Effects of Preventive Nutrition Interventions among Adolescents on Health and Nutritional Status in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective
2.2. Type of Studies and Participants
- Nutrition education and counseling: (refers to education aimed at promoting a healthy diet by increasing the diversity and amount of foods consumed through various platforms including schools, communities, peer-based networks, and computer- and web-based education).
- Micronutrient supplementation (refers to the provision of individual or mixture of nutrients separately from the diet in form of injections, tablets, capsules, syrups/liquids, or powders) and fortification (refers to the process in which micronutrients are added to processed foods).
- Macronutrient supplementation (refers to supplementary feeding, balanced energy and protein supplementation, and lipid-based nutrition supplementation (LNS)).
2.3. Type of Outcomes
2.4. Search Methods
2.5. Data Collection and Analysis
- Duration or intensity of intervention (e.g., short versus long term, one-off versus multiple sessions).
- Individual context versus group context (for nutrition education and counseling only, i.e., children receiving the intervention individually versus those in groups)
- Study setting: school, community, clinic, etc.
- Sex: male and female
- Population (e.g., urban population versus rural population; resource-poor versus resource-rich population)
- We also attempted to conduct subgroup analysis based on the WHO health system building blocks factors (where data was available).
2.6. Quality Assessment
3. Results
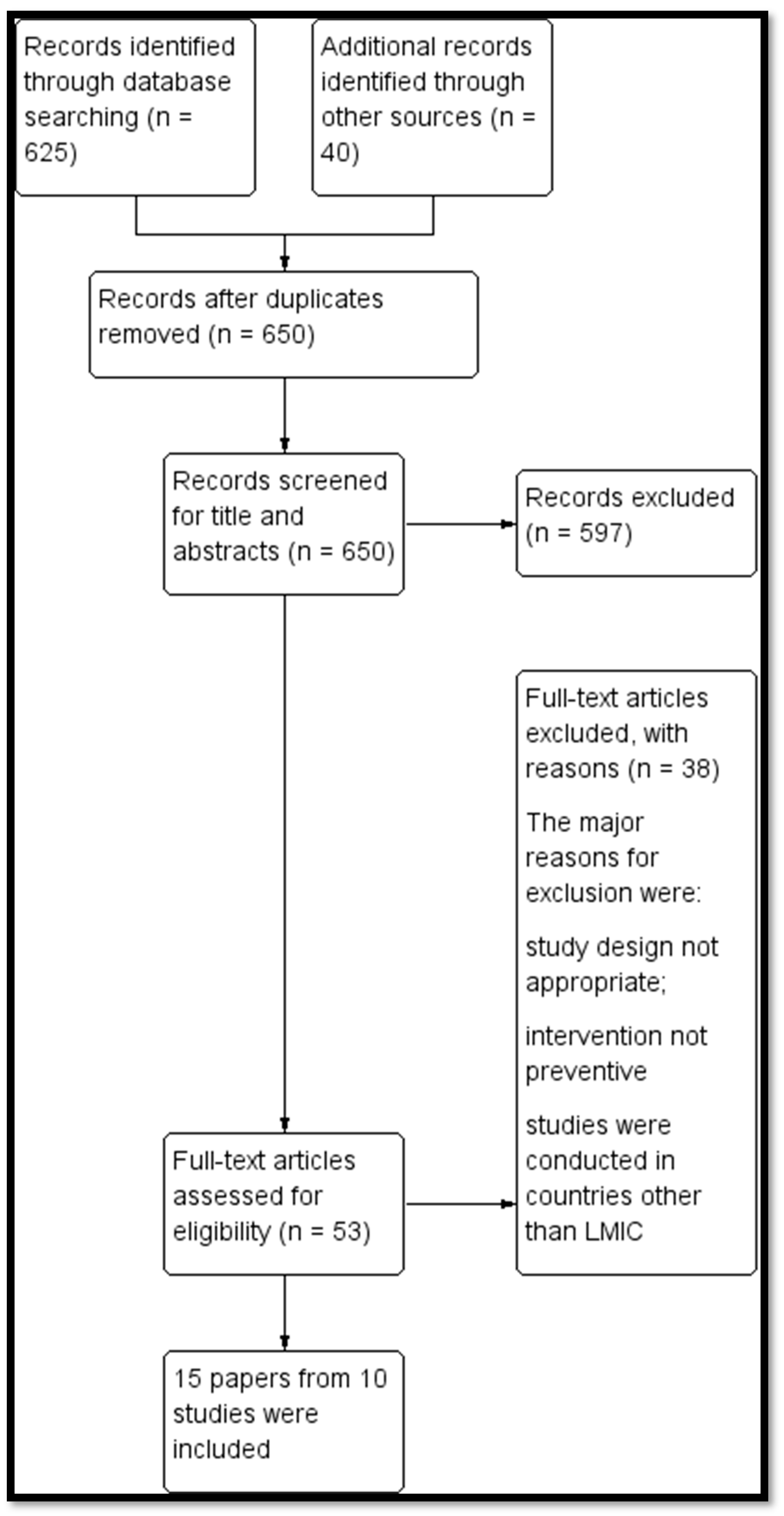
3.1. Results of the Search
3.2. Description of Included Studies
3.3. Contextual Factors Based on WHO Health System Building Blocks
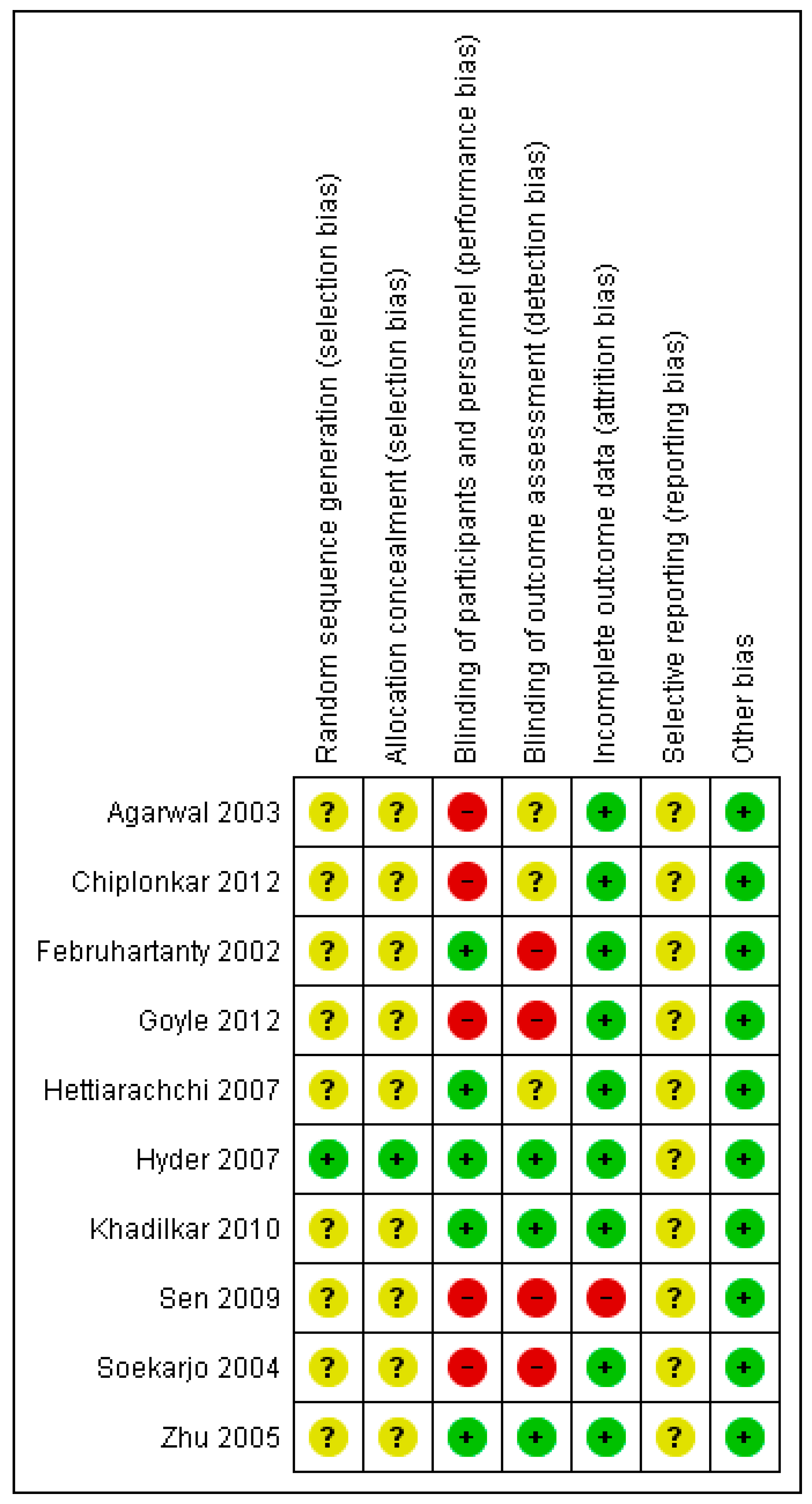
3.4. Risk of Bias
3.5. Effects of Intervention
3.5.1. Comparison 1: Nutrition Education and Counseling
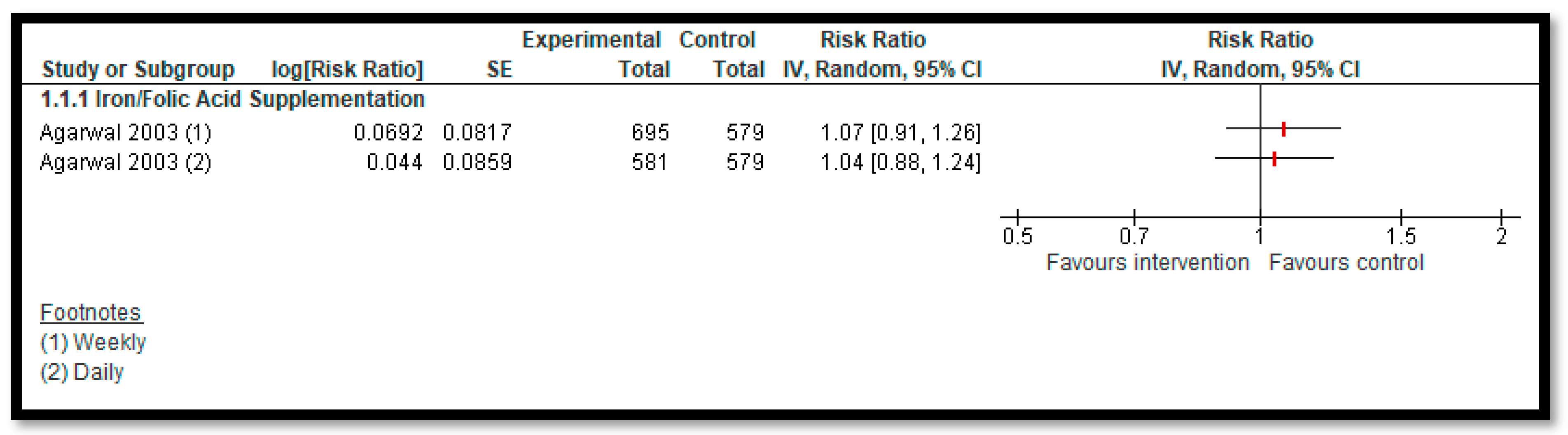
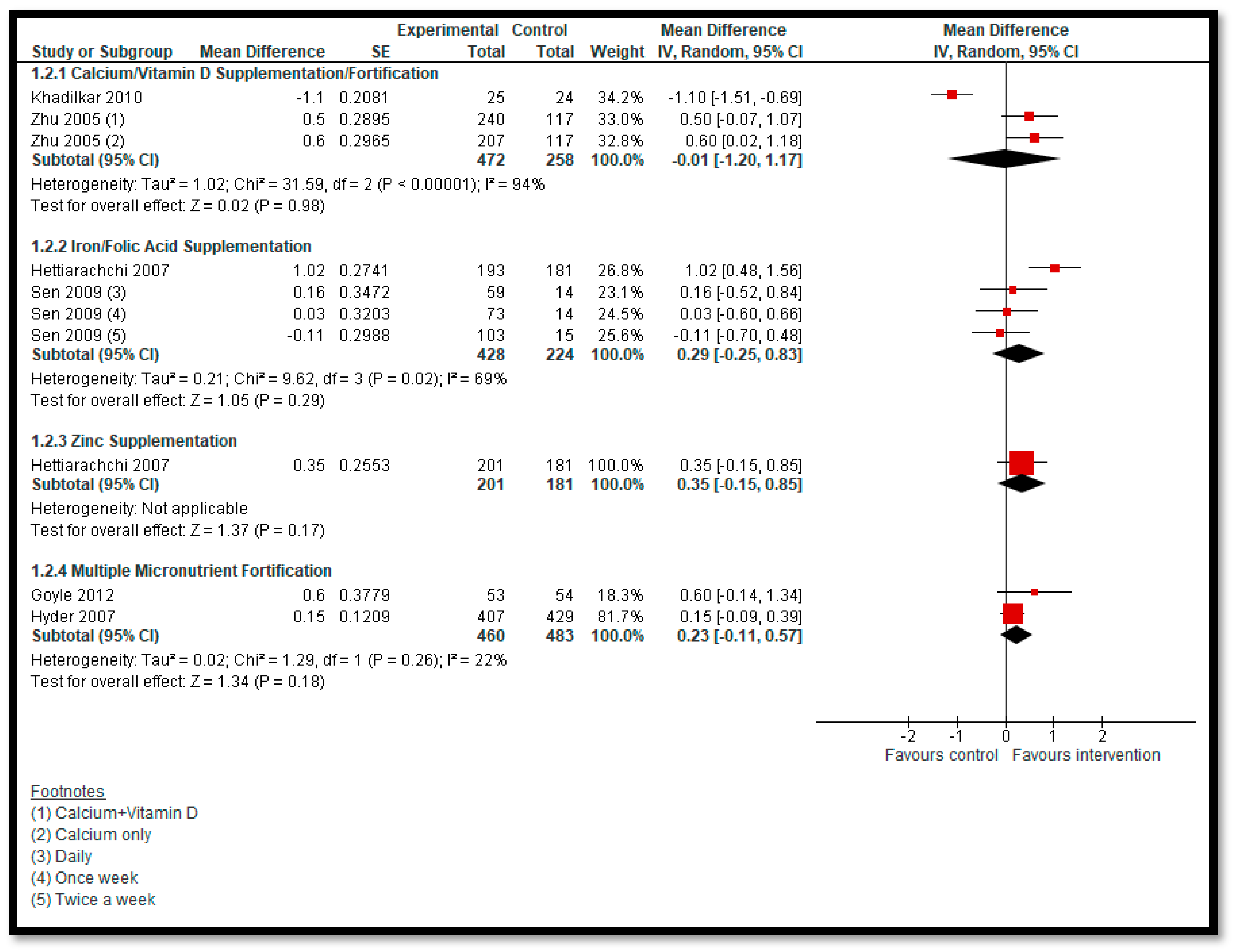
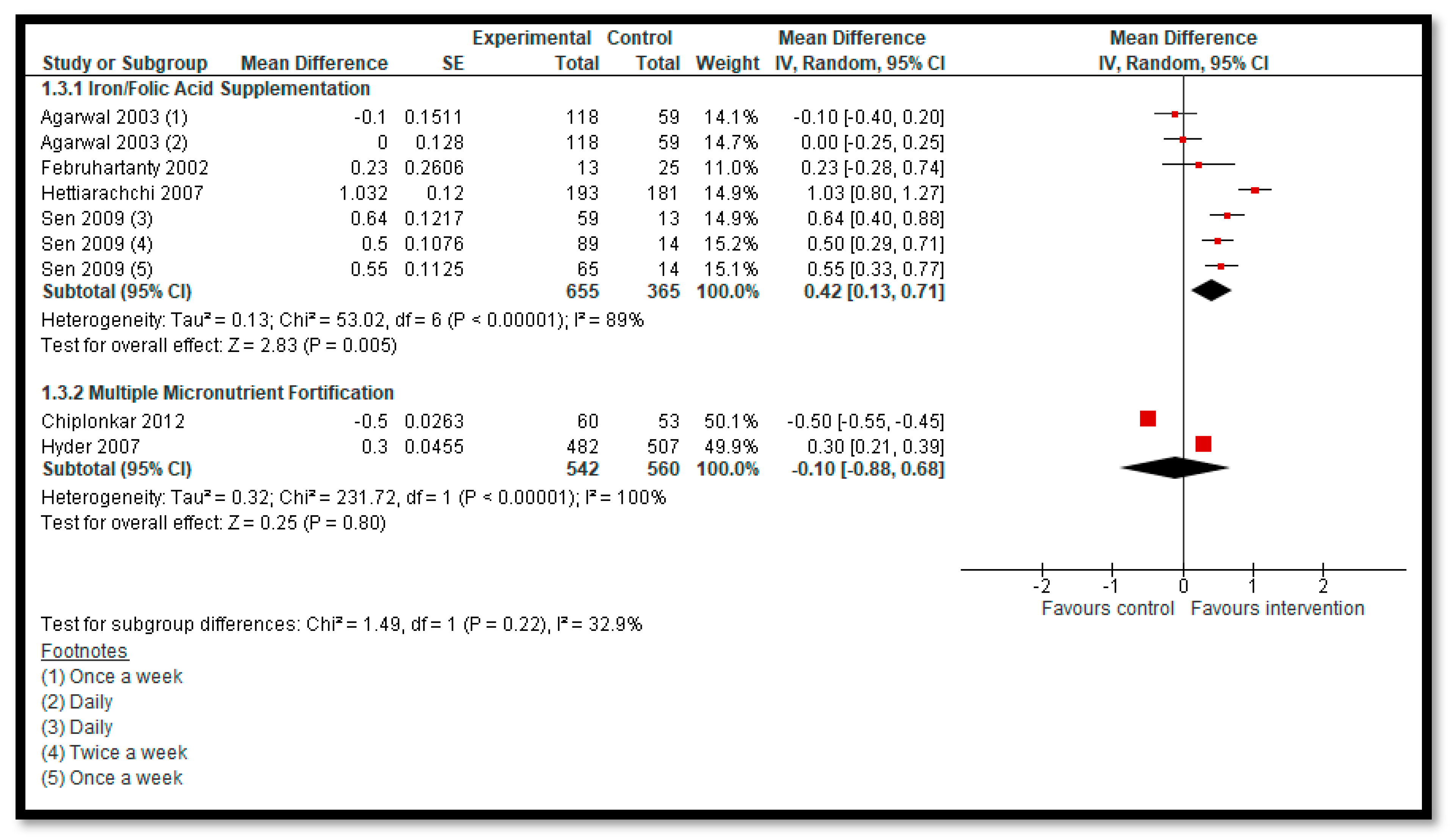
3.5.2. Comparison 2: Micronutrient Supplementation and Fortification (Any Micronutrient Alone or in Combination)
3.5.3. Comparison 3: Macronutrient Supplementation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. Search Strategy
| PubMed Search Strategy: (Titles/Abstracts and Text Words) | ((“Adolescent” [Mesh]) OR (“Child” [Mesh]) OR (Adolescent* OR Adolescence)OR (Teen* OR (Youth*) OR (Puberty) OR (juvenil*)) AND ((“Micronutrients” [Mesh]) OR (“Dietary Supplements” [Mesh]) OR (“Food, Fortified” [Mesh]) OR (“Vitamins” [Mesh]) OR (“Minerals” [Mesh] OR “Trace Elements” [Mesh]) OR (“Ferric compounds” [Mesh] OR “Ferrous Compounds” [Mesh]) OR (Iron* OR Ferric OR Ferrous) OR (“Diet Supplement*” OR “Dietary Supplement*” OR Biofortification) OR (“Folic Acid” [Mesh]) OR (Folic* OR Folate* OR Folvite* OR Folacin*) OR (“Zinc” [Mesh] OR “Zinc Sulfate” [Mesh]) OR (“Calcium” [Mesh]) OR (Calcium) OR (“Vitamin D” [Mesh]) OR (vitamin d) OR (“Vitamin A” [Mesh]) OR (“Vitamin A”) OR (“Ascorbic Acid” [Mesh]) OR (“Vitamin C”) OR (Ascorb* OR “ascorbic acid”) OR (Vitamin* OR multivitamin* OR multi-vitamin* OR MMN OR micro-nutrient* OR mineral* OR multimineral* OR multi-mineral OR multinutrient* OR “multiple micronutrient*” OR “food environment” OR advertisement* OR “mass media” OR “supplementary feeding” OR “energy supplement*” OR “protein supplement*” OR “lipid based nutrition” OR LNS)) AND ((“Adolescent Development” [Mesh]) OR (“Adolescent Growth”) OR (“Serum Haemoglobin” OR “Serum micronutrient*” OR “Anthropometric measurement*”)) |
| EBSCO CINAHL Plus: | ((“Adolescent” [Mesh]) OR (Adolescent* OR Adolescence) OR (Teen* OR Teenager*) OR (Youth*) OR (Puberty) OR (juvenile)) AND ((“Micronutrients” [Mesh]) OR (“Dietary Supplements” [Mesh]) OR (“Food, Fortified” [Mesh]) OR (“Vitamins” [Mesh]) OR (“Minerals” [Mesh] OR “Trace Elements” [Mesh]) OR (“Ferric compounds” [Mesh] OR “Ferrous Compounds” [Mesh]) OR (Iron* OR Ferric OR Ferrous) OR (“Diet Supplement*” OR “Dietary Supplement*” OR Biofortification) OR (“Folic Acid” [Mesh]) OR (Folic* OR Folate* OR Folvite* OR Folacin*) OR (“Zinc” [Mesh] OR “Zinc Sulfate” [Mesh]) OR (“Calcium” [Mesh]) OR (Calcium) OR (“Vitamin D” [Mesh]) OR (vitamin d) OR (“Vitamin A” [Mesh]) OR (“Vitamin A”) OR (“Ascorbic Acid” [Mesh]) OR (“Vitamin C”) OR (Ascorb* OR “ascorbic acid”) OR (Vitamin* OR multivitamin* OR multi-vitamin* OR MMN OR micro-nutrient* OR mineral* OR multimineral* OR multi-mineral OR multinutrient* OR “multiple micronutrient*” OR “food environment” OR advertisement* OR “mass media” OR “supplementary feeding” OR “energy supplement*” OR “protein supplement*” OR “lipid based nutrition” OR LNS)) AND ((“Adolescent Development” [Mesh]) OR (“Adolescent Growth”) OR (“Serum Haemoglobin” OR “Serum micronutrient*” OR “Anthropometric measurement*”)) |
