Low FODMAP Diet: Evidence, Doubts, and Hopes
Abstract
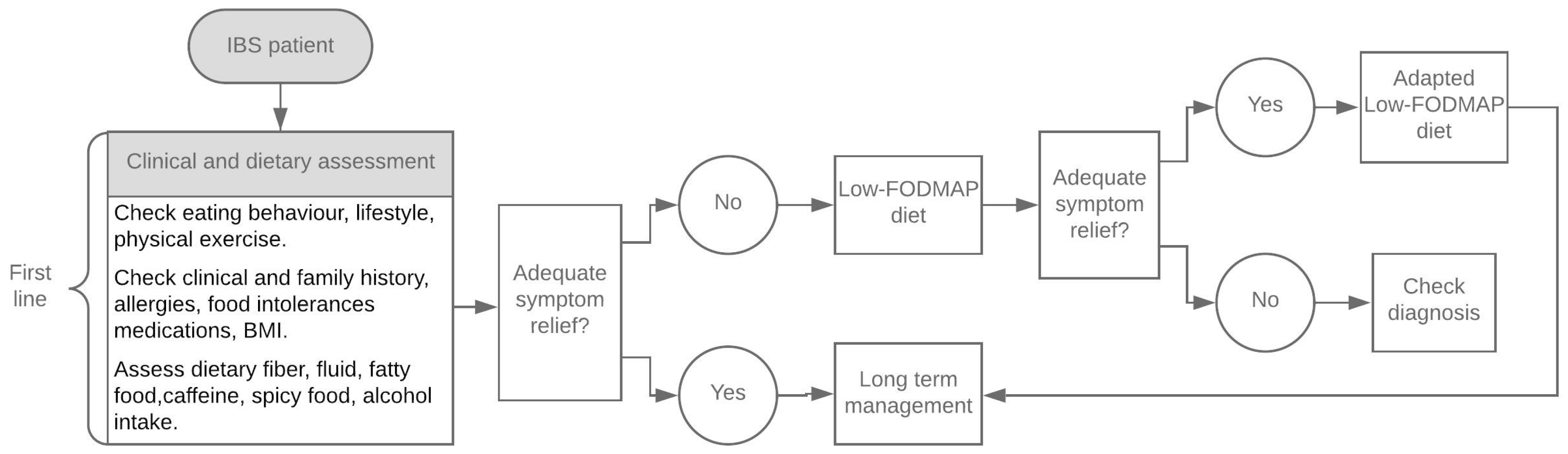
1. Introduction
2. Evidence
2.1. LFD Compared to Other Diets
2.2. LFD Compared to Other Therapies
2.3. LFD Beyond IBS
3. Doubts
- Explaining the nature and the aim of the diet;
- Ensuring nutritional adequacy and avoiding nutritional and caloric imbalance;
- Favoring patients’ compliance with frequent monitoring (e.g., phone calls, emails) and giving timely suggestions;
- Adapting the diet to the normal eating behavior and lifestyle (personal taste, ethnicity, etc.) of the patients.
3.1. Complex and Difficult to Teach and to Learn
3.2. Difficult to Continue and Potentially Expensive
3.3. Reduction of Natural Prebiotics and Impact on Gut Microbiota and Metabolome
3.4. Constipation
3.5. Nutritional Adequacy
- The exclusion of carbohydrates rich in fructans may lead to a reduction in carbohydrate, fiber, and iron intake [42];
- The lower amount of kcalories of the LFD may lead to an excessive weight loss;
- The exclusion of several types of vegetables may lead to a reduction in natural antioxidants, such as flavonoids, carotenoids, and vitamin C, or phenolic acid and anthocyanins;
- The exclusion of dairy products may favor calcium deficiency, both because they are the main food source and because lactose acts as a promoter of its absorption.
