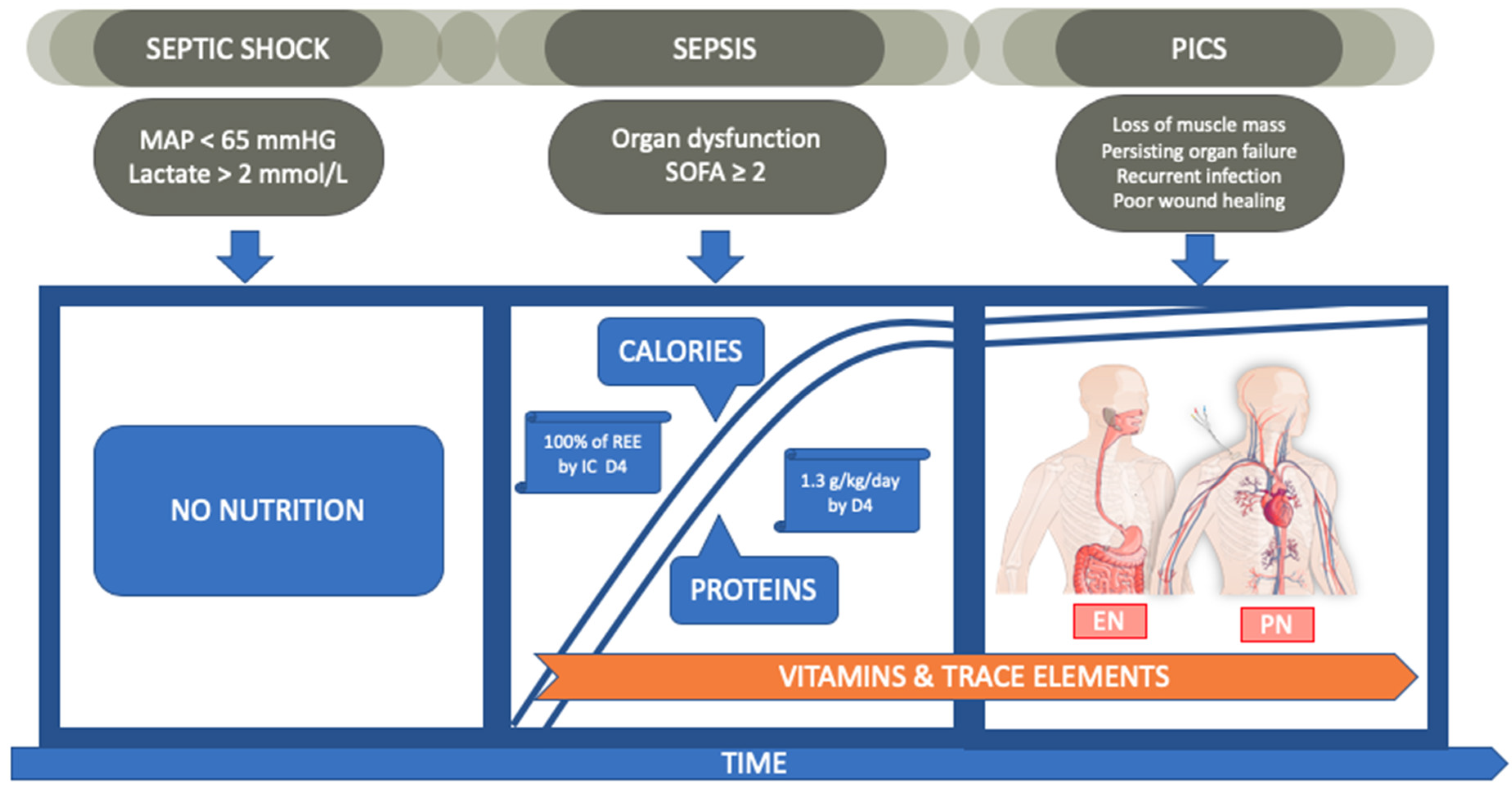
1. Introduction
1.1. Pathophysiology of Sepsis: A Two-Faced Immune-Inflammatory State
1.2. Calories and Proteins
1.2.1. Calories
- Measurement of REE with IC.
- A slow but steady increase of caloric load to reach target values when shock is resolved.
1.2.2. Proteins
- We recommend administration of 0.8 g/kg/day during sepsis and a gradual increase up to 1.3 g/kg/day when shock resolves.
