Influence of Resveratrol on the Immune Response
Abstract
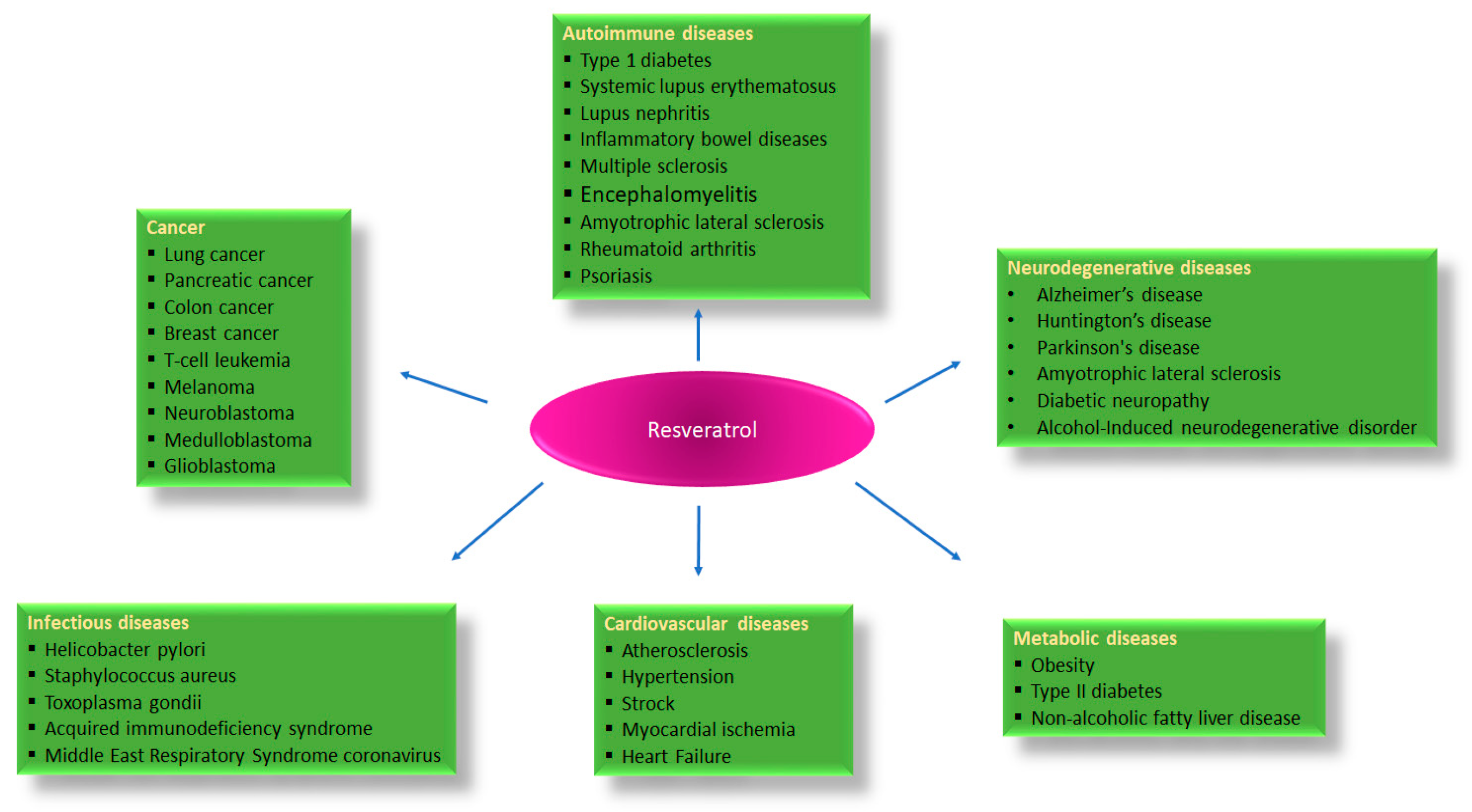
:1. Introduction
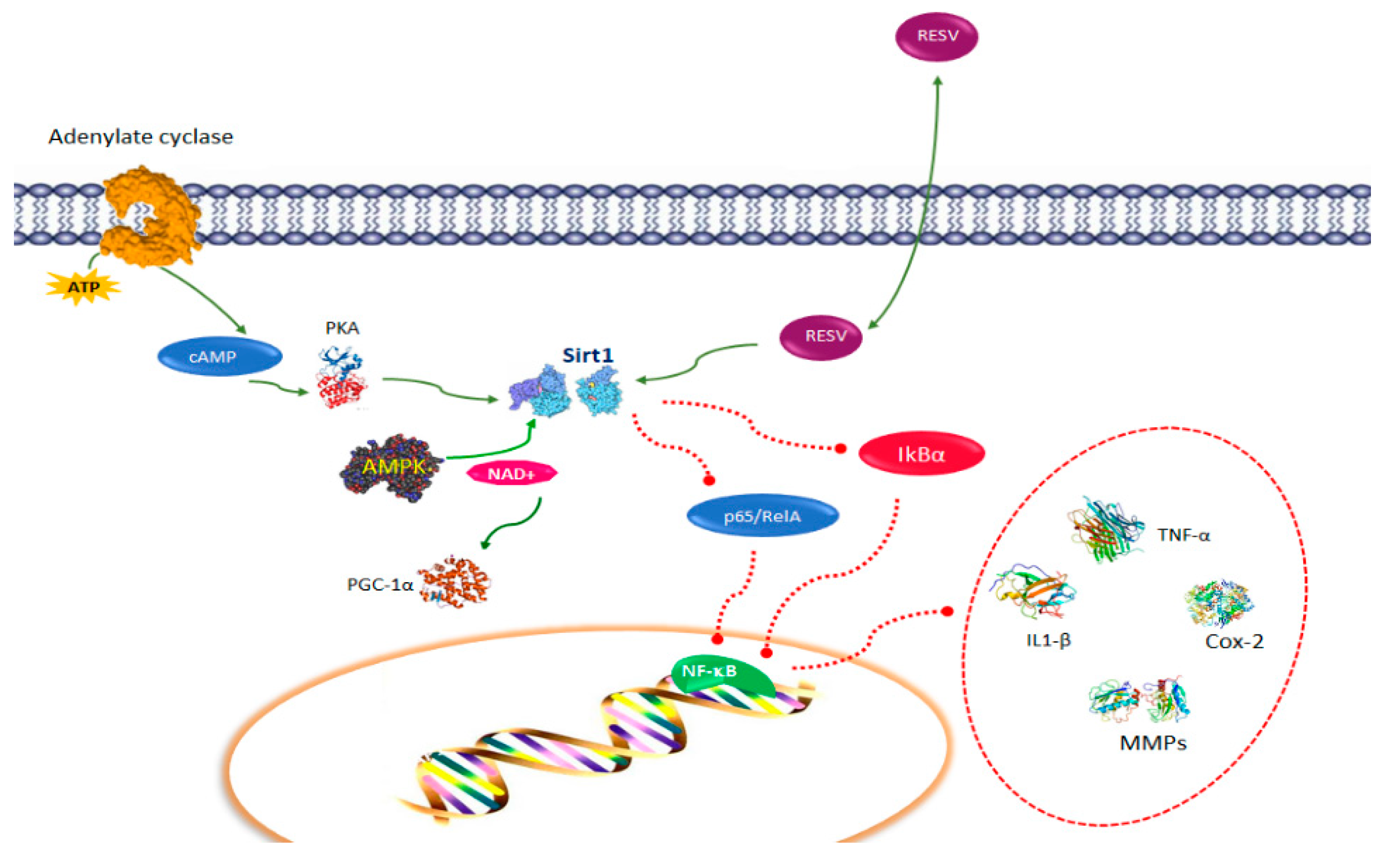
2. Resveratrol Pathways in Immune Function
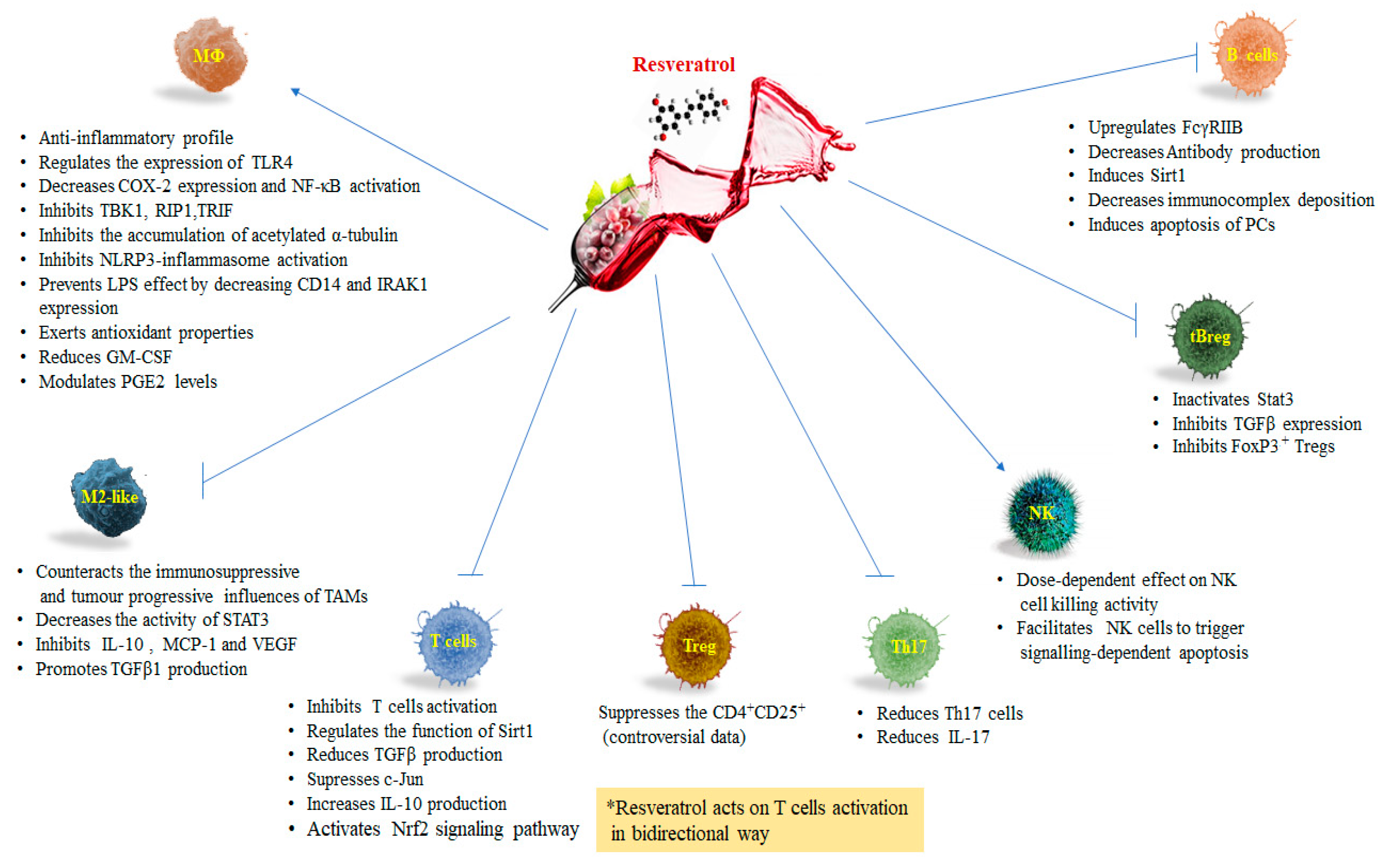
3. Resveratrol and Macrophages
Resveratrol and Tumor Associated Macrophages (TAMs)
4. Resveratrol and T Lymphocytes
5. Resveratrol and Natural Killer Cells
6. Resveratrol and B Lymphocytes
7. Conclusions
Funding
Conflicts of Interest
References
- Jeandet, P.; Bessis, R.; Maume, B.F.; Meunier, P.; Peyron, D.; Trollat, P. Effect of enological practices on the resveratrol isomer content of wine. J. Agric. Food Chem. 1995, 43, 316–319. [Google Scholar] [CrossRef]
- Raal, A.; Pokk, P.; Arend, A.; Aunapuu, M.; Jõgi, J.; Okva, K.; Püssa, T. Trans-resveratrol alone and hydroxystilbenes of rhubarb (Rheum rhaponticum L.) root reduce liver damage induced by chronic ethanol administration: A comparative study in mice. Phytother. Res. 2009, 23, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.M.; Yu, C.; Toma, R.B.; Cho, S.Y.; Reiboldt, W.; Lee, J.; van Breemen, R.B. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 2003, 51, 5867–5870. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3,5,4’-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol Viticult. 1991, 42, 41–46. [Google Scholar]
- Sales, J.M.; Resurreccion, A.V.A. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.B.; Aggarwal, B.B. A multitarget agent for age-associated chronic diseases. Cell Cycle 2008, 15, 1020–1035. [Google Scholar] [CrossRef]
- Pennisi, M.; Bertino, G.; Gagliano, C.; Malaguarnera, M.; Bella, R.; Borzì, A.M.; Madeddu, R.; Drago, F.; Malaguarnera, G. Resveratrol in Hepatitis C Patients Treated with Pegylated-Interferon-α-2b and Ribavirin Reduces Sleep Disturbance. Nutrients 2017, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of Resveratrol. Rev. Mutat. Rese. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Pennisi, M.; Bertino, G.; Motta, M.; Borzì, A.M.; Vicari, E.; Bella, R.; Drago, F.; Malaguarnera, M. Resveratrol in Patients with Minimal Hepatic Encephalopathy. Nutrients 2018, 10, 329. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory activity of Resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef]
- Švajger, U.; Jeras, M. Anti-inflammatory effects of Resveratrol and its potential use in therapy of immune-mediated diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef]
- Sharma, S.; Chopra, K.; Kulkarni, S.K.; Agrewala, J.N. Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin. Exp. Immunol. 2007, 147, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Chen, J.K. Resveratrol enhances perforin expression and NK cell cytotoxicity through NKG2D-dependent pathways. J. Cell. Physiol. 2010, 223, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xue, X.; Guo, R.B.; Sun, X.L.; Hu, G. Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway. CNS Neurosci. Ther. 2012, 18, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Paik, J.H.; Cho, D.; Cho, J.A.; Kim, C.W. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int. Immunopharmacol. 2008, 8, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Mahal, H.S.; Mukherjee, T. Scavenging of reactive oxygen radicals by Resveratrol: Antioxidant effect. Res. Chem. Intermed. 2006, 32, 59–71. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Chung, W.J.; Michaluart, P.; Telang, N.; Tanabe, T.; Inoue, H.; Jang, M.; Pezzuto, J.M.; Dannenberg, A.J. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem. 1998, 273, 21875–21882. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk, L.M.; Forti, L.; Stivala, L.A.; Penning, T.M. Resveratrol is a peroxidase mediated inactivator of COX-1 but not COX-2: A mechanistic approach to the design of COX-1 selective agents. J. Biol. Chem. 2004, 279, 22727–22737. [Google Scholar] [CrossRef]
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. Rev. 2008, 658, 68–94. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide-and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef]
- Alarcon De La Lastra, C.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.Z.; Liu, R.X.; Wang, D.P.; Wang, X.; Dai, C.C. Biocatalysis and biotransformation of Resveratrol in microorganisms. Biotechnol. Lett. 2015, 37, 9–18. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of Resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493. [Google Scholar] [CrossRef] [PubMed]