| Cochrane Library: | ((“Adolescent” [Mesh]) OR (“Child” [Mesh]) OR (Adolescent* OR Adolescence) OR (Teen* OR (Youth*) OR (Puberty) OR (juvenil*)) AND ((“Micronutrients” [Mesh]) OR (“Dietary Supplements” [Mesh]) OR (“Food, Fortified”[Mesh]) OR (“Vitamins” [Mesh]) OR (“Minerals” [Mesh] OR “Trace Elements” [Mesh]) OR (“Ferric compounds” [Mesh] OR “Ferrous Compounds” [Mesh]) OR (Iron* OR Ferric OR Ferrous) OR (“Diet Supplement*” OR “Dietary Supplement*” OR Biofortification) OR (“Folic Acid” [Mesh]) OR (Folic* OR Folate* OR Folvite* OR Folacin*) OR (“Zinc” [Mesh] OR “Zinc Sulfate” [Mesh]) OR (“Calcium” [Mesh]) OR (Calcium) OR (“Vitamin D” [Mesh]) OR (vitamin d) OR (“Vitamin A” [Mesh]) OR (“Vitamin A”) OR (“Ascorbic Acid” [Mesh]) OR (“Vitamin C”) OR (Ascorb* OR “ascorbic acid”) OR (Vitamin* OR multivitamin* OR multi-vitamin* OR MMN OR micro-nutrient* OR mineral* OR multimineral* OR multi-mineral OR multinutrient* OR “multiple micronutrient*” OR “food environment” OR advertisement* OR “mass media” OR “supplementary feeding” OR “energy supplement*” OR “protein supplement*” OR “lipid based nutrition” OR LNS)) AND ((“Adolescent Development” [Mesh]) OR (“Adolescent Growth”) OR (“Serum Haemoglobin” OR “Serum micronutrient*” OR “Anthropometric measurement*”)) |
Appendix B. Reasons for Exclusion
| Study | Reason for Exclusion |
| Abrams, 2005 [43] | Intervention given was prebiotic (inulin-type fructans) |
| Ahmed, 2005 [44] | Participants were anemic at baseline and the intervention was therapeutic |
| Ahmed, 2010 [45] | Participants were anemic at baseline and the intervention was therapeutic |
| Angeles-Agdeppa, 1997 [46] | Participants were asymptomatic anemic individuals and the intervention was therapeutic |
| Beasley, 2000 [47] | Participants were infected with schistosomiasis; infection was believed to affect the outcome, and IFA was taken as therapeutic intervention |
| Castillo-Durán, 2001 [47] | The study was from a non-LMIC country |
| Chan, 2006 [48] | The study was carried out in a non-LMIC country |
| Damsgaard, 2012 [49] | Only slightly overweight individuals were enrolled |
| de Oliveira, 2009 [50] | The study was from a non-LMIC country |
| Deshmukh, 2008 [51] | This study did not have an appropriate control group |
| Diogenes, 2013 [52] | The study was from a non-LMIC country |
| Dongre, 2011 [53] | This study did not have an appropriate control group |
| Eftekhari, 2006 [54] | Participants were iron-deficient at baseline and the intervention was therapeutic |
| Friis, 1997 [55] | 93% of the participants were infected by schistosomiasis; infection was believed to affect the outcome |
| Ganmaa, 2017 [56] | Participants were asymptomatic vitamin D-deficient individuals according to the inclusion criteria; intervention was used as therapeutic intervention |
| Ilich-Ernst, 1998 [57] | The study was carried out in a non-LMIC country |
| Kianfer, 2000 [58] | The intervention was therapeutic |
| Kotecha, 2009 [59] | The study did not have an appropriate control group |
| Lambert, 2009 [60] | The study was carried out in a non-LMIC country |
| Ma, 2014 [61] | The study did not have an appropriate control group |
| Manger, 2008 [62] | The study population included children and adolescents, and the study author suggested that the data for the adolescent subgroup were too small |
| Mann, 2002 [63] | Participants were asymptomatic anemic individuals; grouping was done based on energy intakes |
| McKenna, 1997 [64] | The study was carried out in a non-LMIC country |
| Mwaniki, 2002 [65] | The intervention was therapeutic |
| Pilz, 2017 [66] | The methods described inclusion criteria of age 18–45 years but results showed that the age of participants was between 22 and 29 years; participants were not adolescents |
| Prentice, 2005 [67] | The study was carried out in a non-LMIC country |
| Dibba, 2000 [68] | The study population included children and adolescents; the corresponding authors were contacted for the adolescent subgroup data; however, we did not receive any response |
| Rerksuppaphol, 2016 [69] | The study population included children and adolescents; the corresponding authors were contacted for the adolescent subgroup data; however, we did not receive any response |
| Rousham, 2013 [70] | Intervention was used as therapeutic intervention |
| Sarma, 2006 [71] | The study population included children and adolescents; the corresponding authors were contacted for the adolescent subgroup data; however, we did not receive any response |
| Schou, 2003 [72] | The study was carried out in a non-LMIC country |
| Shah, 2002 [73] | Intervention was used as therapeutic intervention |
| Silk, 2015 [74] | The study was carried out in a non-LMIC country |
| Sunawang, 2009 [75] | The participants were not adolescents |
| Tee, 1999 [76] | Participants were asymptomatic anemic individuals; intervention was used as a therapeutic agent in this study |
| Viljakainen, 2006 [77] | The study was carried out in a non-LMIC country |
| White, 2015 [78] | The study was carried out in a non-LMIC country |
