Energy Expenditure and Changes in Body Composition During Submarine Deployment—An Observational Study “DasBoost 2-2017”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Deployment
2.3. Measurements
2.4. Statistics
3. Results
3.1. Subject Characteristics
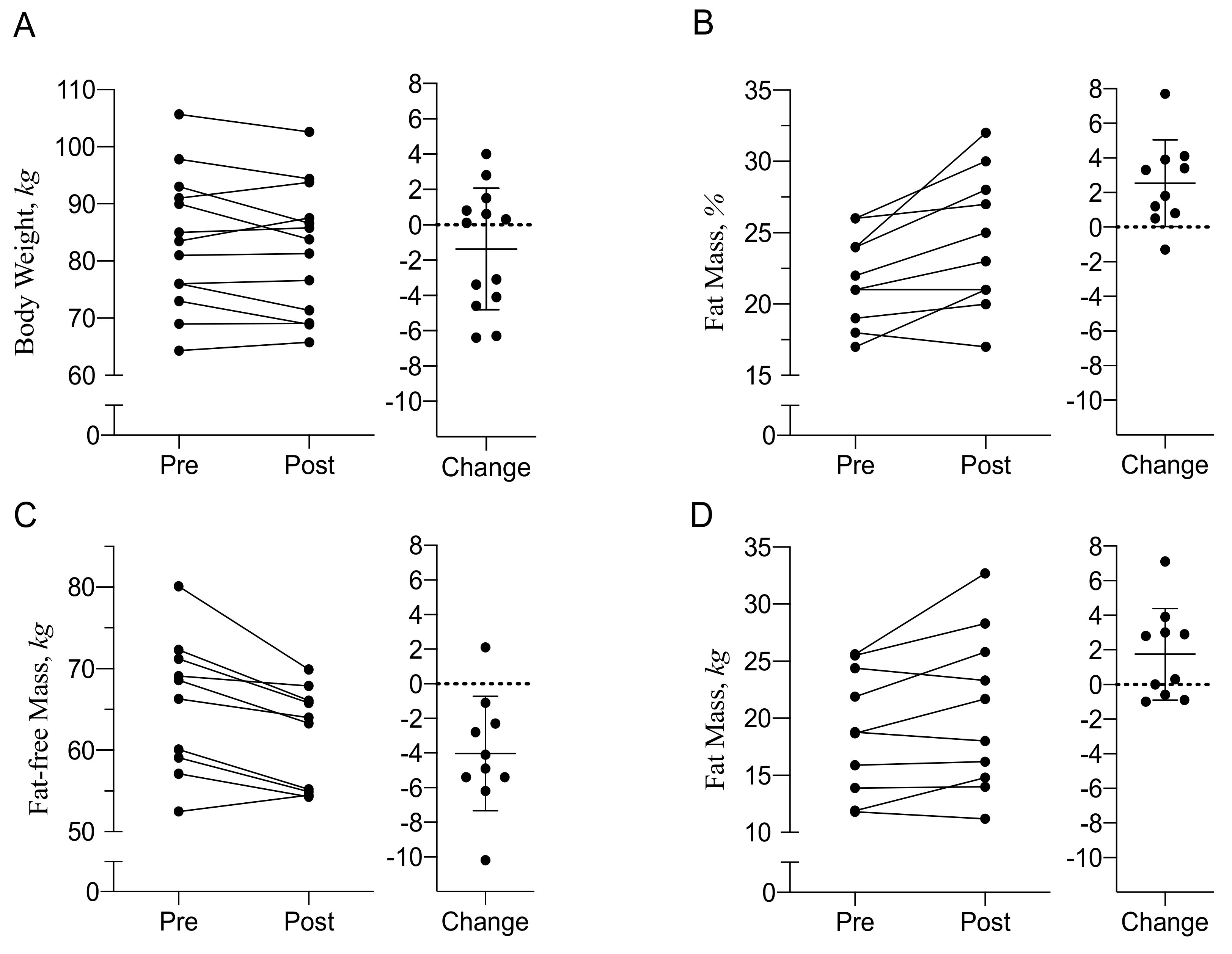
3.2. Body Composition and Energy Balance
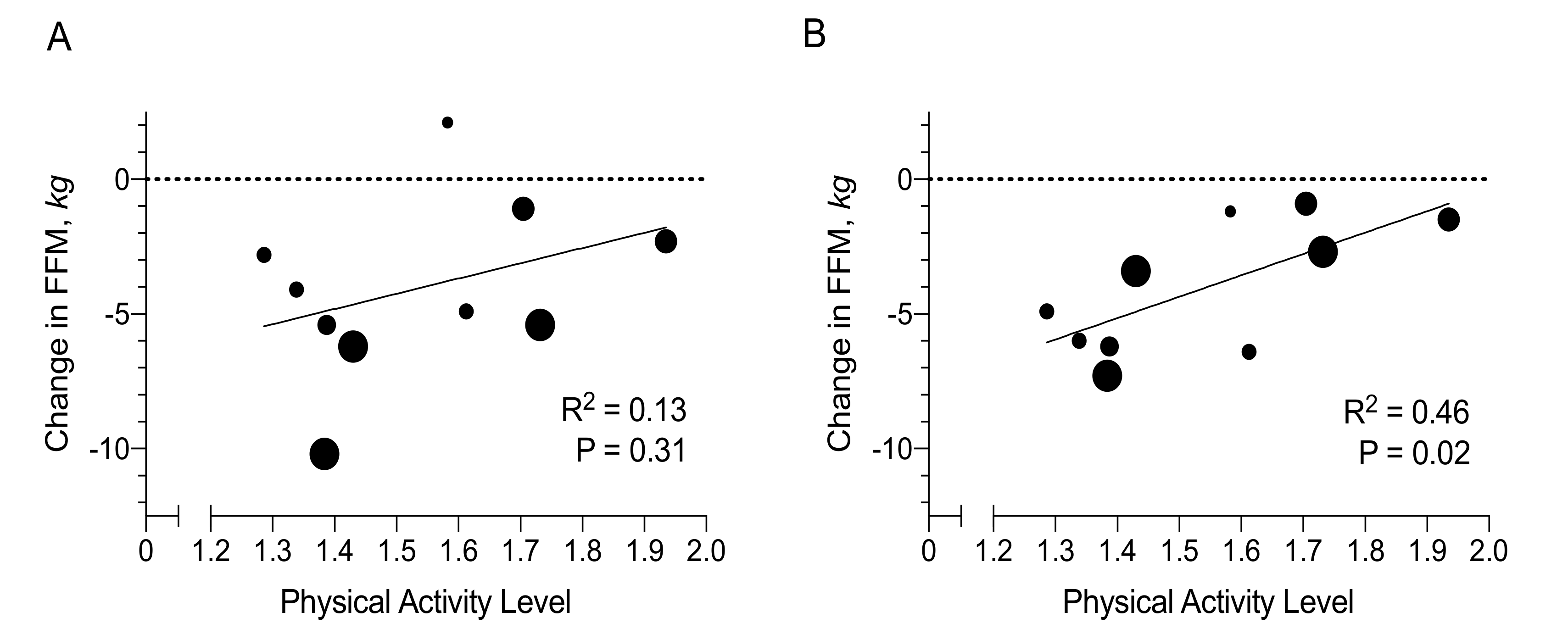
3.3. Determinants of Changes in Fat Mass and Fat-Free Mass
3.4. Cardiometabolic Risk Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wunderlin, S.; Roos, L.; Roth, R.; Faude, O.; Frey, F.; Wyss, T. Trunk muscle strength tests to predict injuries, attrition and military ability in soldiers. J. Sports Med. Phys. Fit. 2015, 55, 535–543. [Google Scholar]
- Knapik, J.J. The Importance of Physical Fitness for Injury Prevention: Part 2. J. Spec. Oper. Med. 2015, 15, 112–115. [Google Scholar] [PubMed]
- Pierce, J.R.; DeGroot, D.W.; Grier, T.L.; Hauret, K.G.; Nindl, B.C.; East, W.B.; McGurk, M.S.; Jones, B.H. Body mass index predicts selected physical fitness attributes but is not associated with performance on military relevant tasks in U.S. Army Soldiers. J. Sci. Med. Sport 2017, 20 (Suppl. 4), S79–S84. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.I.; Greene, N.P.; Dobson, J.P.; Wiggs, M.P.; Gasier, H.G.; Macias, B.R.; Shimkus, K.L.; Fluckey, J.D. Insulin resistance syndrome blunts the mitochondrial anabolic response following resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E466–E474. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E.; Nakayama, A.T.; Guerriere, K.I.; Lutz, L.J.; Walker, L.A.; Staab, J.S.; Scott, J.M.; Gasier, H.G.; McClung, J.P. Calcium and vitamin D supplementation and bone health in Marine recruits: Effect of season. Bone 2019, 123, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Gasier, H.G.; Gaffney-Stomberg, E.; Young, C.R.; McAdams, D.C.; Lutz, L.J.; McClung, J.P. The efficacy of vitamin D supplementation during a prolonged submarine patrol. Calcif. Tissue Int. 2014, 95, 229–239. [Google Scholar] [CrossRef]
- Gasier, H.G.; Young, C.R.; Gaffney-Stomberg, E.; McAdams, D.C.; Lutz, L.J.; McClung, J.P. Cardiometabolic Health in Submariners Returning from a 3-Month Patrol. Nutrients 2016, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Fothergill, D.M.; Sims, J.R. Aerobic performance of Special Operations Forces personnel after a prolonged submarine deployment. Ergonomics 2000, 43, 1489–1500. [Google Scholar] [CrossRef]