3.6. Precipitating Eating Disorder Behavior
3.7. Long-Term Efficacy
4. Hopes for the Future
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American College of Gastroenterology Task Force on Irritable Bowel Syndrome; Brandt, L.J.; Chey, W.D.; Foxx-Orenstein, A.E.; Schiller, L.R.; Schoenfeld, P.S.; Spiegel, B.M.; Talley, N.J.; Quigley, E.M. An evidence-based position statement on the management of irritable bowel syndrome. Am. J. Gastroenterol. 2009, 104 (Suppl. 1), S1–S35. [Google Scholar] [CrossRef]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome—Etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-Related Gastrointestinal Symptoms in the Irritable Bowel Syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Holzel, A.; Schwarz, V.; Sutcliffe, K.W. Defective lactose absorption causing malnutrition in infancy. Lancet 1959, 273, 1126–1128. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought—Western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef]
- Muir, J.G.; Rose, R.; Rosella, O.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R. Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2009, 57, 554–565. [Google Scholar] [CrossRef]
- Muir, J.G.; Shepherd, S.J.; Rosella, O.; Rose, R.; Barrett, J.S.; Gibson, P.R. Fructan and free fructose content of common Australian vegetables and fruit. J. Agric. Food Chem. 2007, 55, 6619–6627. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef]
- Mansueto, P.; Seidita, A.; D’Alcamo, A.; Carroccio, A. Role of FODMAPs in Patients with Irritable Bowel Syndrome. Nutr. Clin. Pract. 2015, 30, 665–682. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Lomer, M.C. Who should deliver the low FODMAP diet and what educational methods are optimal: A review. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 23–26. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, Y.A.; Thompson, J.; Gulia, P.; Lomer, M.C.E. British Dietetic Association systematic review and evidence based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 576–592. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R. History of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.C.; Brown, M.L.; Phillips, S.F. Decreased fluid tolerance, accelerated transit, and abnormal motility of the human colon induced by oleic acid. Gastroenterology 1986, 91, 100–107. [Google Scholar] [CrossRef]
- Murray, K.; Wilkinson-Smith, V.; Hoad, C.; Costigan, C.; Cox, E.; Lam, C.; Marciani, L.; Gowland, P.; Spiller, R.C. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am. J. Gastroenterol. 2014, 109, 110–119. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Vanhoutvin, S.A.; Troost, F.J.; Kilkens, T.O.; Lindsey, P.J.; Hamer, H.M.; Jonkers, D.M.; Venema, K.; Brummer, R.J. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol. Motil. 2009, 21, 952-e76. [Google Scholar] [CrossRef]
- Hill, P.; Muir, J.G.; Gibson, P.R. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol. Hepatol. 2017, 13, 36–45. [Google Scholar]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef]
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Ong, D.K.; Mitchell, S.B.; Barrett, J.S.; Shepherd, S.J.; Irving, P.M.; Biesiekierski, J.R.; Smith, S.; Gibson, P.R.; Muir, J.G. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2010, 25, 1366–1373. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Pedersen, N.; Andersen, N.N.; Végh, Z.; Jensen, L.; Ankersen, D.V.; Felding, M.; Simonsen, M.H.; Burisch, J.; Munkholm, P. Ehealth: Low FODMAP diet vs. Lactobacillus rhamnosus GG in irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 16215–16226. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Cope, J.L.; Hollister, E.B.