| Yusoff, 2012 [79] | The study was from a non-LMIC country |
References
- World Health Organization. Health for the World’s Adolescents: A Second Chance in the Second Decade: Summary; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Akseer, N.; Al-Gashm, S.; Mehta, S.; Mokdad, A.; Bhutta, Z.A. Global and regional trends in the nutritional status of young people: a critical and neglected age group. Ann. N. Y. Acad. Sci. 2017, 1393, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Gore, F.M.; Bloem, P.J.; Patton, G.C.; Ferguson, J.; Joseph, V.; Coffey, C.; Sawyer, S.M.; Mathers, C.D. Global burden of disease in young people aged 10–24 years: A systematic analysis. Lancet 2011, 377, 2093–2102. [Google Scholar] [CrossRef]
- Reiner, R.C.; Olsen, H.E.; Ikeda, C.T.; Echko, M.M.; Ballestreros, K.E.; Manguerra, H.; Martopullo, I.; Millear, A.; Shields, C.; Smith, A. Diseases, injuries, and risk factors in child and adolescent health, 1990 to 2017: findings from the Global Burden of Diseases, Injuries, and Risk Factors 2017 Study. JAMA Pediatrics 2019, 173, e190337. [Google Scholar] [PubMed]
- Christian, P.; Smith, E.R. Adolescent undernutrition: Global burden, physiology, and nutritional risks. Ann. Nutr. Metab. 2018, 72, 316–328. [Google Scholar] [CrossRef]
- Onyango, A.W. Promoting healthy growth and preventing childhood stunting: a global challenge. Matern. Child Nutr. 2013, 9 (Suppl. 2), 1–5. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Patton, G.C.; Sawyer, S.M.; Santelli, J.S.; Ross, D.A.; Afifi, R.; Allen, N.B.; Arora, M.; Azzopardi, P.; Baldwin, W.; Bonell, C.; et al. Our future: A Lancet commission on adolescent health and wellbeing. Lancet 2016, 387, 2423–2478. [Google Scholar] [CrossRef]
- Ladipo, O.A. Nutrition in pregnancy: Mineral and vitamin supplements. Am. J. Clin. Nutr. 2000, 72, 280S–290S. [Google Scholar] [CrossRef]
- Caleyachetty, R.; Thomas, G.N.; Kengne, A.P.; Echouffo-Tcheugui, J.B.; Schilsky, S.; Khodabocus, J.; Uauy, R. The double burden of malnutrition among adolescents: analysis of data from the Global School-Based Student Health and Health Behavior in School-Aged Children surveys in 57 low- and middle-income countries. Am. J. Clin. Nutr. 2018, 108, 414–424. [Google Scholar] [CrossRef]
- Keats, E.C.; Rappaport, A.I.; Shah, S.; Oh, C.; Jain, R.; Bhutta, Z.A. The Dietary Intake and Practices of Adolescent Girls in Low- and Middle-Income Countries: A Systematic Review. Nutrients 2018, 10, 1978. [Google Scholar] [CrossRef]
- Story, M.; Lytle, L.A.; Birnbaum, A.S.; Perry, C.L. Peer-led, school-based nutrition education for young adolescents: feasibility and process evaluation of the TEENS study. J. Sch. Health 2002, 72, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Oenema, A.; Brug, J.; Lechner, L. Web-based tailored nutrition education: results of a randomized controlled trial. Health Educ. Res. 2001, 16, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, W.; Werkman, A.; Brug, J. A systematic review of randomized trials on the effectiveness of computer-tailored education on physical activity and dietary behaviors. Ann. Behav. Med. 2006, 31, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rodrigo, C.; Aranceta, J. School-based nutrition education: lessons learned and new perspectives. Public Health Nutr. 2001, 4, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Blasbalg, T.L.; Wispelwey, B.; Deckelbaum, R.J. Econutrition and utilization of food-based approaches for nutritional health. Food Nutr. Bull. 2011, 32, S4–S13. [Google Scholar] [CrossRef]
- Zlotkin, S.H.; Schauer, C.; Christofides, A.; Sharieff, W.; Tondeur, M.C.; Hyder, S.M. Micronutrient sprinkles to control childhood anaemia. PLoS Med. 2005, 2, e1. [Google Scholar] [CrossRef]
- Sguassero, Y.; de Onis, M.; Bonotti, A.M.; Carroli, G. Community-based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries. Cochrane Database Syst. Rev. 2012, 6, CD005039. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Moin, A.; Das, J.K.; Salam, R.A.; Bhutta, Z.A. Systematic review on evidence-based adolescent nutrition interventions. Ann. N. Y. Acad. Sci. 2017, 1393, 34–50. [Google Scholar] [CrossRef]
- Salam, R.A.; Hooda, M.; Das, J.K.; Arshad, A.; Lassi, Z.S.; Middleton, P.; Bhutta, Z.A. Interventions to Improve Adolescent Nutrition: A Systematic Review and Meta-Analysis. J. Adolesc. Health 2016, 59, S29–S39. [Google Scholar] [CrossRef]
- World Health Organization. Monitoring the Building Blocks of Health Systems: A Handbook of Indicators and Their Measurement Strategies; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- The Nordic Cochrane Centre. Review Manager, 5.3th ed.; Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- EPOC Resources for Review Authors. 2017. Available online: epoc.cochrane.org/resources/epoc-resources-review-authors (accessed on 15 September 2019).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.N.; Gomber, S.; Bisht, H.; Som, M. Anemia prophylaxis in adolescent school girls by weekly or daily iron-folate supplementation. Indian Pediatr. 2003, 40, 296–301. [Google Scholar] [PubMed]