1.2.3. Glucose
1.2.4. Lipids
1.3. Enteral vs. Parenteral Feeding
- EN has no proven clinical benefit over PN and is associated with more digestive disorders. However, when the gut works, we recommend using it.
- Early EN in combination with supplemental PN is the preferred procedure to reach at least 80% of caloric needs by day 3.
1.4. Pharmaco-Nutrition
1.4.1. Glutamine
- Glutamine administration has no benefit and may even be harmful in septic patients.
1.4.2. Arginine
- Lack of firm evidence argues against arginine supplementation in sepsis and septic shock.
1.4.3. Omega-3 Fatty Acids
- The scientific evidence to justify fish oil supplementation in patients with sepsis or septic shock is weak.
- Administering ω-3 PUFAs might improve gas exchange and subsequent weaning from mechanical ventilation in ARDS patients, but it remains unclear whether this therapeutic effect depends on the feeding route (enteral vs. parenteral), method (bolus vs. continuous infusion), or composition (lipid type and amount).
1.4.4. Selenium
- Selenium alone or in combination with other antioxidants is not recommended in sepsis.
1.4.5. Vitamin C
- At present, insufficient evidence supports routine vitamin C administration in sepsis.
1.5. Nutrition Stewardship
1.5.1. Diagnosis
1.5.2. Drug
1.5.3. Dose
1.5.4. Duration
1.5.5. De-escalation
1.5.6. Discharge
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Honore, P.M.; Hoste, E.; Molnár, Z.; Jacobs, R.; Joannes-Boyau, O.; Malbrain, M.L.N.G.; Forni, L.G. Cytokine removal in human septic shock: Where are we and where are we going? Ann. Intensive Care 2019, 9, 56. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.-J.; Joannes-Boyau, O.; Teboul, J.-L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care 2018, 8, 66. [Google Scholar] [CrossRef]
- Blaser, A.R.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intens. Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- De Waele, E.; Opsomer, T.; Honoré, P.M.; Diltoer, M.; Mattens, S.; Huyghens, L.; Spapen, H. Measured versus calculated resting energy expenditure in critically ill adult patients. Do mathematics match the gold standard? Minerva Anestesiol. 2015, 81, 272–282. [Google Scholar]
- Zusman, O.; Kagan, I.; Bendavid, I.; Theilla, M.; Cohen, J.; Singer, P. Predictive equations versus measured energy expenditure by indirect calorimetry: A retrospective validation. Clin. Nutr. 2019, 38, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Bendavid, I.; Singer, P.; Theilla, M.; Themessl-Huber, M.; Sulz, I.; Mouhieddine, M.; Schuh, C.; Mora, B.; Hiesmayr, M. NutritionDay ICU: A 7 year worldwide prevalence study of nutrition practice in intensive care. Clin. Nutr. 2017, 36, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Berger, M.M.; De Waele, E.; Guttormsen, A.B.; Heidegger, C.-P.; Hiesmayr, M.; Singer, P.; Wernerman, J.; Pichard, C. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin. Nutr. 2017, 36, 651–662. [Google Scholar] [CrossRef]
- Berger, M.M.; Pichard, C. Feeding should be individualized in the critically ill patients. Curr. Opin. Crit. Care 2019, 25, 307–313. [Google Scholar] [CrossRef]
- Wischmeyer, P.E. Nutrition Therapy in Sepsis. Crit. Care Clin. 2018, 34, 107–125. [Google Scholar] [CrossRef]
- Coudenys, E.; De Waele, E.; Meers, G.; Collier, H.; Pen, J.J. Inadequate glycemic control in patients receiving parenteral nutrition lowers survival: A retrospective observational trial. Clin. Nutr. Exp. 2018, 17, 1–7. [Google Scholar] [CrossRef]
- Uehara, M.; Plank, L.D.; Hill, G.L. Components of energy expenditure in patients with severe sepsis and major trauma: A basis for clinical care. Crit. Care Med. 1999, 27, 1295–1302. [Google Scholar] [CrossRef]
- Kao, C.C.; Guntupalli, K.K.; Bandi, V.; Jahoor, F. WHOLE-BODY CO2 PRODUCTION AS AN INDEX OF THE METABOLIC RESPONSE TO SEPSIS. Shock 2009, 32, 23–28. [Google Scholar] [CrossRef]