- Saqib, U.; Kelley, T.T.; Panguluri, S.K.; Liu, D.; Savai, R.; Baig, M.S.; Schürer, S.C. Polypharmacology or Promiscuity? Structural Interactions of Resveratrol with Its Bandwagon of Targets. Front. Pharmacol. 2018, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat. Rev. Endocrinol. 2012, 8, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Wong, L.L.; Tse, E.Y.; Liu, H.F.; Leong, V.Y.; Lee, J.M.; Hardie, D.G.; Ng, I.O.; Ching, Y.P. AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res. 2012, 72, 4394–4404. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, H.; Kong, Q.; Li, J.; Lee, S.M.; Gao, B.; Dong, H.; Wei, J.; Song, J.; Zhang, D.D.; et al. USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol. Cell 2012, 46, 484–494. [Google Scholar] [CrossRef]
- Kwon, H.S.; Lim, H.W.; Wu, J.; Schnölzer, M.; Verdin, E.; Ott, M. Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells. J. Immunol. 2012, 188, 2712–2721. [Google Scholar] [CrossRef]
- Gao, B.; Kong, Q.; Kemp, K.; Zhao, Y.S.; Fang, D. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc. Natl. Acad. Sci. USA 2012, 109, 899–904. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, S.M.; Shannon, S.; Gao, B.; Chen, W.; Chen, A.; Divekar, R.; McBurney, M.W.; Braley-Mullen, H.; Zaghouani, H.; et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J. Clin. Investig. 2009, 119, 3048–3058. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by Resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef]
- Lee, S.M.; Yang, H.; Tartar, D.; Gao, B.; Luo, X.; Ye, S.; Zaghouani, H.; Fang, D. Prevention and treatment of diabetes with Resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia 2011, 54, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Xuzhu, G.; Komai-Koma, M.; Leung, B.P.; Howe, H.S.; McSharry, C.; McInnes, I.B.; Xu, D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann. Rheum. Dis. 2012, 71, 129–135. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κB, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB signaling and inhibit osteoclasto-genesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [PubMed]
- Price Nathan, L.; Gomes Ana, P.; Ling Alvin, J.Y.; Duarte Filipe, V.; Martin-Montalvo, A.; North Brian, J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro Joao, S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of Resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Nakayama, H.; Yaguchi, T.; Yoshiya, S.; Nishizaki, T. Resveratrol induces apoptosis MH7A human rheumatoid arthritis synovial cells in a sirtuin 1-dependent manner. Rheumatol. Int. 2012, 32, 151–157. [Google Scholar] [CrossRef]
- Pallarès, V.; Calay, D.; Cedó, L.; Castell-Auví, A.; Raes, M.; Pinent, M.; Ardévol, A.; Arola, L.; Blay, M. Enhanced anti-inflammatory effect of Resveratrol and EPA in treated endotoxin-activated RAW 264.7 macrophages. Br. J. Nutr. 2012, 108, 1562–1573. [Google Scholar] [CrossRef]
- Stout, R.D.; Suttles, J. Functional plasticity of macrophages: Reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004, 76, 509–513. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- David, M.; Jonathan, B.; David, B.R.; Ivan, M.R. Mononuclear Phagocytes in Immune Defense. In Immunology, 8th ed.; David, M., Jonathan, B., David, B.R., Ivan, M.R., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2013; pp. 125–126. [Google Scholar]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, Q.; Shi, Y.; Zheng, M.; Liu, Y.; Chen, F.; Song, G.; Xu, H.; Wan, T.; et al. Resveratrol reduces the proinflammatory effects and lipopolysaccharide- induced expression of HMGB1 and TLR4 in RAW264.7 cells. Cell. Physiol. Biochem. 2014, 33, 1283–1292. [Google Scholar] [CrossRef]
- Narayanankutty, A. Toll like receptors as a novel therapeutic target for natural products against chronic diseases. Curr. Drug Target 2019. [Google Scholar] [CrossRef]
- Youn, H.S.; Lee, J.Y.; Fitzgerald, K.A.; Young, H.A.; Akira, S.; Hwang, D.H. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by Resveratrol: Molecular targets are TBK1 and RIP1 in TRIF complex. J. Immunol. 2005, 175, 3339–3346. [Google Scholar] [CrossRef]
- Jakus, P.B.; Kalman, N.; Antus, C.; Radnai, B.; Tucsek, Z.; Gallyas, F., Jr.; Sumegi, B.; Veres, B. TRAF6 is functional in inhibition of TLR4-mediated NF-κB activation by Resveratrol. J. Nutr. Biochem. 2013, 24, 819–823. [Google Scholar] [CrossRef]
- Hu, P.; Han, Z.; Couvillon, A.D.; Kaufman, R.J.; Exton, J.H. Autocrine tumor necrosis factor a links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-Regulation of TRAF2 expression. Mol. Cell. Biol. 2006, 26, 3071–3084. [Google Scholar] [CrossRef]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Chen, X.; Lee, A.H.; Glimcher, L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010, 11, 411–418. [Google Scholar] [CrossRef]
