Mung Bean Protein Supplement Improves Muscular Strength in Healthy, Underactive Vegetarian Adults
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
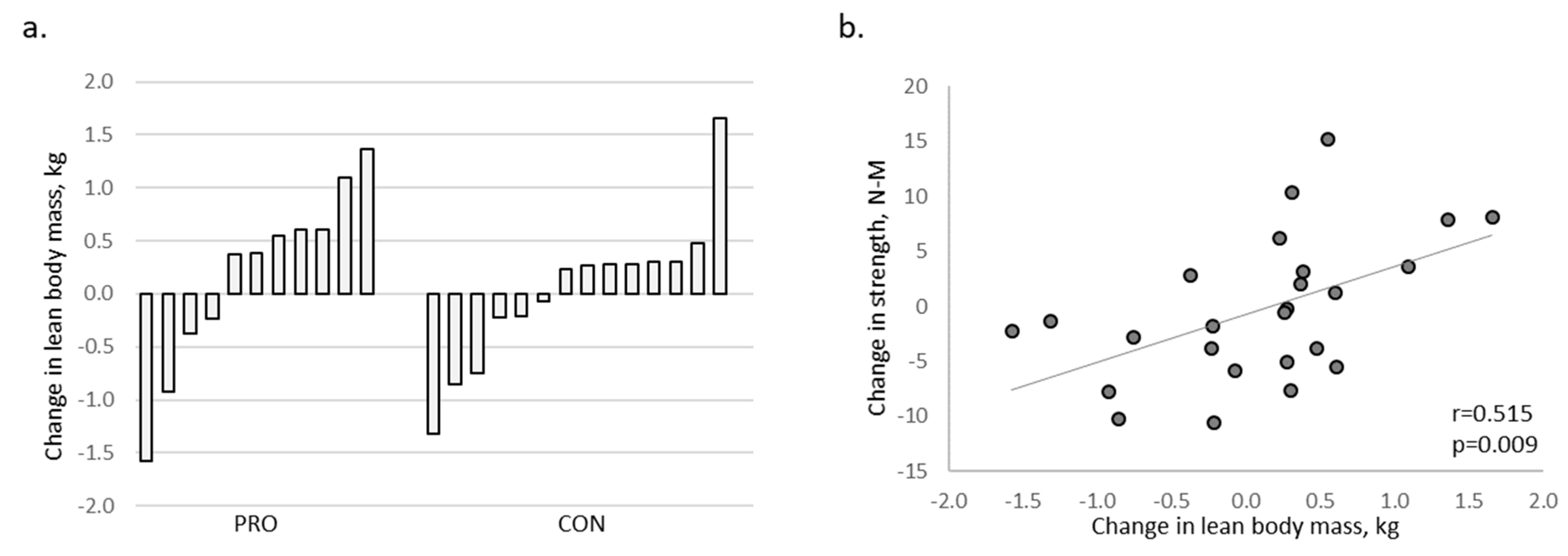
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association between plant-based dietary patterns and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-metabolic benefits of plant-based diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Rose, D.; Heller, M.C.; Roberto, C.A. Position of the Society for Nutrition Education and Behavior: The importance of including environmental sustainability in dietary guidance. J. Nutr. Educ. Behav. 2019, 51, 3–15. [Google Scholar] [CrossRef]
- Appleby, P.N.; Key, T.J. The long-term health of vegetarians and vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef]
- How Many Adults in the U.S. Are Vegetarian and Vegan? Available online: https://www.vrg.org/nutshell/Polls/2016_adults_veg.htm (accessed on 30 July 2019).
- Nielsen. Plant-Based Proteins Are Gaining Dollar Share among North Americans. Available online: https://www.nielsen.com/us/en/insights/news/2017/plant-based-proteins-are-gaining-dollar-share-among-north-americans.html (accessed on 20 August 2019).
- Nielsen. Plant-Based Food Options Are Sprouting Growth for Retailers. Available online: https://www.nielsen.com/us/en/insights/news/2018/plant-based-food-options-are-sprouting-growth-for-retailers.html (accessed on 20 August 2019).