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Versalovic, J.; Shulman, R.J. Randomised clinical trial: Gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 42, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, R.; Koskenpato, J.; Hongisto, S.M.; Loponen, J.; Poussa, T.; Hillilä, M.; Korpela, R. Randomised clinical trial: Low FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and probiotic restores Bifidobacterium species: A randomized controlled trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Muir, J.G.; Gibson, P.R. Review article: Gut-directed hypnotherapy in the management of irritable bowel syndrome and inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 41, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Whelan, K.; Irving, P.M.; Lomer, M.C. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2011, 24, 487–495. [Google Scholar] [CrossRef]
- Peters, S.L.; Yao, C.K.; Philpott, H.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised clinical trial: The efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 447–459. [Google Scholar] [CrossRef]
- Schumann, D.; Langhorst, J.; Dobos, G.; Cramer, H. Randomised clinical trial: Yoga vs. a low-FODMAP diet in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 47, 203–211. [Google Scholar] [CrossRef]
- De Roest, R.H.; Dobbs, B.R.; Chapman, B.A.; Batman, B.; O’Brien, L.A.; Leeper, J.A.; Hebblethwaite, C.R.; Gearry, R.B. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: A prospective study. Int. J. Clin. Pract. 2013, 67, 895–903. [Google Scholar] [CrossRef]
- Maagaard, L.; Ankersen, D.V.; Vegh, Z.; Burisch, J.; Jensen, L.; Pedersen, N.; Munkholm, P. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J. Gastroenterol. 2016, 22, 4009–4019. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.; Jansen, C.; Martin, L.; Williams, M.; Seamark, L.; Staudacher, H.M.; Irving, P.M.; Whelan, K.; Lomer, M.C. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Harvie, R.M.; Chisholm, A.W.; Bisanz, J.E.; Burton, J.P.; Herbison, P.; Schultz, K.; Schultz, M. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J. Gastroenterol. 2017, 23, 4632–4643. [Google Scholar] [CrossRef] [PubMed]
- Weynants, A.; Goossens, L.; Genetello, M.; De Looze, D.; Van Winckel, M. The long-term effect and adherence of a low fermentable oligosaccharides disaccharides monosaccharides and polyols (FODMAP) diet in patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2019. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ohlsson, B.; Ulander, K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS). BMC Gastroenterol. 2007, 7, 16. [Google Scholar] [CrossRef]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- NICE, National Institute for Health and Care Excellence. Available online: https://www.nice.org.uk/guidance/cg61/chapter/1-Recommendations#dietary-and-lifestyle-advice (accessed on 11 August 2019).
- Miller, V.; Carruthers, H.; Morris, J.; Hasan, S.; Archbold, S.; Whorwell, P. Hypnotherapy for irritable bowel syndrome: An audit of one thousand adult patients. Aliment. Pharmacol. Ther. 2015, 41, 844–855. [Google Scholar] [CrossRef]
- Flik, C.E.; Laan, W.; Zuithoff, N.P.A.; van Rood, Y.R.; Smout, A.J.P.M.; Weusten, B.L.A.M.; Whorwell, P.J.; de Wit, N.J. Efficacy of individual and group hypnotherapy in irritable bowel syndrome (IMAGINE): A multicentre randomised controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 20–31. [Google Scholar] [CrossRef]
- Schumann, D.; Anheyer, D.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Effect of Yoga in the Therapy of Irritable Bowel Syndrome: A Systematic Review. Clin. Gastroenterol. Hepatol. 2016, 14, 1720–1731. [Google Scholar] [CrossRef]