- Wang, F.M.; Chen, Y.J.; Ouyang, H.J. Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem. J. 2010, 433, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Misawa, T.; Saitoh, T.; Kozaki, T.; Park, S.; Takahama, M.; Akira, S. Resveratrol inhibits the acetylated α-tubulin-mediated assembly of the NLRP3-inflammasome. Int. Immunol. 2015, 27, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, N.C.; Baek, S.J. The anticancer effects of Resveratrol: Modulation of transcription factors. Nutr. Cancer 2012, 64, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Sebai, H.; Ristorcelli, E.; Sbarra, V.; Hovsepian, S.; Fayet, G.; Aouani, E.; Lombardo, D. Protective effect of Resveratrol against LPS-induced extracellular lipoperoxidation in AR42J cells partly via a Myd88-dependent signaling pathway. Arch. Biochem. Biophys. 2010, 495, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Schafer, N.; Breitenstein, A.; Besler, C.; Winnik, S.; Lohmann, C.; Heinrich, K.; Brokopp, C.E.; Handschin, C.; Landmesser, U.; et al. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging (Albany N. Y.) 2010, 2, 353. [Google Scholar] [CrossRef]
- Schug, T.T.; Xu, Q.; Gao, H.; Peres-da-Silva, A.; Draper, D.W.; Fessler, M.B.; Purushotham, A.; Li, X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell. Biol. 2010, 30, 4712–4721. [Google Scholar] [CrossRef]
- Schwager, J.; Richard, N.; Widmer, F.; Raederstorff, D. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement Altern. Med. 2017, 17, 309. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Fossati, G.; Mazzucchelli, I.; Gritti, D.; Ricevuti, G.; Edwards, S.W.; Moulding, D.A.; Rossi, M.L. In vitro effects of GM-CSF on mature peripheral blood neutrophils. Int. J. Mol. Med. 1998, 1, 943–951. [Google Scholar] [CrossRef]
- Park, D.W.; Baek, K.; Kim, J.R.; Lee, J.J.; Ryu, S.H.; Chin, B.R.; Baek, S.H. Resveratrol inhibits foam cell formation via NADPH oxidase 1- mediated reactive oxygen species and monocyte chemotactic protein-1. Exp. Mol. Med. 2009, 41, 171–179. [Google Scholar] [CrossRef]
- Voloshyna, I.; Hai, O.; Littlefield, M.J.; Carsons, S.; Reiss, A.B. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARγ and adenosine. Eur. J. Pharmacol. 2013, 698, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, X.; Bi, S.; Pan, Z.; Liu, S.; Yu, H.; Lu, H.; Lin, X.; Wang, X.; Ma, T.; et al. Inhibitory effects of Resveratrol on foam cell formation are mediated through monocyte chemotactic protein-1 and lipid metabolism-related proteins. Int. J. Mol. Med. 2014, 33, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of Resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Chen, Y.W.; Chi, P.L.; Lin, C.C.; Hsiao, L.D. Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-κB in human rheumatoid arthritis synovial fibroblasts. Biochem. Pharmacol. 2017, 132, 77–91. [Google Scholar] [CrossRef]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.D.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Brönneke, H.S.; et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 2014, 15, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral Resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, T.; Lawrence, T.; McNeish, I.; Charles, K.A.; Kulbe, H.; Thompson, R.G.; Robinson, S.C.; Balkwill, F.R. “Re-educating” tumor-associated macrophages by targeting NF-kappa B. J. Exp. Med. 2008, 205, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Takeuchi, O.; Vandenbon, A.; Yasuda, K.; Tanaka, Y.; Kumagai, Y.; Miyake, T.; Matsushita, K.; Okazaki, T.; Saitoh, T.; et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 2010, 11, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Szanto, A.; Balint, B.L.; Nagy, Z.S.; Barta, E.; Dezso, B.; Pap, A.; Szeles, L.; Poliska, S.; Oros, M.; Evans, R.M.; et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity 2010, 33, 699–712. [Google Scholar] [CrossRef]
- Liao, X.; Sharma, N.; Kapadia, F.; Zhou, G.; Lu, Y.; Hong, H.; Paruchuri, K.; Mahabeleshwar, G.H.; Dalmas, E.; Venteclef, N.; et al. Krüppel-like factor 4 regulates macrophage polarization. J. Clin. Investig. 2011, 121, 2736–2749. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 14, 139–151. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumor-associated macrophages are a distinct M2 polarised population promoting tumor progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 2006, 427, 17–727. [Google Scholar]
- Wu, P.; Wu, D.; Zhao, L.; Huang, L.; Chen, G.; Shen, G.; Huang, J.; Chai, Y. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: A meta-analysis. Oncotarget 2016, 7, 40451–40460. [Google Scholar] [CrossRef]