- Chiplonkar, S.A.; Kawade, R. Effect of zinc- and micronutrient-rich food supplements on zinc and vitamin A status of adolescent girls. Nutrition 2012, 28, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Februhartanty, J.; Dillon, D.; Khusun, H. Will iron supplementation given during menstruation improve iron status better than weekly supplementation? Asia Pac. J. Clin. Nutr. 2002, 11, 36–41. [Google Scholar] [CrossRef]
- Goyle, A. Effect of micronutrient fortified biscuit supplementation on the weight, height and BMI of adolescent girls. Coll. Antropol. 2012, 36, 573–579. [Google Scholar]
- Hettiarachchi, M.; Liyanage, C.; Wickremasinghe, R.; Hilmers, D.C.; Abrams, S.A. The efficacy of micronutrient supplementation in reducing the prevalence of anaemia and deficiencies of zinc and iron among adolescents in Sri Lanka. Eur. J. Clin. Nutr. 2008, 62, 856–865. [Google Scholar] [CrossRef]
- Khadilkar, A.V.; Sayyad, M.G.; Sanwalka, N.J.; Bhandari, D.R.; Naik, S.; Khadilkar, V.V.; Mughal, M.Z. Vitamin D supplementation and bone mass accrual in underprivileged adolescent Indian girls. Asia Pac. J. Clin. Nutr. 2010, 19, 465–472. [Google Scholar]
- Sen, A.; Kanani, S.J. Impact of iron-folic acid supplementation on cognitive abilities of school girls in Vadodara. Indian Pediatr. 2009, 46, 137–143. [Google Scholar]
- Xueqin, D.; Zhu, K.; Trube, A.; Zhang, Q.; Ma, G.; Hu, X.; Fraser, D.R.; Greenfield, H. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10–12 years in Beijing. Br. J. Nutr. 2004, 92, 159–168. [Google Scholar]
- Ziauddin Hyder, S.; Haseen, F.; Khan, M.; Schaetzel, T.; Jalal, C.S.; Rahman, M.; Lönnerdal, B.; Mannar, V.; Mehansho, H. A multiple-micronutrient-fortified beverage affects hemoglobin, iron, and vitamin A status and growth in adolescent girls in rural Bangladesh. The Journal of nutrition 2007, 137, 2147–2153. [Google Scholar] [CrossRef]
- Soekarjo, D.; De Pee, S.; Kusin, J.; Schreurs, W.; Schultink, W.; Bloem, M. Effectiveness of weekly vitamin A (10 000 IU) and iron (60 mg) supplementation for adolescent boys and girls through schools in rural and urban East Java, Indonesia. Eur. J. Clin. Nutr. 2004, 58, 927. [Google Scholar] [CrossRef] [PubMed]
- Goyle, A.; Prakash, S. Effect of supplementation of micronutrient fortified biscuits on haemoglobin and serum iron levels of adolescent girls from Jaipur city, India. Nutr. Food Sci. 2010, 40, 477–484. [Google Scholar] [CrossRef]
- Sen, A.; Kanani, S.J. Physical Work Capacity of Young Underprivileged School Girls: Impact of Daily vs Intermittent Iron-Folic Acid Supplementation - A Randomized Controlled Trial. Indian Pediatr. 2009, 46, 849–854. [Google Scholar] [PubMed]
- Sen, A.; Kanani, S. Intermittent iron folate supplementation: impact on hematinic status and growth of school girls. ISRN Hematol. 2012, 2012, 482153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, K.; Du, X.; Cowell, C.T.; Greenfield, H.; Blades, B.; Dobbins, T.A.; Zhang, Q.; Fraser, D.R. Effects of school milk intervention on cortical bone accretion and indicators relevant to bone metabolism in Chinese girls aged 10-12 y in Beijing. Am. J. Clin. Nutr. 2005, 81, 1168–1175. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, Q.; Foo, L.H.; Trube, A.; Ma, G.; Hu, X.; Du, X.; Cowell, C.T.; Fraser, D.R.; Greenfield, H. Growth, bone mass, and vitamin D status of Chinese adolescent girls 3 y after withdrawal of milk supplementation. Am. J. Clin. Nutr. 2006, 83, 714–721. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Kumar, R.; Bhutta, Z.A. Micronutrient fortification of food and its impact on woman and child health: a systematic review. Syst. Rev. 2013, 2, 67. [Google Scholar] [CrossRef]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Gunn, S.K.; Gundberg, C.M.; Carpenter, T.O. Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J. Clin. Endocrinol. Metab. 2005, 90, 5576–5581. [Google Scholar] [CrossRef]
- Ahmed, F.; Khan, M.R.; Akhtaruzzaman, M.; Karim, R.; Marks, G.C.; Banu, C.P.; Nahar, B.; Williams, G. Efficacy of twice-weekly multiple micronutrient supplementation for improving the hemoglobin and micronutrient status of anemic adolescent schoolgirls in Bangladesh. Am. J. Clin. Nutr. 2005, 82, 829–835. [Google Scholar] [CrossRef]
- Ahmed, F.; Khan, M.R.; Akhtaruzzaman, M.; Karim, R.; Williams, G.; Torlesse, H.; Darnton-Hill, I.; Dalmiya, N.; Banu, C.P.; Nahar, B. Long-Term Intermittent Multiple Micronutrient Supplementation Enhances Hemoglobin and Micronutrient Status More Than Iron plus Folic Acid Supplementation in Bangladeshi Rural Adolescent Girls with Nutritional Anemia. J. Nutr. 2010, 140, 1879–1886. [Google Scholar] [CrossRef]