- De Backer, C.J.S.; Hudders, L. From Meatless Mondays to Meatless Sundays: Motivations for Meat reduction among vegetarians and semi-vegetarians who mildly or significantly reduce their meat intake. Ecol. Food Nutr. 2014, 53, 639–657. [Google Scholar] [CrossRef]
- Key, T.J.; Fraser, G.E.; Thorogood, M.; Appleby, P.N.; Beral, V.; Reeves, G.; Burr, M.L.; Chang-Claude, J.; Frentzel-Beyme, R.; Kuzma, J.W.; et al. Mortality in vegetarians and nonvegetarians: Detailed findings from a collaborative analysis of 5 prospective studies. Am. J. Clin. Nutr. 1999, 70, 516–524. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N.D. Vegetarian dietary patterns and cardiovascular disease. Prog. Cardiovasc. Dis. 2018, 61, 54–61. [Google Scholar] [CrossRef]
- Kwok, C.S.; Umar, S.; Myint, P.K.; Mamas, M.A.; Loke, Y.K. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: A systematic review and meta-analysis. Int. J. Cardiol. 2014, 176, 680–686. [Google Scholar] [CrossRef]
- Fraser, G.E. Vegetarian diets: What do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 2009, 89, 1607–1612. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Spencer, E.A.; Travis, R.C.; Roddam, A.W.; Allen, N.E. Cancer incidence in vegetarians: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am. J. Clin. Nutr. 2009, 89, 1620–1626. [Google Scholar] [CrossRef]
- Appleby, P.N.; Thorogood, M.; Mann, J.I.; Key, T.J.A. The Oxford vegetarian study: An overview. Am. J. Clin. Nutr. 1999, 70, 525–531. [Google Scholar] [CrossRef]
- De Biase, S.G.; Fernandes, S.F.C.; Gianini, R.J.; Duarte, J.L.G. Vegetarian diet and cholesterol and triglycerides levels. Arq. Bras. Cardiol. 2007, 88, 35–39. [Google Scholar] [CrossRef]
- Toohey, M.L.; Harris, M.A.; Williams, D.; Foster, G.; Schmidt, W.D.; Melby, C.L. Cardiovascular disease risk factors are lower in African-American vegans compared to lacto-ovo-vegetarians. J. Am. Coll. Nutr. 1998, 17, 425–434. [Google Scholar] [CrossRef]
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of Vegetarian Diet, Body Weight, and Prevalence of Type 2 Diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Berkow, S.E.; Barnard, N. Vegetarian diets and weight status. Nutr. Rev. 2006, 64, 175–188. [Google Scholar] [CrossRef]
- Jian, Z.H.; Chiang, Y.C.; Lung, C.C.; Ho, C.C.; Ko, P.C.; Nfor, O.N.; Chang, H.C.; Liaw, Y.C.; Liang, Y.C.; Liaw, Y.P. Vegetarian diet and cholesterol and TAG levels by gender. Public Health Nutr. 2015, 18, 721–726. [Google Scholar] [CrossRef]
- Wang, F.L.; Zheng, J.S.; Yang, B.; Jiang, J.J.; Fu, Y.Q.; Li, D. Effects of vegetarian diets on blood lipids: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Levin, S.M.; Barnard, N.D. Association between plant-based diets and plasma lipids: A systematic review and meta-analysis. Nutr. Rev. 2017, 75, 683–698. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishimura, K.; Barnard, N.D.; Takegami, M.; Watanabe, M.; Sekikawa, A.; Okamura, T.; Miyamoto, Y. Vegetarian diets and blood pressure a meta-analysis. JAMA Intern. Med. 2014, 174, 577–587. [Google Scholar] [CrossRef]
- Harland, J.; Garton, L. An update of the evidence relating to plant-based diets and cardiovascular disease, type 2 diabetes and overweight. Nutr. Bull. 2016, 41, 323–338. [Google Scholar] [CrossRef] [Green Version]