- Hung, K.-Y.; Chen, Y.-M.; Wang, C.-C.; Wang, Y.-H.; Lin, C.-Y.; Chang, Y.-T.; Huang, K.-T.; Lin, M.-C.; Fang, W.-F. Insufficient Nutrition and Mortality Risk in Septic Patients Admitted to ICU with a Focus on Immune Dysfunction. Nutrients 2019, 11, 367. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: The development and initial validation of a novel risk assessment tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 367. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.; Peake, S.L.; Bellomo, R.; Davies, A.; Deane, A.; Horowitz, M.; Hurford, S.; Lange, K.; Little, L.; Mackle, D.; et al. Energy-Dense versus Routine Enteral Nutrition in the Critically Ill. New Eng. J. Med. 2018, 379, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.-C. High protein intake during the early phase of critical illness: Yes or no? Crit. Care 2018, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.; Looijaard, W.G.; Beishuizen, A.; Girbes, A.R.; Oudemans-van Straaten, H.M. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit. Care 2014, 18, 701. [Google Scholar] [CrossRef]
- Bendavid, I.; Zusman, O.; Kagan, I.; Theilla, M.; Cohen, J.; Singer, P. Early Administration of Protein in Critically Ill Patients: A Retrospective Cohort Study. Nutrients 2019, 11, 106. [Google Scholar] [CrossRef]
- Weijs, P.J.M.; Mogensen, K.M.; Rawn, J.D.; Christopher, K.B. Protein Intake, Nutritional Status and Outcomes in ICU Survivors: A Single Center Cohort Study. J. Clin. Med. 2019, 8, 43. [Google Scholar] [CrossRef]
- Koekkoek, W.A.C.K.; van Setten, C.H.C.; Olthof, L.E.; Kars, J.C.N.H.; van Zanten, A.R.H. Timing of PROTein INtake and clinical outcomes of adult critically ill patients on prolonged mechanical VENTilation: The PROTINVENT retrospective study. Clin. Nutr. 2019, 38, 883–890. [Google Scholar] [CrossRef]
- Patel, J.J.; Rice, T.; Compher, C.; Heyland, D.K. Do We Have Clinical Equipoise (or Uncertainty) About How Much Protein to Provide to Critically Ill Patients? Nutr. Clin. Pract. 2019. [Google Scholar] [CrossRef]
- Gunst, J.; De Bruyn, A.; Van den Berghe, G. Glucose control in the ICU. Curr. Opin. in Anaesth. 2019, 32, 156–162. [Google Scholar] [CrossRef]
- Mueller, C.M.; American Society of Parenteral and Enteral Nutrition (Eds.) The ASPEN Adult Nutrition Support Core Curriculum, 2nd ed.; Silver Spring: Berlin, Germany, 2012. [Google Scholar]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesnayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Seres, D.S.; Valcarcel, M.; Guillaume, A. Advantages of enteral nutrition over parenteral nutrition. Ther. Adv. Gastroenter. 2013, 6, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Mancl, E.E.; Muzevich, K.M. Tolerability and safety of enteral nutrition in critically ill patients receiving intravenous vasopressor therapy. JPEN-Parenter. Enter. 2013, 37, 641–651. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Rice, T.W.; Wheeler, A.P.; Thompson, B.T.; Steingrub, J.; Hite, R.D.; Moss, M.; Morris, A.; Dong, N.; Rock, P. Initial trophic vs full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA 2012, 307, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Heighes, P.T.; Allingstrup, M.J.; Doig, G.S. Early Enteral Nutrition Provided Within 24 Hours of ICU Admission: A Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2018, 46, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Cromphaut, S.V.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus Late Parenteral Nutrition in Critically Ill Adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Wilmer, A.; Hermans, G.; Wouters, P.J.; Mesotten, D.; Van den Berghe, G. Role of Disease and Macronutrient Dose in the Randomized Controlled EPaNIC Trial. Am. J. Respir. Crit. Care Med. 2013, 187, 247–255. [Google Scholar] [CrossRef]
- De Waele, E.; Spapen, H.; Honoré, P.M.; Mattens, S.; Van Gorp, V.; Diltoer, M.; Huyghens, L. Introducing a new generation indirect calorimeter for estimating energy requirements in adult intensive care unit patients: Feasibility, practical considerations, and comparison with a mathematical equation. J. Crit. Care 2013, 28, 884.e1–884.e6. [Google Scholar] [CrossRef]