- Mei, J.; Xiao, Z.; Guo, C.; Pu, Q.; Ma, L.; Liu, C.; Lin, F.; Liao, H.; You, Z.; Liu, L. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget 2016, 7, 34217–34228. [Google Scholar] [CrossRef]
- Yin, S.; Huang, J.; Li, Z.; Zhang, J.; Luo, J.; Lu, C.; Xu, H.; Xu, H. The Prognostic and Clinicopathological Significance of Tumor-Associated Macrophages in Patients withGastric Cancer: A Meta-Analysis. PLoS ONE 2017, 12, e0170042. [Google Scholar]
- Qian, B.Z.; Zhang, H.; Li, J.; He, T.; Yeo, E.J.; Soong, D.Y.; Carragher, N.O.; Munro, A.; Chang, A.; Bresnick, A.R.; et al. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J. Exp. Med. 2015, 212, 1433–1448. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Z.; Zhang, X.M.; Nakamura, M.; Sun, M.; Hartman, J.; Harris, R.A.; Sun, Y.; Cao, Y. Novel mechanism of macrophage-mediated metastasis revealed in a zebrafish model of tumor development. Cancer Res. 2015, 75, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.K.; Yang, K.; Park, Y.S.; Choi, Y.J.; Oh, S.J.; Lee, C.W.; Lee, K.Y.; Jeong, M.H.; Jo, W.S. Interferon gamma induced by Resveratrol analog, HS-1793, reverses the properties of tumor associated macrophages. Int. Immunopharmacol. 2014, 22, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Gkouveris, I.; Nikitakis, N.; Sauk, J. STAT3 signaling in cancer. J. Cancer Ther. 2015, 6, 709–726. [Google Scholar] [CrossRef]
- Ries, C.H.; Cannarile, M.A.; Hoves, S.; Benz, J.; Wartha, K.; Runza, V.; Rey-Giraud, F.; Pradel, L.P.; Feuerhake, F.; Klaman, I.; et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014, 25, 846–859. [Google Scholar] [CrossRef]
- Sun, L.; Chen, B.; Jiang, R.; Li, J.; Wang, B. Resveratrol inhibits lung cancer growth by suppressing M2-like polarization of tumor associated macrophages. Cell Immunol. 2017, 311, 86–93. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Resveratrol Prevents Tumor Growth and Metastasis by Inhibiting Lymphangiogenesis and M2 Macrophage Activation and Differentiation in Tumor-associated Macrophages. Nutr. Cancer 2016, 68, 667–678. [Google Scholar] [CrossRef]
- Hengartner, H.; Odermatt, B.; Schneider, R.; Schreyer, M.; Walle, G.; MacDonald, H.R.; Zinkernagel, R.M. Deletion of self-reactive T cells before entry into the thymus medulla. Nature 1988, 336, 388–390. [Google Scholar] [CrossRef]
- Jenkinson, E.J.; Kingston, R.; Smith, C.A.; Williams, G.T.; Owen, J.J. Antigen-induced apoptosis in developing T cells: A mechanism for negative selection of the T cell receptor repertoire. Eur. J. Immunol. 1989, 19, 2175–2177. [Google Scholar] [CrossRef]
- Shimon, S.; Noriko, S.; Jun, S.; Sayuri, Y.; Toshiko, S.; Misako, I.; Kuniyasu, Y.; Nomura, T.; Toda, M.; Takahashi, T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001, 182, 18–32. [Google Scholar]
- Fu, S.; Zhang, N.; Yopp, A.C.; Chen, D.; Mao, M.; Zhang, H.; Ding, Y.; Bromberg, J.S. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25—Precursors. Am. J. Transplant. 2004, 4, 1614–1627. [Google Scholar] [CrossRef]
- Tai, Y.; Wang, Q.; Korner, H.; Zhang, L.; Wei, W. Molecular Mechanisms of T Cells Activation by Dendritic Cells in Autoimmune Diseases. Front. Pharmacol. 2018, 9, 642. [Google Scholar] [CrossRef]
- Caza, T.; Landas, S. Functional and Phenotypic Plasticity of CD4(+) T Cell Subsets. Biomed. Res. Int. 2015, 521957. [Google Scholar] [CrossRef]
- Astry, B.; Venkatesha, S.H.; Moudgil, K.D. Involvement of the IL-23/IL-17 axis and the Th17/Treg balance in the pathogenesis and control of autoimmune arthritis. Cytokine 2015, 74, 54–61. [Google Scholar] [CrossRef]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- Petro, T.M. Regulatory role of Resveratrol on Th17 in autoimmune disease. Int. Immunopharmacol. 2011, 11, 310–318. [Google Scholar] [CrossRef]
- Jeong, M.H.; Yang, K.M.; Choi, Y.J.; Kim, S.D.; Yoo, Y.H.; Seo, S.Y.; Lee, S.H.; Ryu, S.R.; Lee, C.M.; Suh, H.; et al. Resveratrol analog, HS-1793 enhance anti-tumor immunity by reducing the CD4+CD25+ regulatory T cells in FM3A tumor bearing mice. Int. Immunopharmacol. 2012, 14, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pastrana, J.; Shao, Y.; Chernaya, V.; Wang, H.; Yang, X.F. Epigenetic enzymes are the therapeutic targets for CD4+CD25+/highFoxp3+ regulatory T cells. Transl. Res. 2015, 165, 221–240. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Zhang, Z.; Wang, J.; Yang, H.; Liu, G. Intercellular interplay between Sirt1 signalling and cell metabolism in immune cell biology. Immunology 2015, 145, 455–467. [Google Scholar] [CrossRef]