- Chuang, S.Y.; Chiu, T.H.T.; Lee, C.Y.; Liu, T.T.; Tsao, C.K.; Hsiung, C.A.; Chiu, Y.F. Vegetarian diet reduces the risk of hypertension independent of abdominal obesity and inflammation: A prospective study. J. Hypertens. 2016, 34, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Tantamango-Bartley, Y.; Jaceldo-Siegl, K.; Fan, J.; Fraser, G. Vegetarian diets and the incidence of cancer in a low-risk population. Epidemiol. Prev. Biomark. 2013, 22, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovas. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. 2011, 28, 549–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.A.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Jung, T.; Inkeles, S.B. Diet and exercise in the treatment of NIDDM—The need for early emphasis. Diabetes Care 1994, 17, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.A.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A.; Jenkins, A.L.; Augustin, L.S.A.; Ludwig, D.S.; Barnard, N.D.; Anderson, J.W. Type 2 diabetes and the vegetarian diet. Am. J. Clin. Nutr. 2003, 78, 610–616. [Google Scholar] [CrossRef]
- Crane, M.G.; Sample, C. Regression of diabetic neuropathy with total vegetarian (vegan) diet. J. Nutr. Med. 1994, 4, 431–439. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Temple, N.; Woodside, J.V. Vegetarian diets, low-meat diets and health: A review. Public Health Nutr. 2012, 15, 2287–2294. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian diets. J. Acad. Nutr. Diet 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. (Eds.) The Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; Institute of Medicine, National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Kniskern, M.A.; Johnston, C.S. Protein dietary reference intakes may be inadequate for vegetarians if low amounts of animal protein are consumed. Nutrition 2011, 27, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Lesser, S. The 2013 FAO report on dietary protein quality evaluation in human nutrition: Recommendations and implications. Nutr. Bull. 2013, 38, 421–428. [Google Scholar] [CrossRef]
- Aubertin-Leheudre, M.; Adlercreutz, H. Relationship between animal protein intake and muscle mass index in healthy women. Br. J. Nutr. 2009, 102, 1803–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novakova, K.; Kummer, O.; Bouitbir, J.; Stoffel, S.D.; Hoerler-Koerner, U.; Bodmer, M.; Roberts, P.; Urwyler, A.; Ehrsam, R.; Krahenbuhl, S. Effect of L-carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetarians. Eur. J. Nutr. 2016, 55, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Van Loon, L.J.C. Dietary protein for athletes: From requirements to optimum adaptation. J. Sport Sci. 2011, 29, 29–38. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan diets: Practical advice for athletes and exercisers. J. Int. Soc. Sport Nutr. 2017, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Thalacker-Mercer, A.E.; Drummond, M.J. The importance of dietary protein for muscle health in inactive, hospitalized older adults. Ann. N.Y. Acad. Sci. 2014, 1328, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef]
- Li, R.; Xia, J.; Zhang, X.; Gathirua-Mwangi, W.G.; Guo, J.J.; Li, Y.F.; McKenzie, S.; Song, Y.Q. Associations of muscle mass and strength with all-cause mortality among us older adults. Med. Sci. Sport Exer. 2018, 50, 458–467. [Google Scholar] [CrossRef]
- Forrest, K.Y.Z.; Williams, A.M.; Leeds, M.J.; Robare, J.F.; Bechard, T.J. Patterns and Correlates of Grip Strength in Older Americans. Curr. Aging Sci. 2018, 11, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Wang, W.J.; Liu, T.W.; Zhang, D.F. association of grip strength with risk of all-cause mortality, cardiovascular diseases, and cancer in community-dwelling populations: A meta-analysis of prospective cohort studies. J. Am. Med. Dir. Assoc. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, L.; Brindisi, J.; Kleppinger, A.; Sullivan, R.; Mangano, K.M.; Bihuniak, J.D.; Kenny, A.M.; Kerstetter, J.E.; Insogna, K.L. Adequate dietary protein is associated with better physical performance among post-menopausal women 60-90 years. J. Nutr. Health Aging 2014, 18, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Goldman, J.D.; Sahyoun, N.R.; Moshfegh, A.J. Association between dietary protein intake and grip strength among adults aged 51 years and over: What we eat in america, national health and nutrition examination survey 2011–2014. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Silventoinen, K.; Magnusson, P.K.E.; Tynelius, P.; Batty, G.D.; Rasmussen, F. Association of body size and muscle strength with incidence of coronary heart disease and cerebrovascular diseases: A population-based cohort study of one million Swedish men. Int. J. Epidemiol. 2009, 38, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.Y.N.; Key, T.J.; Sobiecki, J.G.; Bradbury, K.E. Anthropometric and physiologic characteristics in white and British Indian vegetarians and nonvegetarians in the UK Biobank. Am. J. Clin. Nutr. 2018, 107, 909–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vliet, S.; Burd, N.A.; van Loon, L.J.C. The skeletal muscle anabolic response to plant—Versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W.; Barton, M.L.; Cyr-Campbell, D.; Davey, S.L.; Beard, J.L.; Parise, G.; Evans, W.J. Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. Am. J. Clin. Nutr. 1999, 70, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Godin, G.; Shephard, R.J. A simple method to assess exercise behavior in the community. Can J. Appl. Sport Sci. 1985, 10, 141–146. [Google Scholar] [PubMed]
- Ren, Z.; Huang, C.; Momma, H.; Cui, Y.; Niu, K.; Sugiyama, S.; Nanno, M.; Nagatomi, R. High Tomato and Tomato Product Consumption is Protective Against the Decline in Handgrip Strength Among Japanese Adults: The Oroshisho Study. J. Epidemiol. 2018, 28, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigotsky, A.D.; Contreras, B.; Beardsley, C. Biomechanical implications of skeletal muscle hypertrophy and atrophy: A musculoskeletal model. PeerJ 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sport Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Tsauo, J.Y.; Wu, Y.T.; Cheng, C.P.; Chen, H.C.; Huang, Y.C.; Liou, T.H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Res, P.T.; de Groot, L.; Saris, W.H.M.; van Loon, L.J.C. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Kutty, V.R.; Lanas, F.; Hui, C.; Xiang, Q.Y.; Qian, Z.Z.; Tang, J.H.; Noorhassim, I.; et al. Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: A prospective urban rural epidemiologic (PURE) study. J. Cachexia Sarcopenia Muscle 2016, 7, 535–546. [Google Scholar] [CrossRef]
- Mubarak, A.E. Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 2005, 89, 489–495. [Google Scholar] [CrossRef]
- Tang, D.Y.; Dong, Y.M.; Ren, H.K.; Li, L.; He, C.F. A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem. Cent. J. 2014, 8. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Przybylski, R.; Sultana, B.; Ashraf, M. Chemical composition and antioxidant activity of seeds of different cultivars of mungbean. J. Food. Sci. 2007, 72, 503–510. [Google Scholar] [CrossRef]
- Xu, X.P.; Liu, H.; Tian, L.H.; Dong, X.B.; Shen, S.H.; Qu, L.Q. Integrated and comparative proteomics of high-oil and high-protein soybean seeds. Food Chem. 2015, 172, 105–116. [Google Scholar] [CrossRef]
- FAO/WHO. Energy and Protein Requirements. Report of FAO Nutritional Meeting Series No 52; FAO: Rome, Italy, 1973. [Google Scholar]
- Yi-Shen, Z.; Shuai, S.; FitzGerald, R. Mung bean proteins and peptides: Nutritional, functional and bioactive properties. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.K.; Linnemann, A.R.; Van Boekel, M.; Khetarpaul, N.; Grewal, R.B.; Nout, M.J.R. Mung bean: Technological and nutritional potential. Cri. Rev. Food Sci. 2015, 55, 670–688. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Varkey, A.; Sheshshayee, M.S.; Preston, T.; Kurpad, A.V. Measurement of protein digestibility in humans by a dual-tracer method. Am. J. Clin. Nutr. 2018, 107, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef]