- Kang, J.; Song, Y.M. The association between submarine service and multimorbidity: A cross-sectional study of Korean naval personnel. BMJ Open 2017, 7, e017776. [Google Scholar] [CrossRef] [Green Version]
- Church, D.D.; Gwin, J.A.; Wolfe, R.R.; Pasiakos, S.M.; Ferrando, A.A. Mitigation of Muscle Loss in Stressed Physiology: Military Relevance. Nutrients 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Song, Y.M. Metabolic syndrome and its components among Korean submariners: A retrospective cross-sectional study. Endocrine 2018, 59, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, L.A.; Ravussin, E.; Bray, G.A.; Han, H.; Redman, L.M. An objective estimate of energy intake during weight gain using the intake-balance method. Am. J. Clin. Nutr. 2014, 100, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Tataranni, P.A.; Harper, I.T.; Snitker, S.; Del Parigi, A.; Vozarova, B.; Bunt, J.; Bogardus, C.; Ravussin, E. Body weight gain in free-living Pima Indians: Effect of energy intake vs. expenditure. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1578–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. 1961. Nutrition 1993, 9, 480–491. [Google Scholar]
- Anderson, K.M.; Wilson, P.W.; Odell, P.M.; Kannel, W.B. An updated coronary risk profile. A statement for health professionals. Circulation 1991, 83, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Westerterp, K.R.; Wouters, L.; van Marken Lichtenbelt, W.D. The Maastricht protocol for the measurement of body composition and energy expenditure with labeled water. Obes. Res. 1995, 3 (Suppl. 1), 49–57. [Google Scholar] [CrossRef] [Green Version]
- Schoeller, D.A.; Ravussin, E.; Schutz, Y.; Acheson, K.J.; Baertschi, P.; Jequier, E. Energy expenditure by doubly labeled water: Validation in humans and proposed calculation. Am. J. Physiol. 1986, 250, R823–R830. [Google Scholar] [CrossRef]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Black, A.E.; Prentice, A.M.; Coward, W.A. Use of food quotients to predict respiratory quotients for the doubly-labelled water method of measuring energy expenditure. Hum. Nutr. Clin. Nutr. 1986, 40, 381–391. [Google Scholar]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Westerterp, K.R. Physical activity and physical activity induced energy expenditure in humans: Measurement, determinants, and effects. Front. Physiol. 