- Lewis, S.R.; Schofield-Robinson, O.J.; Alderson, P.; Smith, A.F. Enteral versus parenteral nutrition and enteral versus a combination of enteral and parenteral nutrition for adults in the intensive care unit. Cochrane Db. Syst. Rev. 2018, 6, CD012276. [Google Scholar] [CrossRef]
- Harvey, S.E.; Parrott, F.; Harrison, D.A.; Bear, D.E.; Segaran, E.; Beale, R.; Bellingan, G.; Leonard, R.; Mythen, M.G.; Rowan, K.M.; et al. Trial of the Route of Early Nutritional Support in Critically Ill Adults. New Engl. J. Med. 2014, 371, 1673–1684. [Google Scholar] [CrossRef]
- Reignier, J.; Boisramé-Helms, J.; Brisard, L.; Lascarrou, J.-B.; Ait Hssain, A.; Anguel, N.; Argaud, L.; Asehnoune, K.; Asfar, P.; Bellec, F.; et al. Enteral versus parenteral early nutrition in ventilated adults with shock: A randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018, 391, 133–143. [Google Scholar] [CrossRef]
- Pierre, J.F.; Heneghan, A.F.; Lawson, C.M.; Wischmeyer, P.E.; Kozar, R.A.; Kudsk, K.A. Pharmaconutrition Review. JPEN Parenter. Enter. 2013, 37, 51S–65S. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.S.; Martindale, R.G. Immunonutrition in Critical Illness: What Is the Role? Nutr. Clin. Pract. 2018, 33, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E. Glutamine: Mode of action in critical illness. Crit. Care Med. 2007, 35, S541–S544. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Andrews, P.J.D.; Avenell, A.; Noble, D.W.; Campbell, M.K.; Croal, B.L.; Simpson, W.G.; Vale, L.D.; Battison, C.G.; Jenkinson, D.; Cook, J.A.; et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011, 342, d1542. [Google Scholar] [CrossRef]
- Wernerman, J.; Kirketeig, T.; Andersson, B.; Berthelson, H.; Ersson, A.; Friberg, H.; Guttormsen, A.B.; Hendrikx, S.; Pettilä, V.; Rossi, P.; et al. Scandinavian glutamine trial: A pragmatic multi-centre randomised clinical trial of intensive care unit patients. Acta Anaesth. Scand. 2011, 55, 812–818. [Google Scholar] [CrossRef]
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G. Canadian Critical Care Trials Group. A Randomized Trial of Glutamine and Antioxidants in Critically Ill Patients. New Engl. J. Med. 2013, 368, 1489–1497. [Google Scholar] [CrossRef]
- Heyland, D.K.; Elke, G.; Cook, D.; Berger, M.M.; Wischmeyer, P.E.; Albert, M.; Muscedere, J.; Jones, G.; Day, A.G.; Canadian Critical Care Trials Group. Glutamine and Antioxidants in the Critically Ill Patient. JPEN Parenter. Enter. 2015, 39, 401–409. [Google Scholar] [CrossRef]
- van Zanten, A.R.H.; Sztark, F.; Kaisers, U.X.; Zielmann, S.; Felbinger, T.W.; Sablotzki, A.R.; De Waele, J.J.; Timsit, J.F.; Honing, M.L.; Keh, D.; et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: A randomized clinical trial. JAMA 2014, 312, 514–524. [Google Scholar] [CrossRef]
- Wischmeyer, P.E. The glutamine debate in surgery and critical care. Curr. Opin. Crit. Care 2019, 25, 322–328. [Google Scholar] [CrossRef]
- Davis, J.S.; Anstey, N.M. Is plasma arginine concentration decreased in patients with sepsis? A systematic review and meta-analysis*. Crit. Care Med. 2011, 39, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Poeze, M.; Ramsay, G.; Deutz, N.E.P. The role of arginine in infection and sepsis. JPEN-Parenter. Enter. 2005, 29, S70–S74. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Poeze, M.; Deutz, N.E. Arginine infusion in patients with septic shock increases nitric oxide production without haemodynamic instability. Clin. Sci. 2015, 128, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Iapichino, G.; Radrizzani, D.; Facchini, R.; Simini, B.; Bruzzone, P.; Zanforlin, G.; Tognoni, G. Early enteral immunonutrition in patients with severe sepsis. Intens. Care Med. 2003, 29, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Sevransky, J.E.; Myers, D.E.; Esposito, C.; Vandivier, R.W.; Eichacker, P.; Susla, G.M.; Solomon, S.B.; Csako, G.; Costello, R.; et al. Preclinical trial of L-arginine monotherapy alone or with N-acetylcysteine in septic shock. Crit. Care Med. 2006, 34, 2719–2728. [Google Scholar] [CrossRef]