- Colombel, J.F.; Shin, A.; Gibson, P.R. AGA Clinical Practice Update on Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 380–390.e1. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Prince, A.C.; Myers, C.E.; Irving, P.M.; Lindsay, J.O.; Lomer, M.C.; Whelan, K. Fermentable Carbohydrates FODMAPs. Exacerbate Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: A Randomised, Double-blind, Placebo-controlled, Cross-over, Re-challenge Trial. J. Crohn’s Colitis 2017, 11, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.C.; Myers, C.E.; Joyce, T.; Irving, P.; Lomer, M.; Whelan, K. Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R. Use of the low-FODMAP diet in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 40–42. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Sheperd, S.J.; Muir, J.G.; Gibson, P.R. Consistent prebiotic effect on gut microbiota with altered FODMAP intake in patients with Crohn’s disease: A randomised, controlled cross-over trial of well-defined diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef]
- Damas, O.M.; Garces, L.; Abreu, M.T. Diet as adjunctive treatment for inflammatory bowel disease: Review and update of the latest literature. Curr. Treat. Options Gastroenterol. 2019. [Google Scholar] [CrossRef]
- Bodini, G.; Zanella, C.; Crespi, M.; Lo Pumo, S.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67–68, 110542. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef]
- Smith, E.; Pauling, J.D. The efficacy of dietary intervention on gastrointestinal involvement in systemic sclerosis: A systematic literature review. Semin. Arthritis Rheum. 2018. [Google Scholar] [CrossRef]
- McMahan, Z.H.; Hummers, L.K. Gastrointestinal involvement in systemic sclerosis: Diagnosis and management. Curr. Opin. Rheumatol. 2018, 30, 533–540. [Google Scholar] [CrossRef]
- Marum, A.P.; Moreira, C.; Tomas-Carus, P.; Saraiva, F.; Guerreiro, C.S. A low fermentable oligo-di-mono-saccharides and polyols (FODMAP) diet is a balanced therapy for fibromyalgia with nutritional and symptomatic benefits. Nutr. Hosp. 2017, 34, 667–674. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marum, A.P.; Moreira, C.; Saraiva, F.; Tomas-Carus, P.; Sousa-Guerreiro, C. A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients. Scand. J. Pain 2016, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Rossi, A. Is a low FODMAP diet dangerous? Tech. Coloproctol. 2018, 22, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Gibson, P.R. Fructose malabsorption and symptoms of irritable bowel syndrome: Guidelines for effective dietary management. J. Am. Diet. Assoc. 2006, 106, 1631–1639. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Bazzichi, L.; Bassotti, G.; Mumolo, M.G.; Fani, B.; Costa, F.; Ricchiuti, A.; De Bortoli, N.; Mosca, M.; et al. Bioelectrical impedance vector analysys in patients with irritable bowel syndrome on a low FODMAP diet: A pilot study. Tech. Coloproctol. 2017, 21, 451–459. [Google Scholar] [CrossRef]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food composition, defining cutoff values and international application. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 53–61. [Google Scholar] [CrossRef]
- Barrett, J.S. How to institute the low-FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 8–10. [Google Scholar] [CrossRef]
- Trott, N.; Aziz, I.; Rej, A.; Surendran Sanders, D. How Patients with IBS Use Low FODMAP Dietary Information Provided by General Practitioners and Gastroenterologists: A Qualitative Study. Nutrients 2019, 11, 1313. [Google Scholar] [CrossRef]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Sheperd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J. Crohns Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef]
- Méance, S.; Giordano, J.; Chuang, E.; Schneider, H. Food regulations: Low FODMAP labeling and communication goals. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 62–63. [Google Scholar] [CrossRef]