- Angeles-Agdeppa, I.; Schultink, W.; Sastroamidjojo, S.; Gross, R.; Karyadi, D. Weekly micronutrient supplementation to build iron stores in female Indonesian adolescents. Am. J. Clin. Nutr. 1997, 66, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Beasley, N.M.; Tomkins, A.M.; Hall, A.; Lorri, W.; Kihamia, C.M.; Bundy, D.A. The impact of weekly iron supplementation on the iron status and growth of adolescent girls in Tanzania. Trop. Med. Int. Health 2000, 5, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.M.; McElligott, K.; McNaught, T.; Gill, G. Effects of dietary calcium intervention on adolescent mothers and newborns: A randomized controlled trial. Obstet. Gynecol. 2006, 108, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Damsgaard, C.T.; Molgaard, C.; Matthiessen, J.; Gyldenlove, S.N.; Lauritzen, L. The effects of n-3 long-chain polyunsaturated fatty acids on bone formation and growth factors in adolescent boys. Pediatr. Res. 2012, 71, 713–719. [Google Scholar] [CrossRef]
- De Oliveira, K.d.J.F.; Donangelo, C.M.; de Oliveira Jr, A.V.; da Silveira, C.L.P.; Koury, J.C. Effect of zinc supplementation on the antioxidant, copper, and iron status of physically active adolescents. Cell Biochem. Funct. Cell. Biochem. Modul. Act. Agents Dis. 2009, 27, 162–166. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Garg, B.S.; Bharambe, M.S. Effectiveness of weekly supplementation of iron to control anaemia among adolescent girls of Nashik, Maharashtra, India. J. Health Popul. Nutr. 2008, 26, 74–78. [Google Scholar]
- Diogenes, M.E.; Bezerra, F.F.; Rezende, E.P.; Taveira, M.F.; Pinhal, I.; Donangelo, C.M. Effect of calcium plus vitamin D supplementation during pregnancy in Brazilian adolescent mothers: a randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2013, 98, 82–91. [Google Scholar] [CrossRef]
- Dongre, A.R.; Deshmukh, P.R.; Garg, B.S. Community-led initiative for control of anemia among children 6 to 35 months of age and unmarried adolescent girls in rural Wardha, India. Food Nutr. Bull. 2011, 32, 315–323. [Google Scholar] [CrossRef]
- Eftekhari, M.H.; Simondon, K.B.; Jalali, M.; Keshavarz, S.A.; Elguero, E.; Eshraghian, M.R.; Saadat, N. Effects of administration of iron, iodine and simultaneous iron-plus-iodine on the thyroid hormone profile in iron-deficient adolescent Iranian girls. Eur. J. Clin. Nutr. 2006, 60, 545–552. [Google Scholar] [CrossRef]
- Friis, H.; Ndhlovu, P.; Mduluza, T.; Kaondera, K.; Sandstrom, B.; Michaelsen, K.F.; Vennervald, B.J.; Christensen, N.O. The impact of zinc supplementation on growth and body composition: A randomized, controlled trial among rural Zimbabwean schoolchildren. Eur. J. Clin. Nutr. 1997, 51, 38–45. [Google Scholar] [CrossRef]
- Ganmaa, D.; Stuart, J.J.; Sumberzul, N.; Ninjin, B.; Giovannucci, E.; Kleinman, K.; Holick, M.F.; Willett, W.C.; Frazier, L.A.; Rich-Edwards, J.W. Vitamin D supplementation and growth in urban Mongol school children: Results from two randomized clinical trials. PLoS ONE 2017, 12, e0175237. [Google Scholar] [CrossRef]
- Ilich-Ernst, J.Z.; McKenna, A.A.; Badenhop, N.E.; Clairmont, A.C.; Andon, M.B.; Nahhas, R.W.; Goel, P.; Matkovic, V. Iron status, menarche, and calcium supplementation in adolescent girls. Am. J. Clin. Nutr. 1998, 68, 880–887. [Google Scholar] [CrossRef]
- Kianfar, H.; Kimiagar, M.; Ghaffarpour, M. Effect of daily and intermittent iron supplementation on iron status of high school girls. Int. J. Vitam. Nutr. Res. 2000, 70, 172–177. [Google Scholar] [CrossRef]
- Kotecha, P.V.; Nirupam, S.; Karkar, P.D. Adolescent girls’ Anaemia Control Programme, Gujarat, India. Indian J. Med. Res. 2009, 130, 584–589. [Google Scholar]
- Lambert, H.L.; Eastell, R.; Karnik, K.; Russell, J.M.; Barker, M.E. Calcium supplementation and bone mineral accretion in adolescent girls: an 18-mo randomized controlled trial with 2-y follow-up. Am. J. Clin. Nutr. 2008, 87, 455–462. [Google Scholar] [CrossRef]
- Ma, X.M.; Huang, Z.W.; Yang, X.G.; Su, Y.X. Calcium supplementation and bone mineral accretion in Chinese adolescents aged 12–14 years: A 12-month, dose-response, randomised intervention trial. Br. J. Nutr. 2014, 112, 1510–1520. [Google Scholar] [CrossRef]
- Manger, M.S.; McKenzie, J.E.; Winichagoon, P.; Gray, A.; Chavasit, V.; Pongcharoen, T.; Gowachirapant, S.; Ryan, B.; Wasantwisut, E.; Gibson, R.S. A micronutrient-fortified seasoning powder reduces morbidity and improves short-term cognitive function, but has no effect on anthropometric measures in primary school children in northeast Thailand: a randomized controlled trial. Am. J. Clin. Nutr. 2008, 87, 1715–1722. [Google Scholar] [CrossRef]
- Mann, S.K.; Kaur, S.; Bains, K. Iron and energy supplementation improves the physical work capacity of female college students. Food Nutr. Bull. 2002, 23, 57–64. [Google Scholar] [CrossRef]
- McKenna, A.A.; Ilich, J.Z.; Andon, M.B.; Wang, C.; Matkovic, V. Zinc balance in adolescent females consuming a low- or high-calcium diet. Am. J. Clin. Nutr. 1997, 65, 1460–1464. [Google Scholar] [CrossRef]