- Marik, P.E.; Zaloga, G.P. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intens. Care Med. 2008, 34, 1980–1990. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids, inflammation, and immunity. Lipids 2001, 36, 1007–1024. [Google Scholar] [CrossRef]
- Galbán, C.; Montejo, J.C.; Mesejo, A.; Marco, P.; Celaya, S.; Sánchez-Segura, J.M.; Farré, M.; Bryg, D.J. An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit. Care Med. 2000, 28, 643–648. [Google Scholar] [CrossRef]
- Pontes-Arruda, A.; Demichele, S.; Seth, A.; Singer, P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: A meta-analysis of outcome data. JPEN Parenter. Enter. 2008, 32, 596–605. [Google Scholar] [CrossRef]
- Beale, R.J.; Sherry, T.; Lei, K.; Campbell-Stephen, L.; McCook, J.; Smith, J.; Venetz, M.; Alteheld, B.; Stehle, P.; Schneider, H. Early enteral supplementation with key pharmaconutrients improves Sequential Organ Failure Assessment score in critically ill patients with sepsis: Outcome of a randomized, controlled, double-blind trial. Crit. Care Med. 2008, 36, 131–144. [Google Scholar] [CrossRef]
- Pontes-Arruda, A.; Martins, L.F.; de Lima, S.M.; Isola, A.M.; Toledo, D.; Rezende, E.; Maia, M.; Magnan, G.B.; Investigating Nutritional Therapy with EPA, GLA and Antioxidants Role in Sepsis Treatment (INTERSET) Study Group. Enteral nutrition with eicosapentaenoic acid, γ-linolenic acid and antioxidants in the early treatment of sepsis: Results from a multicenter, prospective, randomized, double-blinded, controlled study: The INTERSEPT study. Crit. Care 2011, 15, R144. [Google Scholar] [CrossRef] [PubMed]
- Hofman, Z.; Swinkels, S.; van Zanten, A.R.H. Glutamine, fish oil and antioxidants in critical illness: MetaPlus trial post hoc safety analysis. Ann. Intensive Care 2016, 6, 119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tao, W.; Li, P.-S.; Shen, Z.; Shu, Y.-S.; Liu, S. Effects of omega-3 fatty acid nutrition on mortality in septic patients: A meta-analysis of randomized controlled trials. BMC Anesthesiol. 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Sharma, S.; McIntyre, L.; Rhodes, A.; Evans, L.; Almenawer, S.; Leduc, L.; Angus, D.C.; Alhazzani, W. Omega-3 supplementation in patients with sepsis: A systematic review and meta-analysis of randomized trials. Ann. Intensive Care 2017, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, S.; Zhao, Y.; Luo, Y.; Tong, H.; Su, L. Correlation analysis of omega-3 fatty acids and mortality of sepsis and sepsis-induced ARDS in adults: Data from previous randomized controlled trials. Nutr. J. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Kristine Koekkoek, W.; Panteleon, V.; van Zanten, A.R. Current evidence on ω-3 fatty acids in enteral nutrition in the critically ill: A systematic review and meta-analysis. Nutrition 2019, 59, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, Y.; Li, S.; Gan, L.; Feng, H.; Nie, W. Enteral omega-3 fatty acid supplementation in adult patients with acute respiratory distress syndrome: A systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Intens. Care Med. 2014, 40, 504–512. [Google Scholar] [CrossRef]
- Santacruz, C.A.; Orbegozo, D.; Vincent, J.-L.; Preiser, J.C. Modulation of Dietary Lipid Composition During Acute Respiratory Distress Syndrome: Systematic Review and Meta-Analysis. JPEN Parenter. Enter. 2015, 39, 837–846. [Google Scholar] [CrossRef]
- Langlois, P.L.; D’Aragon, F.; Hardy, G.; Manzanares, W. Omega-3 polyunsaturated fatty acids in critically ill patients with acute respiratory distress syndrome: A systematic review and meta-analysis. Nutrition 2019, 61, 84–92. [Google Scholar] [CrossRef]
- Forceville, X.; Vitoux, D.; Gauzit, R.; Combes, A.; Lahilaire, P.; Chappuis, P. Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit. Care Med. 1998, 26, 1536–1544. [Google Scholar] [CrossRef]