- Zou, T.; Yang, Y.; Xia, F.; Huang, A.; Gao, X.; Fang, D.; Xiong, S.; Zhang, J. Resveratrol Inhibits CD4+ T cell activation by enhancing the expression and activity of Sirt1. PLoS ONE 2013, 8, e75139. [Google Scholar] [CrossRef]
- Wu, S.L.; Yu, L.; Jiao, X.Y.; Meng, K.W.; Pan, C.E. The suppressive effect of Resveratrol on protein kinase C theta in peripheral blood T lymphocytes in a rat liver transplantation model. Transplant. Proc. 2006, 38, 3052–3054. [Google Scholar] [CrossRef]
- Karlsson, E.A.; Sheridan, P.A.; Beck, M.A. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J. Immunol. 2010, 184, 3127–3133. [Google Scholar] [CrossRef]
- Eliakim, A.; Schwindt, C.; Zaldivar, F.; Casali, P.; Cooper, D.M. Reduced tetanus antibody titers in overweight children. Autoimmunity 2006, 39, 137–141. [Google Scholar] [CrossRef]
- Wang, B.; Sun, J.; Li, L.; Zheng, J.; Shi, Y.; Le, G. Regulatory effects of Resveratrol on glucose metabolism and T-lymphocyte subsets in the development of high-fat diet-induced obesity in C57BL/6 mice. Food Funct. 2014, 5, 1452–1463. [Google Scholar] [CrossRef]
- Liu, L.L.; Chao, P.L.; Zhang, H.L.; Tong, M.L.; Liu, G.L.; Lin, L.R.; Su, Y.H.; Wu, J.Y.; Dong, J.; Zheng, W.H.; et al. Analysis of lymphocyte subsets in HIV-negative neurosyphilis patients. Diagn. Microbiol. Infect. Dis. 2013, 75, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Redecha, P.B.; Vukelic, M.; Qing, X.; Perino, G.; Salmon, J.E.; Koo, G.C. Engraftment of peripheral blood mononuclear cells from systemic lupus erythematosus and antiphospholipid syndrome patient donors into BALB-RAG-2−/− IL-2Rγ−/− mice: A promising model for studying human disease. Arthr. Rheum. 2011, 63, 2764–2773. [Google Scholar] [CrossRef]
- Robertson, M.J.; Ritz, J. Biology and clinical relevance of human natural killer cells. Blood 1990, 76, 2421–2438. [Google Scholar]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef]
- Lee, S.-H.; Miyagi, T.; Biron, C.A. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007, 28, 252–259. [Google Scholar] [CrossRef]
- Biron, C.A.; Brossay, L. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 2001, 13, 458–464. [Google Scholar] [CrossRef]
- Krzewski, K.; Strominger, J.L. The killer’s kiss: The many functions of NK cell immunological synapses. Curr. Opin. Cell. Biol. 2008, 20, 597–605. [Google Scholar] [CrossRef]
- Walzer, T.; Dalod, M.; Robbins, S.H.; Zitvogel, L.; Vivier, E. Natural-killer cells and dendritic cells: ‘l’union fait la force’. Blood 2005, 106, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Narni-Mancinelli, E.; Ugolini, S.; Vivier, E. Tuning the threshold of natural killer cell responses. Curr. Opin. Immunol. 2013, 25, 53–58. [Google Scholar] [CrossRef]
- Falchetti, R.; Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Ravagnan, G. Effects of Resveratrol on human immune cell function. Life Sci. 2001, 70, 81–96. [Google Scholar] [CrossRef]
- Quoc Trung, L.; Espinoza, J.L.; Takami, A.; Nakao, S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS ONE 2013, 8, e55183. [Google Scholar] [CrossRef]
- Li, Q.; Huyan, T.; Ye, L.-J.; Li, J.; Shi, J.-L.; Huang, Q.-S. Concentration-dependent biphasic effects of Resveratrol on human natural killer cells in vitro. J. Agric. Food Chem. 2014, 62, 10928–10935. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Trivedi, P.P.; Ge, B.; Krzewski, K.; Strominger, J.L. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc. Natl. Acad. Sci. USA 2007, 104, 6329–6334. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-C.; Lai, H.-C.; Hsieh, S.-C.; Chen, J.-K. Resveratrol ameliorates Serratia marcescens-induced acute pneumonia in rats. J. Leukoc. Biol. 2008, 83, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cao, D.; Li, Y.; He, Y.; Guo, K. Resveratrol sensitized leukemia stem cell-like KG-1a cells to cytokine-induced killer cells-mediated cytolysis through NKG2D ligands and TRAIL receptors. Cancer Biol. Ther. 2012, 13, 516–526. [Google Scholar] [CrossRef]
- Shankar, S.; Siddiqui, I.; Srivastava, R. Molecular mechanisms of Resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol. Cell. Biochem. 2007, 304, 273–285. [Google Scholar] [CrossRef]
- Ivanov, V.N.; Partridge, M.A.; Johnson, G.E.; Huang, S.X.L.; Zhou, H.; Hei, T.K. Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp. Cell Res. 2008, 314, 1163–1176. [Google Scholar] [CrossRef]
- Jacquemin, G.; Shirley, S.; Micheau, O. Combining naturally occurring polyphenols with TNF-related apoptosis-inducing ligand: A promising approach to kill resistant cancer cells? Cell. Mol. Life Sci. 2010, 67, 3115–3130. [Google Scholar] [CrossRef]