- Mwaniki, D.; Omondi, B.; Muniu, E.; Thiong’o, F.; Ouma, J.; Magnussen, P.; Geissler, P.W.; Michaelsen, K.F.; Friis, H. Effects on serum retinol of multi-micronutrient supplementation and multi-helminth chemotherapy: a randomised, controlled trial in Kenyan school children. Eur. J. Clin. Nutr. 2002, 56, 666–673. [Google Scholar] [CrossRef]
- Pilz, S.; Hahn, A.; Schon, C.; Wilhelm, M.; Obeid, R. Effect of Two Different Multimicronutrient Supplements on Vitamin D Status in Women of Childbearing Age: A Randomized Trial. Nutrients 2017, 9, 30. [Google Scholar] [CrossRef]
- Prentice, A.; Ginty, F.; Stear, S.J.; Jones, S.C.; Laskey, M.A.; Cole, T.J. Calcium supplementation increases stature and bone mineral mass of 16-to 18-year-old boys. J. Clin. Endocrinol. Metab. 2005, 90, 3153–3161. [Google Scholar] [CrossRef]
- Dibba, B.; Prentice, A.; Ceesay, M.; Stirling, D.M.; Cole, T.J.; Poskitt, E.M. Effect of calcium supplementation on bone mineral accretion in gambian children accustomed to a low-calcium diet. Am. J. Clin. Nutr. 2000, 71, 544–549. [Google Scholar] [CrossRef]
- Rerksuppaphol, S.; Rerksuppaphol, L. Effect of zinc plus multivitamin supplementation on growth in school children. Pediatr. Int. 2016, 58, 1193–1199. [Google Scholar] [CrossRef]
- Rousham, E.K.; Uzaman, B.; Abbott, D.; Lee, S.F.; Mithani, S.; Roschnik, N.; Hall, A. The effect of a school-based iron intervention on the haemoglobin concentration of school children in north-west Pakistan. Eur. J. Clin. Nutr. 2013, 67, 1188–1192. [Google Scholar] [CrossRef]
- Sarma, K.V.R.; Udaykumar, P.; Balakrishna, N.; Vijayaraghavan, K.; Sivakumar, B. Effect of micronutrient supplementation on health and nutritional status of schoolchildren: growth and morbidity. Nutrition 2006, 22, S8–S14. [Google Scholar] [CrossRef]
- Schou, A.J.; Heuck, C.; Wolthers, O.D. A randomized, controlled lower leg growth study of vitamin D supplementation to healthy children during the winter season. Ann. Hum. Biol. 2003, 30, 214–219. [Google Scholar] [CrossRef]
- Shah, B.K.; Gupta, P. Weekly vs daily iron and folic acid supplementation in adolescent Nepalese girls. Arch. Pediatr. Adolesc. Med. 2002, 156, 131–135. [Google Scholar] [CrossRef]
- Silk, L.N.; Greene, D.A.; Baker, M.K.; Jander, C.B. Tibial bone responses to 6-month calcium and vitamin D supplementation in young male jockeys: A randomised controlled trial. Bone 2015, 81, 554–561. [Google Scholar] [CrossRef]
- Sunawang; Utomo, B.; Hidayat, A.; Kusharisupeni; Subarkah. Preventing low birthweight through maternal multiple micronutrient supplementation: A cluster-randomized, controlled trial in Indramayu, West Java. Food Nutr. Bull. 2009, 30, S488–S495. [Google Scholar] [CrossRef]
- Tee, E.S.; Kandiah, M.; Awin, N.; Chong, S.M.; Satgunasingam, N.; Kamarudin, L.; Milani, S.; Dugdale, A.E.; Viteri, F.E. School-administered weekly iron-folate supplements improve hemoglobin and ferritin concentrations in Malaysian adolescent girls. Am. J. Clin. Nutr. 1999, 69, 1249–1256. [Google Scholar] [CrossRef]
- Viljakainen, H.T.; Natri, A.M.; Karkkainen, M.; Huttunen, M.M.; Palssa, A.; Jakobsen, J.; Cashman, K.D.; Molgaard, C.; Lamberg-Allardt, C. A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention. J. Bone Miner. Res. 2006, 21, 836–844. [Google Scholar] [CrossRef]
- White, D.J.; Cox, K.H.; Peters, R.; Pipingas, A.; Scholey, A.B. Effects of Four-Week Supplementation with a Multi-Vitamin/Mineral Preparation on Mood and Blood Biomarkers in Young Adults: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2015, 7, 9005–9017. [Google Scholar] [CrossRef]
- Yusoff, H.; Daud, W.N.; Ahmad, Z. Nutrition education and knowledge, attitude and hemoglobin status of Malaysian adolescents. Southeast Asian J. Trop. Med. Public Health 2012, 43, 192–200. [Google Scholar]
| Study | Study Design | Setting | Participants | Intervention/Control | Outcomes |
|---|---|---|---|---|---|
| Agarwal, 2003 [27] | Cluster randomized trial | Four government senior secondary schools, Delhi, India | 2088 adolescent girls | 100 mg elemental iron and 500 μg folic acid in the form of oral tablets was provided for 100 days Group 1: Daily treatment (N = 702) Group 2: Weekly treatment: (N = 695) Control: Control group did not receive any tablets during the intervention period (N = 691) | Hemoglobin, plasma ferritin, anemia |
| Chiplonkar, 2012 [28] | Individually randomized trial | A secondary girl’s school in Pune City, Maharashtra, India | 180 adolescent girls | Intervention group 1 (N = 60) Supplement was provided in the form of six different snacks to each girl with one snack (average amount 100 g/serving) per day for 6 school days in a week. The average zinc content of the food supplements was 2.2 ± 0.4 mg/serving Intervention group 2 (N = 59) The ayurvedic zinc tablet containing 20 mg of jasad bhasma, equivalent to 16.6 mg of elemental zinc, was given to each girl every day for 6 school days/week under the guidance of an ayurvedic doctor Control (N = 53) No supplement was given to the control group | Dietary intake, hemoglobin, plasma zinc, plasma beta-carotene, plasma retinol, plasma vitamin C |