- Alhazzani, W.; Jacobi, J.; Sindi, A.; Hartog, C.; Reinhart, K.; Kokkoris, S.; Gerlach, H.; Andrews, P.; Drabek, T.; Manzanares, W.; et al. The effect of selenium therapy on mortality in patients with sepsis syndrome: A systematic review and meta-analysis of randomized controlled trials. Crit. Care Med. 2013, 41, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Landucci, F.; Mancinelli, P.; De Gaudio, A.R.; Virgili, G. Selenium supplementation in critically ill patients: A systematic review and meta-analysis. J. Crit. Care 2014, 29, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-S.; Shyu, Y.-C.; Chen, H.-Y.; Lin, L.-M.; Lo, C.-Y.; Yuan, S.-S.; Chen, P.-J. Effect of Parenteral Selenium Supplementation in Critically Ill Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e54431. [Google Scholar] [CrossRef] [PubMed]
- Chelkeba, L.; Ahmadi, A.; Abdollahi, M.; Najafi, A.; Ghadimi, M.H.; Mosaed, R.; Mojtahedzadeh, M. The effect of parenteral selenium on outcomes of mechanically ventilated patients following sepsis: A prospective randomized clinical trial. Ann. Intens. Care 2015, 5, 29. [Google Scholar] [CrossRef]
- Bloos, F.; Trips, E.; Nierhaus, A.; Briegel, J.; Heyland, D.K.; Jaschinski, U.; Moerer, O.; Weyland, A.; Marx, G.; Gründling, M.; et al. Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients With Severe Sepsis or Septic Shock: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1266–1276. [Google Scholar] [CrossRef]
- Li, S.; Tang, T.; Guo, P.; Zou, Q.; Ao, X.; Hu, L.; Tan, L. A meta-analysis of randomized controlled trials. Medicine 2019, 98, e14733. [Google Scholar] [CrossRef]
- Wilson, J.X.; Wu, F. Vitamin C in Sepsis. Sub-cell. Biochem. 2012, 56, 67–83. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of Vitamin C in the Function of the Vascular Endothelium. Antioxid. Redox Sign. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef]
- Zhang, M.; Jativa, D.F. Vitamin C supplementation in the critically ill: A systematic review and meta-analysis. SAGE Open Med. 2018, 6, 205031211880761. [Google Scholar] [CrossRef]
- Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-van Straaten, H.M. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit. Care 2018, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Peeters, Y.; Vandervelden, S.; Wise, R.; Malbrain, M.L.N.G. An overview on fluid resuscitation and resuscitation endpoints in burns: Past, present and future. Part 1 - historical background, resuscitation fluid and adjunctive treatment. Anaesthesiol. Intensive Ther. 2015, 47, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.; Rice, T.W.; Mythen, M.; Wuyts, S. It is time for improved fluid stewardship. ICU Manag. Pract. 2018, 18, 158–162. [Google Scholar]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Blaauw, R.; Coats, A. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Calder, P.; Cury-Boaventura, M.; De Waele, E.; Jakubowski, J.; Zaloga, G. Biological and Clinical Aspects of an Olive Oil-Based Lipid Emulsion—A Review. Nutrients 2018, 10, 776. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, L.; Schneider, S.M.; Stanga, Z.; et al. ESPEN guideline on home enteral nutrition. Clin. Nutr. 2020, 39, 5–22. [Google Scholar] [CrossRef]
| Nutrient | Recommended Dose |
|---|---|
| Caloric needs | Determined by indirect calorimetry |
| Protein | 0.8–1.3 g/kg/day |
| Lipids | 0.7–1.5 g/kg/day |
| Glucose | 1–1.5 g/kg/day |
| Glutamine | <0.35 g/kg/day IV or <0.5 g/kg/day enterally in TPN fed patients |
| Fluid | 1 mL/kg/h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Waele, E.; Malbrain, M.L.N.G.; Spapen, H. Nutrition in Sepsis: A Bench-to-Bedside Review. Nutrients 2020, 12, 395. https://doi.org/10.3390/nu12020395
De Waele E, Malbrain MLNG, Spapen H. Nutrition in Sepsis: A Bench-to-Bedside Review. Nutrients. 2020; 12(2):395. https://doi.org/10.3390/nu12020395
Chicago/Turabian StyleDe Waele, Elisabeth, Manu L.N.G. Malbrain, and Herbert Spapen. 2020. "Nutrition in Sepsis: A Bench-to-Bedside Review" Nutrients 12, no. 2: 395. https://doi.org/10.3390/nu12020395
APA StyleDe Waele, E., Malbrain, M. L. N. G., & Spapen, H. (2020). Nutrition in Sepsis: A Bench-to-Bedside Review. Nutrients, 12(2), 395. https://doi.org/10.3390/nu12020395