2013, 4, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daskalopoulou, C.; Stubbs, B.; Kralj, C.; Koukounari, A.; Prince, M.; Prina, A.M. Physical activity and healthy ageing: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2017, 38, 6–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Weggemans, R.M.; Backx, F.J.G.; Borghouts, L.; Chinapaw, M.; Hopman, M.T.E.; Koster, A.; Kremers, S.; van Loon, L.J.C.; May, A.; Mosterd, A.; et al. The 2017 Dutch Physical Activity Guidelines. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 58. [Google Scholar] [CrossRef]
- Thompson, B.J.; Stock, M.S.; Banuelas, V.K. Effects of Accumulating Work Shifts on Performance-Based Fatigue Using Multiple Strength Measurements in Day and Night Shift Nurses and Aides. Hum. Factors 2017, 59, 346–356. [Google Scholar] [CrossRef]
- Choi, Y.I.; Park, D.K.; Chung, J.W.; Kim, K.O.; Kwon, K.A.; Kim, Y.J. Circadian rhythm disruption is associated with an increased risk of sarcopenia: A nationwide population-based study in Korea. Sci. Rep. 2019, 9, 12015. [Google Scholar] [CrossRef] [Green Version]
- Gunton, J.E.; Girgis, C.M. Vitamin D and muscle. Bone Rep. 2018, 8, 163–167. [Google Scholar] [CrossRef]
- Hildebrand, R.A.; Miller, B.; Warren, A.; Hildebrand, D.; Smith, B.J. Compromised Vitamin D Status Negatively Affects Muscular Strength and Power of Collegiate Athletes. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 558–564. [Google Scholar] [CrossRef]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.C.; Carbone, J.W.; Combs, G.F., Jr.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef] [Green Version]
- Schoenfeld, B.J.; Aragon, A.A.; Krieger, J.W. Effects of meal frequency on weight loss and body composition: A meta-analysis. Nutr. Rev. 2015, 73, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Alencar, M.K.; Beam, J.R.; McCormick, J.J.; White, A.C.; Salgado, R.M.; Kravitz, L.R.; Mermier, C.M.; Gibson, A.L.; Conn, C.A.; Kolkmeyer, D.; et al. Increased meal frequency attenuates fat-free mass losses and some markers of health status with a portion-controlled weight loss diet. Nutr. Res. 2015, 35, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Gunner, F.; Lindasy, M.; Brown, P.E.H.; Shaw, A.M.; Britland, S.E.; Davey, T.; Fallowfield, J.L. Surgeon General’s Armed Forces Feeding Project. J. R. Nav. Med Serv. 2009, 95, 37. [Google Scholar]
- Institute of Naval Medicine. Body Composition, Physical Fitness and Dietary Intake Following a Submarine Deployment; Institute of Naval Medicine; MOD Abbey Wood South: Bristol, UK, 2016; pp. 1–71. [Google Scholar]