- Clement, M.V.; Hirpara, J.L.; Chawdhury, S.H.; Pervaiz, S. Chemopreventive agent Resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998, 92, 996–1002. [Google Scholar]
- Van Es, N.; Sturk, A.; Middeldorp, S.; Nieuwland, R. Effects of cancer on platelets. Semin. Oncol. 2014, 41, 311–318. [Google Scholar] [CrossRef]
- Nieswandt, B.; Hafner, M.; Echtenacher, B.; Männel, D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999, 59, 1295–1300. [Google Scholar] [PubMed]
- Toliopoulos, I.K.; Simos, Y.V.; Oikonomidis, S.; Karkabounas, S.C. Resveratrol diminishes platelet aggregation and increases susceptibility of K562 tumor cells to natural killer cells. Indian J. Biochem. Biophys. 2013, 50, 14–18. [Google Scholar]
- Mauri, C.; Menon, M. Human regulatory B cells in health and disease: Therapeutic potential. J. Clin. Investig. 2017, 127, 772–779. [Google Scholar] [CrossRef]
- Ray, A.; Basu, S.; Williams, C.B.; Salzman, N.H.; Dittel, B.N. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J. Immunol. 2012, 188, 3188–3198. [Google Scholar] [CrossRef]
- Bodogai, M.; Lee-Chang, C.; Wejksza, K.; Lai, J.P.; Merino, M.; Wersto, R.P.; Gress, R.E.; Chan, A.C.; Hesdorffer, C.; Biragyn, A. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res. 2013, 73, 2127–2138. [Google Scholar] [CrossRef]
- Olkhanud, P.B.; Damdinsuren, B.; Bodogai, M.; Gress, R.E.; Sen, R.; Wejksza, K.; Malchinkhuu, E.; Wersto, R.P.; Biragyn, A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4 T cells to T-regulatory cells. Cancer Res. 2011, 71, 3505–3515. [Google Scholar] [CrossRef] [PubMed]
- Lee-Chang, C.; Bodogai, M.; Martin-Montalvo, A.; Wejksza, K.; Sanghvi, M.; Moaddel, R.; de Cabo, R.; Biragyn, A. Inhibition of breast cancer metastasis by Resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J. Immunol. 2013, 191, 4141–4151. [Google Scholar] [CrossRef] [PubMed]
- Jhou, J.P.; Chen, S.J.; Huang, H.Y.; Lin, W.W.; Huang, D.Y.; Tzeng, S.J. Upregulation of FcγRIIB by Resveratrol via NF-κB activation reduces B-cell numbers and ameliorates lupus. Exp. Mol. Med. 2017, 49, e381. [Google Scholar] [CrossRef] [PubMed]
- Hiepe, F.; Radbruch, A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat. Rev. Nephrol. 2016, 12, 232–240. [Google Scholar] [CrossRef]
- Wang, Z.L.; Luo, X.F.; Li, M.T.; Xu, D.; Zhou, S.; Chen, H.Z.; Gao, N.; Chen, Z.; Zhang, L.L.; Zeng, X.F. Resveratrol possesses protective effects in a pristane-induced lupus mouse model. PLoS ONE 2014, 9, e114792. [Google Scholar] [CrossRef]
- Isaák, A.; Gergely, P., Jr.; Szekeres, Z.; Prechl, J.; Poór, G.; Erdei, A.; Gergely, J. Physiological up-regulation of inhibitory receptors FcγRII and CR1 on memory B cells is lacking in SLE patients. Int. Immunol. 2008, 20, 185–192. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Clément, C.; Nabavi, S.F.; Habtemariam, S.; Nabavi, S.M.; Cordelier, S. Engineering stilbene metabolic pathways in microbial cells. Biotechnol. Adv. 2018, 36, 2264–2283. [Google Scholar] [CrossRef]
| Study Type | Subjects | Dose | Effect | Ref. |
|---|---|---|---|---|
| In vitro | Splenic lymphocytes, CTLs and LAKs | 25–50 μM | Suppresses mitogen-, IL-2-, and alloantigen-induced proliferation of splenic lymphocytes; development of antigen-specific CTLs; LAK cells were less sensitive. | [10] |
| In vitro | T lymphocytes and Macrophages | 1–20 µM | Suppresses: T cells proliferation and secretion of IFN-γ and IL-4; B cells proliferation and production of IgG1 and IgG2a isotypes; IL-1, IL-6, TNF-α. Enhances: IL-10; down-regulates the expression of CD28 on CD4+ T cells and of CD80 on macrophages. | [13] |
| In vitro | NK92 cell line | 1.5 µM | Enhances perforin expression and cytotoxic activity acting via NKG2D-dependent JNK and ERK-1/2 pathways. | [12] |
| Ex vivo In vivo | Splenocytes C57BL/6 and BALB/c mice | 25–75 µM 4 mg/kg, i.p. | Suppresses the CD4+CD25+ subsets; downregulated secretion of TGF-β. Enhances IFN-γ expression in CD8+ T cells. | [15] |
| In vitro | RAW 264.7 cell line and BV-2 cell line | 50 μM | Suppresses IL-6, M-CSF, MCP-1, MCP-5, CD54, IL-1ra, IL-27, and TNF-α in both cell lines. Inhibits the TLR4/NF-κB/STAT signaling cascade | [20] |
| In vivo | NOD mice were given | 250 mg/kg | Decreases in expression of CCR-6. Inhibits CD11b+F4/80hi macrophages. It reduces CCR6+ IL-17-producing cells and CD11b+F4/80hi in the pancreas. It reduces migration of splenocytes toward media containing CCL20. Prevents type 1 diabetes in NOD mice. | [32] |