- Staudacher, H.M. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 16–19. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Vegh, Z.; Burisch, J.; Jensen, L.; Ankersen, D.V.; Felding, M.; Andersen, N.N.; Munkholm, P. Ehealth monitoring in irritable bowel syndrome patients treated with low fermentable oligo-, di-, mono-saccharides and polyols diet. World J. Gastroenterol. 2014, 20, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Scott, K.P.; Duncan, S.H.; Louis, P.; Flint, H.J. Nutritional influences on the gut microbiota and the consequences for gastrointestinal health. Biochem. Soc. Trans. 2011, 39, 1073–1078. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, J.; Ran, Z.H. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol. Res. Pract. 2014, 2014, 872725. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Hollister, E.B.; Oezguen, N.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Savidge, T.C.; Versalovic, J.; Shulman, R.J. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes 2014, 5, 165–175. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Ross, F.S.; Briscoe, Z.M.; Irving, P.M.; Whelan, K.; Lomer, M.C. PTU-183 Advice from a dietitian regarding the low fodmap diet broadly maintains nutrient intake and does not alter fibre intake. Gut 2015, 64, A143–A144. [Google Scholar] [CrossRef]
- Catassi, G.; Lionetti, E.; Gatti, S.; Catassi, C. The Low FODMAP Diet: Many Question Marks for a Catchy Acronym. Nutrients 2017, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Dolan, R.D.; Ball, S.C.; Jackson, K.; Chey, W. The Impact of a 4-Week Low-FODMAP and mNICE Diet on Nutrient Intake in a Sample of US Adults with Irritable Bowel Syndrome with Diarrhea. J. Acad. Nutr. Diet. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tham, A.; Katz, T.E.; Sutherland, R.E.; Garg, M.; Liu, V.; Tong, C.W.; Brunner, R.; Quintano, J.; Collins, C.; Ooi, C.Y. Micronutrient intake in children with cystic fibrosis in Sydney, Australia. J. Cyst. Fibros. 2019. [Google Scholar] [CrossRef] [PubMed]
- Iacovou, M. Adapting the low FODMAP diet to special populations: Infants and children. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 43–45. [Google Scholar] [CrossRef] [PubMed]
- Dugum, M.; Barco, K.; Garg, S. Managing irritable bowel syndrome: The low-FODMAP diet. Clevel. Clin. J. Med. 2016, 83, 655–662. [Google Scholar] [CrossRef]
- Hod, K.; Ringel, Y.; van Tilburg, M.A.L.; Ringel-Kulka, T. Bloating in Irritable Bowel Syndrome Is Associated with Symptoms Severity, Psychological Factors, and Comorbidities. Dig. Dis. Sci. 2018. [CrossRef]
- Satherley, R.; Howard, R.; Higgs, S. Disordered eating practices in gastrointestinal disorders. Appetite 2015, 84, 240–250. [Google Scholar] [CrossRef]
- Stasi, C.; Caserta, A.; Nisita, C.; Cortopassi, S.; Fani, B.; Salvadori, S.; Pancetti, A.; Bertani, L.; Gambaccini, D.; de Bortoli, N.; et al. The complex interplay between gastrointestinal and psychiatric symptoms in irritable bowel syndrome: A longitudinal assessment. J. Gastroenterol. Hepatol. 2019, 34, 713–719. [Google Scholar] [CrossRef]
- Mari, A.; Hosadurg, D.; Martin, L.; Zarate-Lopez, N.; Passananti, V.; Emmanuel, A. Adherence with a low-FODMAP diet in irritable bowel syndrome: Are eating disorders the missing link? Eur. J. Gastroenterol. Hepatol. 2019, 31, 178–182. [Google Scholar] [CrossRef]
- Chey, W.D. Elimination Diets for Irritable Bowel Syndrome: Approaching the End of the Beginning. Am. J. Gastroenterol. 2019, 114, 201–203. [Google Scholar] [CrossRef]
- Mitchell, H.; Porter, J.; Gibson, P.R.; Barrett, J.; Garg, M. Review article: Implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Lindsay, J.O.; Irving, P.; Probert, C.; Whelan, K. Volatile organic compounds in feces associate with response to dietary intervention in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2018, 16, 385–391.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gemming, L.; Hanning, R.; Allman-Farinelli, M. Smartphone apps and the nutrition care process: Current perspectives and future considerations. Patient Educ. Couns. 2018, 101, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.M. Restriction of FODMAP in the management of bloating in irritable bowel syndrome. Singap. Med. J. 2016, 57, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- Manning, L.P.; Biesiekierski, J.R. Use of dietary interventions for functional gastrointestinal disorders. Curr. Opin. Pharmacol. 2018, 43, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wiffin, M.; Smith, L.; Antonio, J.; Johnstone, J.; Beasley, L.; Roberts, J. Effect of a short-term low fermentable oligiosaccharide, disaccharide, monosaccharide and polyol (FODMAP) diet on exercise-related gastrointestinal symptoms. J. Int. Soc. Sports Nutr. 2019, 16, 1. [Google Scholar] [CrossRef]