- Ullah, R.; Ullah, Z.; Al-Deyab, S.S.; Adnan, M.; Tariq, A. Nutritional assessment and antioxidant activities of different varieties of vigna radiata. Sci. World J. 2014, 2014, 871753. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.C. Leucine as a pharmaconutrient in health and disease. Curr. Opin. Clin. Nutr. 2012, 15, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.G.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J.C. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Varte, L.R.; Rawat, S.; Singh, I.; Pal, M.S.; Majumdar, D. Diet preference with regional variation of body mass index and hand grip strength of Indian females. Asian J. Med Sci. 2013, 4, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Visrutha, K.V.; Rao, A.P.; Prarthana, K.G.; Chaitra, U. Evaluation of hand grip strength in vegetarian and non vegetarian table tennis players. Indian J. Clin. Anat. Physiol. 2016, 3, 83–85. [Google Scholar] [CrossRef]
- Verlaan, S.; Van Ancum, J.M.; Pierik, V.D.; Van Wijngaarden, J.P.; Scheerman, K.; Meskers, C.G.M.; Maier, A.B. Muscle measures and nutritional status at hospital admission predict survival and independent living of older patients—The empower study. J. Frailty Aging 2017, 6, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Stessman, J.; Rottenberg, Y.; Fischer, M.; Hammerman-Rozenberg, A.; Jacobs, J.M. Handgrip strength in old and very old adults: Mood, cognition, function, and mortality. J. Am. Geriatr. Soc. 2017, 65, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobaus, N.; Gonzalez, M.C.; Schulzke, J.D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Varoni, E.M. Pulses, Healthy, and Sustainable Food Sources for Feeding the Planet. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
| Age (year) | Weight (kg) | BMI (kg/m2) | Lean Body Mass (kg) | Average Grip, kg | Protein (g/kg/ day) | |
|---|---|---|---|---|---|---|
| Vegan (n = 25; 2/23 M/F) | 31.3 ± 9.3 | 63.0 ± 13.5 | 23.4 ± 4.1 | 39.0 ± 8.0 | 26.8 ± 8.0 | 0.74 ± 0.30 |
| Vegetarian (n = 12; 1/11 M/F) | 31.0 ± 9.5 | 70.8 ± 19.2 | 25.2 ± 5.7 | 40.6 ± 9.1 | 24.0 ± 5.4 | 0.79 ± 0.29 |
| PRO (n = 11; 1/10 M/F) | CON (n = 14; 1/13 M/F) | p | Effect | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | ∆ | Pre | Post | ∆ | Value | Size | |
| Lean body mass, kg | 39.7 ± 8.3 | 39.3 ± 8.8 | +0.2 ± 0.9 | 39.9 ± 8.3 | 39.4 ± 8.7 | 0.0 ± 0.7 | 0.598 | 0.012 |
| Average grip, kg | 24.2 ± 5.5 | 24.7 ± 4.5 | +0.5 ± 2.1 | 26.3 ± 9.5 | 26.2 ± 9.4 | −0.1 ± 1.6 | 0.409 | 0.030 |
| Knee flexor (90°/s), N-M | 72.4 ± 19.9 | 74.0 ± 18.4 | +1.5 ± 6.4 | 72.6 ± 24.9 | 70.8 ± 23.7 | −1.8 ± 6.4 | 0.211 | 0.067 |
| Knee extensor (90°/s), N-M | 96.8 ± 30.0 | 98.0 ± 33.7 | +1.2 ± 14.1 | 96.6 ± 38.6 | 90.1 ± 36.3 | −6.5 ± 12.1 | 0.153 | 0.087 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartholomae, E.; Incollingo, A.; Vizcaino, M.; Wharton, C.; Johnston, C.S. Mung Bean Protein Supplement Improves Muscular Strength in Healthy, Underactive Vegetarian Adults. Nutrients 2019, 11, 2423. https://doi.org/10.3390/nu11102423
Bartholomae E, Incollingo A, Vizcaino M, Wharton C, Johnston CS. Mung Bean Protein Supplement Improves Muscular Strength in Healthy, Underactive Vegetarian Adults. Nutrients. 2019; 11(10):2423. https://doi.org/10.3390/nu11102423
Chicago/Turabian StyleBartholomae, Eric, April Incollingo, Maricarmen Vizcaino, Christopher Wharton, and Carol S. Johnston. 2019. "Mung Bean Protein Supplement Improves Muscular Strength in Healthy, Underactive Vegetarian Adults" Nutrients 11, no. 10: 2423. https://doi.org/10.3390/nu11102423