| Februhartanty, 2002 [29] | Individually randomized trial | Junior high schools in Kupang, East Nusa Tenggara, in the eastern part of Indonesia | 150 female adolescents | The iron tablet used in this study contained 60 mg elemental iron and 0.25 mg folic acid in the form of 200 mg ferrous sulfate Group 1: Weekly iron tablets (N = 50). Group 2: Iron tablet for four consecutive days during their menstruation cycle (N = 50) Control: Placebo tablet (N = 50) | Hemoglobin, ferritin level |
| Goyle, 2012 [30,37] | Individually randomized trial | Government school near university of Rajasthan, Jaipur, India | 107 adolescent girls | Intervention group (N = 53): 100 g of biscuits fortified with one RDA levels of vitamin A, iron, folic acid, vitamin C, and iodine were provided for all working days during 4 months Control (placebo) (N = 54): 100 g of biscuits furnishing 497 kcal and 11.36 g of protein per day were provided to the control group for 4 months | Body mass index (BMI), BMI Z-score, weight-for-height, height-for-age |
| Hettiarrachchi, 2007 [31] | Individually randomized trial | School in the Galle district, Sri Lanka | 821 school children | Children were supplemented with two capsules per day containing the following: Group 1: Iron (50 mg/day) in the form of ferrous fumarate (N = 202) Group 2: Zinc (14 mg/day) in the form of zinc sulfate (N = 213) Group 3: Combined (iron + zinc) (N = 216) Group 4: Placebo made of anhydrous lactose (N = 190) | Height, weight, body mass index (BMI), height-for-age, weight-for-age, stunted, underweight, hemoglobin, serum zinc, serum ferritin |
| Hyder, 2007 [35] | Individually randomized trial | Conducted in 54 non-formal primary education schools operated by the Bangladesh Rural Advancement Committee (BRAC) in Sherpur district, Dhaka | 1125 adolescent girls | Group 1 (N = 559): Powdered beverage fortified with multiple micronutrients and packaged in sachets Control (N = 566): Placebo beverage | Weight, height, mid-upper arm circumference (MUAC), body mass index (BMI), hemoglobin, serum ferritin, serum retinol, serum zinc |
| Khadilkar, 2010 [32] | Individually randomized trial | State run school in Pune, India | 50 adolescent girls | Group 1 (N = 25): Subjects in the treatment group were administered 6 vitamin D2 (ergocalciferol; Celltech, UK) tablets each containing 1.25 mg (50,000 IU) orally at 1, 4, 7, and 10 months Group 2 (N = 25): Placebo group; the local pharmacist prepared tablets containing only sucrose | Total body bone mineral content, lumbar spine bone mineral content and lumbar spine bone mineral apparent density, total body lean, fat mass, and serum concentrations of biochemical parameters |
| Sen, 2009 [33,38,39] | Cluster randomized trial | Municipal primary schools in Vadodara, India | 358 girls | Group 1 (N = 94): The participants were given IFA tablets (100 mg elemental iron + 0.5 mg folic acid) once weekly Group 2 (N = 118): The participants were given IFA tablets (100 mg elemental iron + 0.5 mg folic acid) twice weekly Group 3 (N = 81): The participants were given IFA tablets (100 mg elemental iron + 0.5 mg folic acid) daily Group 4 (N = 65): Control group did not receive any intervention | Hemoglobin, body mass index (BMI), cognitive test score) |
| Soekarjo, 2004 [36] | Cluster randomized trial | Schools in Indonesia from both urban and rural locations | 5166 adolescents aged 12–15 years | Group 1 (N = 1033): weekly 10,000 IU vitamin A Group 2 (N = 1045): weekly 60 mg elemental iron (as ferrous sulfate) plus 250 mg folate Group 3 (N = 1130): weekly 10,000 IU vitamin A and 60 mg elemental iron plus 250 mg folate Group 4 (N = 1958): Did not receive any supplement | Hemoglobin concentration, serum retinol concentrations |
| Zhu, 2005 [34,40,41] | Individually randomized trial | Schools in urban Beijing, China | 757 adolescent girls | Group 1 (N = 238): Girls consumed a carton of 330 mL milk fortified with Ca on school days over the study period Group 2 (N = 260): Girls received the same quantity of milk additionally fortified with 5 or 8 mg cholecalciferol Group 3 (N = 259): Control girls did not receive any intervention | Nutrient intake, bone mineral content, bone mineral density, serum PTH, serum calcium, height, weight and vitamin D levels |
| Studies | Service Delivery | Health Workforce | Information Systems | Access to Supplies | Financing | Leadership |
|---|---|---|---|---|---|---|
| Agarwal, 2003 | Delivery of iron supplements in school | Probably through school teachers | Not specified | Iron/folate supplements were provided by researchers | UNICEF, New Delhi | Researchers |
| Chiplonkar, 2012 | Delivery of food supplements and zinc tablets in school | Probably through school teachers | Not specified | Food supplements and zinc tablets provided by researchers | Zensar Foundation, Pune, India | Researchers |