- Uno, Y. Radiation-induced enteropathy—How a low-FODMAP diet might help. Scand. J. Gastroenterol. 2018, 53, 37–378. [Google Scholar] [CrossRef]
- Priyanka, P.; Gayam, S.; Kupec, J.T. The Role of a Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyol Diet in Nonceliac Gluten Sensitivity. Gastroenterol. Res. Pract. 2018, 2018, 1561476. [Google Scholar] [CrossRef]
| Trial Characteristics | Methods | Length of Follow-Up | Evaluated Parameters | Results | Grade of Evidence | |
|---|---|---|---|---|---|---|
| Short-term efficacy | ||||||
| McIntosh et al. [18] 2017 | LFD = 19 HFD = 18 Rome III | Single blinded parallel | 3 weeks | IBS-SSS | Lower IBS-SSS in LFD group for gastrointestinal symptoms and abdominal pain. | Low |
| Ong et al. [26] 2010 | LFD or HFD IBS = 15 Healthy controls = 15 Rome III | Single blinded, crossover | 2 days | Likert scale (GI symptoms severity) | IBS patients under HFD had more severe symptoms compared to those on LFD. | Low |
| Staudacher et al. [27] 2012 | LFD = 19 Habitual diet = 22 Rome III | Single blinded, controlled | 4 weeks | GSRS BSC | LFD group had better adequate symptom control, lower stool frequency, less abdominal pain, and less overall symptoms. | Low |
| Pedersen et al. [28] 2014 | LFD = 42 Probiotic = 41 Habitual diet = 40 Rome III | Unblinded parallel | 6 weeks | IBS-SSS IBS-QOL | Greater reduction in IBS-SSS in LFD group compared to habitual diet. No differences in IBS QOL. | Very low |
| Halmos et al. [29] 2014 | LFD or Typical (Australian) diet IBS = 30 Healthy controls = 8 Rome III | Single blinded, controlled crossover | 21 days | VAS (GI symptoms severity) KSC FWC | Lower VAS in LFD group. Lower stool frequency and lower KSC score in IBS-D during LFD. | Low |
| Bohn et al. [30] 2015 | LFD = 33 NICE = 34 Rome III | Single blinded, multicentre parallel, controlled | 4 weeks | IBS-SSS HADS BSC Visceral sensitivity index | IBS symptoms reduced in both diets, with no difference between groups. | Low |
| Chumpitazi et al. [31] 2015 | Pediatric patients LFD = 16 TACD = 17 Rome III | Double blinded, crossover | 48 hours | Pain and stool diary Likert scale (Pain severity and associated GI symptoms) BSC | Fewer abdominal pain episodes and less severity during LFD. Total composite GI score lower in LFD. | High |
| Eswaran et al. [32] 2016 | LFD = 45 mNICE = 39 Rome III | Unblinded parallel | 4 weeks | AR BSC | Greater reduction in abdominal pain and stool consistency in LFD group. No differences between groups regarding adequate symptom relief. | Very low |
| Laatikainen et al. [33] 2016 | Rye bread = 43 Low FODMAP rye bread = 44 Rome III | Double blinded controlled crossover | 4 weeks | IBS-SSS VAS (GI symptoms severity) IBS-QOL | Less abdominal pain, flatulence, stomach rumbling, and intestinal cramps in the Low-FODMAP rye bread group. | High |
| Staudacher et al. [34] 2017 | Sham diet/placebo = 27 Sham diet/probiotic = 26 LFD/placebo = 24 LFD/probiotic = 27 Rome III | Single blinded, multicentre, placebo-controlled, | 4 weeks | GSRS IBS-SSS BSC IBS-QOL SF-36 | Lower IBS-SSS and better IBS QOL in LFD group. | High |
| Hustoft et al. [35] 2017 | LFD and maltodextrin = 20 LFD and FOS = 20 Rome III | Double blinded, placebo-controlled, crossover | 9 weeks | IBS-SSS VAS (associated symptoms) AR | Lower IBS-SSS and more patients reporting symptom relief in the group supplemented with maltodextrin | High |
| Peters et al. [36] 2015 | LFD = 24 Hypnotherapy = 25 Combined = 25 Rome III | Unblinded | 6 weeks | VAS (GI symptoms severity) IBS-QOL HADS STPI | Lower VAS in LFD and hypnotherapy. IBS-QOL improved in all groups with no statistical differences. | Very low |