- Abdulla, H.; Smith, K.; Atherton, P.J.; Idris, I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: A systematic review and meta-analysis. Diabetologia 2016, 59, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2019. [Google Scholar] [CrossRef] [Green Version]
- Holy, X.; Collombet, J.M.; Labarthe, F.; Granger-Veyron, N.; Begot, L. Effects of seasonal vitamin D deficiency and respiratory acidosis on bone metabolism markers in submarine crewmembers during prolonged patrols. J. Appl. Physiol. 2012, 112, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Duplessis, C.A.; Harris, E.B.; Watenpaugh, D.E.; Horn, W.G. Vitamin D supplementation in underway submariners. Aviat. Space Environ. Med. 2005, 76, 569–575. [Google Scholar]
- Aslinia, F.; Mazza, J.J.; Yale, S.H. Megaloblastic anemia and other causes of macrocytosis. Clin. Med. Res. 2006, 4, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.A. If high folic acid aggravates vitamin B12 deficiency what should be done about it? Nutr. Rev. 2007, 65, 451–458. [Google Scholar] [CrossRef]
| Pre | Post | Change | p | ||
|---|---|---|---|---|---|
| Glucose Homeostasis | |||||
| Glucose, mmol/L | 5.4 ± 0.4 | 5.2 ± 0.5 | −0.3 ± 0.4 | −5% | 0.03 |
| Insulin, mU/L | 5.0 ± 4.8 | 3.5 ± 2.7 | −1.5 ± 2.7 | −30% | 0.07 |
| HOMA-IR | 1.3 ± 1.3 | 0.8 ± 0.7 | −0.4 ± 0.8 | −34% | 0.06 |
| HOMA-ß | 14.2 ± 16.0 | 9.7 ± 10.0 | −4.5 ± 8.7 | −32% | 0.09 |
| Cardiovascular Biomarkers | |||||
| Triglycerides, mmol/L | 1.2 ± 0.5 | 0.9 ± 0.3 | −0.3 ± 0.4 | −27% | 0.01 |
| Free fatty acids, mmol/L | 0.3 ± 0.2 | 0.6 ± 0.3 | 0.3 ± 0.4 | 76% | 0.04 |
| Cholesterol, total, mmol/L | 4.5 ± 0.6 | 4.5 ± 0.6 | −0.05 ± 0.6 | −1% | 0.79 |
| Cholesterol, HDL, mmol/L | 1.4 ± 0.3 | 1.3 ± 0.3 | −0.1 ± 0.2 | −7% | 0.10 |
| Cholesterol, LDL, mmol/L | 2.6 ± 0.7 | 2.8 ± 0.6 | 0.2 ± 0.5 | 8% | 0.21 |
| Systolic blood pressure, mmHg | 123.0 ± 8.7 | 120.0 ± 9.0 | −3.0 ± 4.5 | −2% | 0.08 |
| Diastolic blood pressure, mmHg | 75.2 ± 6.5 | 77.8 ± 5.6 | 2.6 ± 8.2 | 3% | 0.38 |
| Mean arterial blood pressure, mmHg | 91.2 ± 4.4 | 92.0 ± 5.7 | 0.8 ± 6.1 | 1% | 0.71 |
| 10-year CVD risk, % | 0.9 ± 1.3 | 0.9 ± 1.1 | −0.04 ± 0.3 | −5% | 0.66 |
| N | Pre | Post | Change | p | ||
|---|---|---|---|---|---|---|
| P Vitamin D3, nmol/L | 12 | 200 ± 41 | 173 ± 35 | −25 ± 39 | −14% | 0.04 |
| P Calcium, mmol/L | 11 | 2.4 ± 0.1 | 3.6 ± 1.5 | 1.2 ± 1.5 | 51% | 0.02 |