| Februhartanty, 2002 | Delivery of iron supplements in schools | Delivered through school teachers | Not specified | Iron supplements were provided by researchers | SEAMEO-TROPMED Regional Center for Community Nutrition in Jakarta | Researchers |
| Goyle, 2012 | Supplement biscuits in schools | Probably through school teachers | Not specified | Biscuits were supplied through researcher | University Grants Commission, New Delhi, India | Researchers |
| Hettiarachchi, 2007 | Iron and zinc supplements provided in schools | Delivered through teachers and investigators | Not specified | Supplements were provided by the researchers | The study was funded by the International Atomic Energy Agency | Researchers |
| Hyder, 2007 | Iron fortified beverage provided in school | Delivered through school teachers with the assistance of the Bangladesh Rural Advancement Committee (BRAC) community health workers | Not specified | Supplements were provided by the researchers | Supported by the Micronutrient Initiative, Ottawa, Canada | Bangladesh Rural Advancement Committee (BRAC) |
| Khadilkar, 2010 | Vitamin D supplements were provided in school | The tablets were supplied to participants monthly by trial staff | Not specified | Supplements were provided by the researchers | Not specified | Researchers |
| Sen, 2009 | Iron/folic acid supplements were provided in schools | Investigators, monitors, class teachers | Not specified | Supplements were provided by the researchers | None | Researchers |
| Soekarjo, 2004 | Vitamin A, iron, and folate supplements were provided in the schools | Field workers supervised the supplement intake | Not specified | Supplements were produced locally and provided by the researcher | This study was funded by USAID through the OMNI project | Researchers |
| Zhu, 2005 | Milk supplementation given in schools | Probably through school teachers | Not specified | Milk supplementation given in schools | Australian Dairy Research and Development Corporation, Murray Goulburn Co-operative Co. Limited, and the Nestle’ Foundation provided financial support for the laboratory analyses | Researchers |
| Patient or Population: Adolescents Settings: Schools Intervention: Micronutrient supplementation/fortification Comparison: Placebo/no supplementation/no fortification | |||||
| Outcomes | Illustrative Comparative Risks * (95% CI) | Relative Effect (95% CI) | No of Participants (Studies) | Quality of the Evidence (GRADE) | |
| Assumed Risk | Corresponding Risk | ||||
| Placebo/No Supplementation | Micronutrient Supplementation/Fortification | ||||
| Daily Iron Supplementation with or without Folic Acid: Anemia | Study population | RR 1.04 (0.88 to 1.24) | 1160 (one study) | ⊕⊕⊝⊝ low 1,2 | |
| 206 of 579 | 216 of 581 | ||||
| Weekly Iron Supplementation with or without Folic Acid: Anemia | Study population | RR 1.07 (0.91 to 1.26) | 1274 (one study) | ⊕⊕⊝⊝ low 1,2 | |
| 206 of 579 | 265 of 695 | ||||
| Calcium/Vitamin D Supplementation/Fortification: BMI | Study population | MD −0.01 (−1.2 to 1.17) | 730 (2 studies) | ⊕⊝⊝⊝ very low 1,2,3 | |
| The mean BMI ranged between 18.15 and 18.5 | The mean BMI ranged between 17.05 and 19.1 | ||||
| Iron Supplementation with or without Folic Acid: BMI | Study population | MD 0.29 (−0.25 to 0.83) | 652 (2 studies) | ⊕⊝⊝⊝ very low 1,2,3 | |
| The mean BMI ranged between 15.78 and 16.23 | The mean BMI ranged between 15.67 and 17.25 | ||||
| Zinc Supplementation: BMI | Study population | MD 0.35 (−0.15 to 0.85) | 382 (one study) | ⊕⊝⊝⊝ very low 1,2,3 | |
| The mean BMI was 16.23 | The mean BMI was 16.58 | ||||
| MMN Fortification: BMI | Study population | MD 0.23 (−0.11 to 0.57) | 943 (2 studies) | ⊕⊝⊝⊝ very low 1,2,3 | |
| The mean BMI ranged between 15.27 and 16.5 | The mean BMI ranged between 15.42 and 17.1 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salam, R.A.; Das, J.K.; Ahmed, W.; Irfan, O.; Sheikh, S.S.; Bhutta, Z.A. Effects of Preventive Nutrition Interventions among Adolescents on Health and Nutritional Status in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 49. https://doi.org/10.3390/nu12010049
Salam RA, Das JK, Ahmed W, Irfan O, Sheikh SS, Bhutta ZA. Effects of Preventive Nutrition Interventions among Adolescents on Health and Nutritional Status in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(1):49. https://doi.org/10.3390/nu12010049
Chicago/Turabian StyleSalam, Rehana A, Jai K Das, Wardah Ahmed, Omar Irfan, Sana Sadiq Sheikh, and Zulfiqar A Bhutta. 2020. "Effects of Preventive Nutrition Interventions among Adolescents on Health and Nutritional Status in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis" Nutrients 12, no. 1: 49. https://doi.org/10.3390/nu12010049
APA StyleSalam, R. A., Das, J. K., Ahmed, W., Irfan, O., Sheikh, S. S., & Bhutta, Z. A. (2020). Effects of Preventive Nutrition Interventions among Adolescents on Health and Nutritional Status in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients, 12(1), 49. https://doi.org/10.3390/nu12010049