| In vitro | U-937 Jurkat HeLa and H4 cells lines | 0.5–25 μM | Suppresses TNF-induced phosphorylation and nuclear translocation of the p65 subunit of NFκ B, and NFκ B-dependent reporter gene transcription. It suppresses TNF-induced NFκ B activation. Blocks NFκ B activation induced by PMA, LPS, H2O2, and okadaic acid. Suppresses AP-1. Inhibits the TNF-induced activation of MEK and JNK. Abrogates TNF-induced cytotoxicity and caspase activation. Suppresses ROI generation and lipid peroxidation. | [37] |
| In vitro | Bone-derived cell cultures and MC3T3-E1 cell lines | 5 μM | Inhibits RANKL-induced acetylation and nuclear translocation of NFκ B. Induces Sirt1-p300 association in bone-derived and preosteoblastic cells, leading to deacetylation of RANKL-induced NFκ B, inhibition of NFκ B transcriptional activation, and osteoclastogenesis. It activates the bone transcription factors Cbfa-1 and Sirt1 and induces the formation of Sirt1-Cbfa-1 complexes. It regulates the balance between the osteoclastic versus osteoblastic activity. It could exert a therapeutic potential for treating osteoporosis and rheumatoid arthritis-related bone loss. | [38] |
| In vitro | MH7A cell lines | 100 μM | Induces MH7A cell apoptosis by activating caspase-9 and the effector caspase-3, reduces Bcl-XL expression, allowing cytochrome c release from the mitochondria into the cytosol, in a sirtuin 1-dependent manner. It could suppress hyperplasia of synovial cells, a critical factor of rheumatoid arthritis. | [40] |
| In vitro | RAW264.7 and HEK 293T cell lines | 30, 50, 75, 100 μM | Inhibits TRIF signaling in the TLR3 and TLR4 pathway by targeting TANK-binding kinase 1 and RIP1 in TRIF complex. Modulates TLR-derived signaling and inflammatory target gene expression. It could alter susceptibility to microbial infection and chronic inflammatory diseases. | [46] |
| In vitro | RAW 264.7 cell line | 50 μM | Suppresses LPS-induced TRAF6 expression and ubiquitination, attenuates the LPS-induced TLR4–TRAF6, MAPK, and AKT pathways. It could exert anti-inflammatory effects. | [47] |
| In vitro | Mouse bone-marrow cells J774 cell line | 5 μM | Inhibits the accumulation of acetylated α-tubulin and suppressing NLRP3-inflammasome assembly. It prevents the NLRP3-related inflammatory diseases. | [53] |
| In vitro | AR42J cell line | 10–100 μM | It decreases CD14 and IRAK1 expression and increases the p38 MAPK protein phosphorylation. It exerts antioxidant properties either by a Myd88-dependent way not involving IRAK1 or by a TRIF dependent pathway. | [55] |
| In vitro | RAW 264.7 THP-1 HUVEC cell lines and PBLs | 6.25–12.5–25–50 μM 3.13–6.25–12.5–25 μM 10–20–30 μM 6.25–12.5–25 μM | Modulates many mediators of the inflammatory response. Its effects are context-dependent, influencing chemokines and cytokines in opposite ways in different cells. | [58] |
| In vitro | Macrophages | 2.5 μM | Suppresses LPS-induced phosphorylation of FoxO3a. Blocks the LPS-induced PI3K-AKT pathway and affects FoxO3a phosphorylation. Inhibits Nox1 and MCP-1 expression. Could modulate the activations of important macrophage functions associated with atherosclerosis. | [61] |
| In vitro | TPH1 cell line | 25 μM | Promotes apoA-1 and HDL-mediated efflux, downregulates oxLDL uptake, and diminishes foam cell formation. Regulates expression of the cholesterol metabolizing enzyme CYP27A1, and helps cholesterol elimination. | [62] |
| In vitro | TPH1 cell line | 2.5 μM | Inhibits foam cells formation by regulating the expression of the inflammatory cytokine, MCP-1, and by activating the AMPK-Sirt1-PPAR signaling pathway. | [63] |
| In vitro | Granulocytes Monocytes RAW 264.7 cell line | 5–100 μM | Inhibits oxidative burst and CD11b expression in granulocytes and monocytes. Inhibits the production of NO and PGE2, but did not reduce iNOS, TNFα, or IL-1β gene expression in LPS-stimulated RAW 264.7. Induces NRf2 nuclear translocation and reduced miR-146a expression in LPS treated RAW 264.7. | [64] |
| In vitro | Human rheumatoid arthritis synovial fibroblasts | 20 μM | Suppresses the bradykinin-induced COX-2/PGE2. Inhibits the phosphorylation and acetylation of p65, c-Jun, and Fos and reduces the binding to the COX-2 promoter, thereby attenuated the COX-2 expression. Could be used for inflammatory arthritis therapy. | [65] |
| In vivo In vitro | C3H/He mice Splenocytes | 1.5 mg/Kg 1.25–2.5–5 μM | Reprograms M-2 phenotype (TAM) countering the immunosuppressive and tumor progressive influences of TAM. | [83] |
| In vitro | M2 polarization of human monocyte derived macrophages | 20 μM | Decreases STAT3. It inhibits F4/80 positive expressing cells and M2 polarization in the tumors. | [86] |