| Long-term efficacy | ||||||
| Staudacher et al. [37] 2011 | LFD = 43 NICE diet = 39 No aLFD Dietitian-led education | Retrospective observational | 2–6 months | Likert scale (symptom changes and satisfaction with dietary advice) | LFD group reported improvement in bloating, abdominal pain, flatulence, nausea, and energy levels, and more satisfaction with the treatment. | Very low |
| Peters et al. [38] 2016 | LFD + aLFD = 24 Hypnotherapy = 25 Combination = 25 Dietitian-led education | Unblinded, randomized | 6 weeks + 6 months | VAS IBS-SSS STPI HADS IBS-QOL | Improvements in overall symptoms for hypnotherapy, LFD and combination, maintained at 6 months. Hypnotherapy superior regarding psychological indices. | Very low |
| Schumann et al. [39] 2018 | LFD for 12 weeks + aLFD = 29 Yoga 12 weeks = 30 Dietitian-led education | Single blinded randomized controlled trial | 6 months | IBS-SSS IBS-QOL SF-36 HADS CPSS PSQ BAQ BRS AR | IBS-SSS scores decreased both for LFD and yoga, with no statistically significant group differences. HADS scores were lower in yoga group, especially on the subscale for anxiety. | Low |
| de Roest et al. [40] 2013 | LFD = 90 Dietitian-led education | Prospective observational | 15.7 (±9.0) months | GI symptom rating scale Likert scale (symptoms intensity and adherence) | Positive change in most of the investigated symptoms, including abdominal pain, bloating, flatulence, and diarrhea. Fructose malabsorption was associated with response to the diet. 75.6% were adherent to LFD. | Very low |
| Maagaard et al. [41] 2016 | IBS = 131 IBD = 49 LFD for 6-8 weeks + aLFD = 180 Dietitian-led education | Retrospective cross-sectional | 16 months (range: 2–80) | VAS FARS BSC IBS-SSS IBS-QOL SIBDQ | Partial or full efficacy of bloating and abdominal pain. One third were adherent to the diet. LFD was reported to be more expensive and complicated than usual diet. | Very low |
| O’Keeffe et al. [42] 2018 | NICE IBS criteria LFD for 6 weeks + aLFD = 103 Dietitian-led education | Prospective observational | 6–18 months | Global symptom response GSRS BSC Likert scale (acceptability and impact on daily life) | Abdominal pain, bloating and flatulence decreased at long-term follow up. Satisfactory symptom relief was reported at follow-up. aLFD was found to be more expensive and difficult than habitual diet. | Very low |
| Harvie et al. [43] 2017 | LFD = 23 Habitual diet = 27 aLFD = 23 LFD = 27 Dietitian-led education | Randomized, parallel, cross-over | 6 months | IBS-SSS IBS-QOL | Lower reduction of IBS-SSS and better QoL in LFD (3 months) and sustained by aLFD (6 months). | Low |
| Weynants et al. [44] 2019 | LFD for 6–8 weeks + aLFD = 90 Dietitian-led education | Retrospective cross-sectional | 49–168 weeks | IBS-QOL IBS-SSS Self-developed adherence and symptoms questionnaire | Patients who still followed the diet had less severe abdominal pain. 80% of patients were adherent to the LFD. No significant difference in QOL was found. | Very low |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. https://doi.org/10.3390/nu12010148
Bellini M, Tonarelli S, Nagy AG, Pancetti A, Costa F, Ricchiuti A, de Bortoli N, Mosca M, Marchi S, Rossi A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients. 2020; 12(1):148. https://doi.org/10.3390/nu12010148
Chicago/Turabian StyleBellini, Massimo, Sara Tonarelli, Attila G. Nagy, Andrea Pancetti, Francesco Costa, Angelo Ricchiuti, Nicola de Bortoli, Marta Mosca, Santino Marchi, and Alessandra Rossi. 2020. "Low FODMAP Diet: Evidence, Doubts, and Hopes" Nutrients 12, no. 1: 148. https://doi.org/10.3390/nu12010148
APA StyleBellini, M., Tonarelli, S., Nagy, A. G., Pancetti, A., Costa, F., Ricchiuti, A., de Bortoli, N., Mosca, M., Marchi, S., & Rossi, A. (2020). Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients, 12(1), 148. https://doi.org/10.3390/nu12010148