| U Calcium, mmol/L | 11 | 4.6 ± 2.2 | 2.5 ± 0.1 | −2.1 ± 2.2 | −45% | 0.01 |
| P Magnesium, mmol/L | 13 | 0.83 ± 0.06 | 0.86 ± 0.06 | 0.04 ± 0.05 | 5% | 0.02 |
| U Magnesium, mmol/L | 11 | 5.2 ± 1.7 | 4.2 ± 1.4 | −1.1 ± 2.1 | −20% | 0.12 |
| P Parathyroid Hormone, mmol/L | 13 | 2.8 ± 0.8 | 2.7 ± 1.5 | −0.1 ± 1.1 | −3% | 0.80 |
| P Alkaline Phosphatase, IU/L | 13 | 76 ± 17 | 77 ± 16 | 1 ± 9 | 1% | 0.80 |
| P Phosphorus, mmol/L | 13 | 1.1 ± 0.1 | 1.1 ± 0.2 | 0.1 ± 0.2 | 5% | 0.29 |
| U Phosphorus, mmol/L | 11 | 31 ± 8 | 37 ± 10 | 6 ± 12 | 18% | 0.17 |
| P Sodium, mmol/L | 13 | 141 ± 1 | 141 ± 1 | 0.3 ± 1.8 | 0% | 0.56 |
| U Sodium, mmol/L | 11 | 128 ± 73 | 141 ± 52 | 13 ± 89 | 10% | 0.63 |
| P Potassium, mmol/L | 13 | 4.5 ± 0.2 | 4.4 ± 0.2 | −0.1 ± 0.3 | −2% | 0.33 |
| U Potassium, mmol/L | 11 | 57 ± 22 | 46 ± 13 | −11 ± 16 | −19% | 0.05 |
| P Chloride, mmol/L | 13 | 105 ± 2 | 104 ± 1 | −1 ± 2 | −1% | 0.02 |
| U Chloride, mmol/L | 11 | 143 ± 84 | 129 ± 56 | −14 ± 92 | −10% | 0.63 |
| B Haemoglobin, mmol/L | 12 | 9.4 ± 0.8 | 9.4 ± 0.7 | 0.03 ± 0.39 | 0% | 0.77 |
| B Haematocrit, % | 12 | 46.6 ± 3.3 | 44.4 ± 2.1 | −2.2 ± 2.1 | −5% | 0.005 |
| B Ferritin, ng/mL | 12 | 122 ± 60 | 200 ± 127 | 78 ± 75 | 64% | 0.004 |
| B Vitamin B12, pmol/L | 12 | 281 ± 95 | 158 ± 65 | −123 ± 98 | −44% | 0.001 |
| B Folic Acid, ng/mL | 11 | 5.5 ± 1.5 | 13.1 ± 5.7 | 7.5 ± 5.8 | 136% | 0.002 |
| U Osmolality, mOls/kg | 11 | 846 ± 225 | 882 ± 209 | 36 ± 201 | 4% | 0.56 |
| U Creatinine, mmol/day | 11 | 19 ± 11 | 21 ± 6 | 2 ± 12 | 10% | 0.60 |
| U Urea, mmol/L | 11 | 418 ± 122 | 469 ± 137 | 50 ± 103 | 12% | 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rietjens, G.; Most, J.; Joris, P.J.; Helmhout, P.; Plasqui, G. Energy Expenditure and Changes in Body Composition During Submarine Deployment—An Observational Study “DasBoost 2-2017”. Nutrients 2020, 12, 226. https://doi.org/10.3390/nu12010226
Rietjens G, Most J, Joris PJ, Helmhout P, Plasqui G. Energy Expenditure and Changes in Body Composition During Submarine Deployment—An Observational Study “DasBoost 2-2017”. Nutrients. 2020; 12(1):226. https://doi.org/10.3390/nu12010226
Chicago/Turabian StyleRietjens, Gerard, Jasper Most, Peter J. Joris, Pieter Helmhout, and Guy Plasqui. 2020. "Energy Expenditure and Changes in Body Composition During Submarine Deployment—An Observational Study “DasBoost 2-2017”" Nutrients 12, no. 1: 226. https://doi.org/10.3390/nu12010226