| In vivo | C3H/He mice | 0.5, 1 and 1.5 mg/kg | Reduces Tregs (CD4 + CD25 + Foxp3 + cells) and the production of TGF-β. Increases IFN-γ-expressing CD8 + T cells. Upregulates IFN-γ production and enhances the cytotoxicity of splenocytes against FM3A tumor cells. | [97] |
| In vitro In vivo | T cell C57/BL6 and DBA1 mice | 0.5 μM or 25 μM 25 mg/kg | Upregulates Sirt1 expression. Decreases c-Jun acetylation and its translocation. Reduces the incidence and severity of collagen-induced arthritis in mice. | [100] |
| In vivo | Wistar rats | 100 mg kg-1 ml | Downregulates PKC9 level in T lymphocytes | [101] |
| In vivo | C57BL/6 mice | HFD supplemented with 0.06% resveratrol | Activates the PI3K and Sirt1 signaling transduction. Activates the Nrf2-regulated adaptive response. Increases the CD3+CD4+/CD3+CD8+ subsets percentages and the Tregs. Maintains glucose homeostasis alleviating inflammation. | [104] |
| In vitro | PBMCs | 0.625–2.5–5–10 μM | Modulates the functional activities of both T and NK effector cells, with stimulation at low concentrations and suppression at high concentrations. Affects cytokine-production by activated CD41 and CD81 T cells. | [114] |
| In vitro | KHYG-1, NKL, NK-92, and NK-YS cell lines | 3.125–6.25–12.5– 25–50 μM | Suppresses STAT3 and inhibits JAK2 phosphorylation. Induces downregulation of the anti-apoptotic proteins MCL1 and survivin. Induces apoptotic and antiproliferative activities of L-asparaginase against KHYG-1, NKL and NK-92 cells. | [115] |
| In vitro | Human NKs Jurkat cell line | 0.5−50 μM | At high concentration promotes apoptosis of NK cells and of Jurkat cells. At low concentration increases the NK cells cytotoxicity via up-regulating the expression of NKG2D and IFN-γ. | [116] |
| In vitro | KG-1a cells PBMCs | 25–100 μM | Inhibits KG-1a cell growth but has the least growth-inhibition effect PBMCs. Makes KG-1a cells susceptible to CIKs-mediated cytolysis correlated with an increase in cell-surface expression of NKG2D ligands and DR4, coupled with a downregulation of cell-surface expression of DcR1. | [13] |
| In vitro | DU145, and PC3 cells | 5–30 μM | Induces apoptosis in prostate cancer cells. Downregulates Bcl-2, Bcl-XL, and surviving. Upregulates Bax, Bak, PUMA, Noxa, and Bim, TRAIL-R1/DR4 and TRAIL-R2/DR5 expression. Activates caspase-3 and caspase-9 and induces apoptosis. | [119] |
| In vitro | cell lines LU120 cell line | 25–100 μM | Decreases STAT3 and NF-κB activation. Suppresses expression of cFLIP and Bcl-xL proteins and increases sensitivity to exogenous TRAIL in DR5-positive melanomas. In combination with TRAIL it could have a significant efficacy in the treatment of human melanomas. | [121] |
| In vitro | HL60 T47D cell line | 32 μM | Induces cell death mediated by intracellular caspases Dose-dependent increase in proteolytic cleavage of caspase substrate PARP. Enhances CD95L expression on both HL60 cells T47D breast carcinoma cells. | [123] |
| In vivo In vitro | BALB/c or C57BL/6 mice tBregs | 20 or 50 mg/mouse 12.5 mM | Inhibits lung metastasis in mice. Inactivates Stat3, preventing the generation and function of tBregs, including expression of TGF-β. It reduces antitumor effector immune responses by disabling tBreg-induced conversion of Foxp3+ Tregs. Could control cancer escape-promoting tBregs/Tregs without nonspecific inactivation of effector immune cells. | [131] |
| In vivo | MRL/lpr mice BJAB B cells | 20 mg kg−1 per day | Increases the expression of FcγRIIB in B cells. Decreases serum autoantibody titers in MRL/lpr mice. The upregulation of FcγRIIB causes an increase of Sirt1 protein and deacetylation of p65 NF-κB. Reduces plasma cells in MRL/lpr mice, leading to improvement of nephritis and prolonged survival. | [132] |
| In vivo | BALB/c mice | 20 mg/kg | Reduces proteinuria, immunoglobulin deposition in kidney, and in serum in pristane-induced lupus mice. Inhibits CD69 and CD71 expression on CD4+ T cells and CD4+ T cell proliferation. Induces CD4+ T cell apoptosis, and decreased CD4 IFNc+ Th1 cells and the ratio of Th1/Th2 cells in vitro. Inhibits antibody production and proliferation of B cells in vitro. | [134] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. https://doi.org/10.3390/nu11050946
Malaguarnera L. Influence of Resveratrol on the Immune Response. Nutrients. 2019; 11(5):946. https://doi.org/10.3390/nu11050946
Chicago/Turabian StyleMalaguarnera, Lucia. 2019. "Influence of Resveratrol on the Immune Response" Nutrients 11, no. 5: 946. https://doi.org/10.3390/nu11050946
APA StyleMalaguarnera, L. (2019). Influence of Resveratrol on the Immune Response. Nutrients, 11(5), 946. https://doi.org/10.3390/nu11050946




