Vitamin D Daily versus Monthly Administration: Bone Turnover and Adipose Tissue Influences
Abstract
1. Introduction
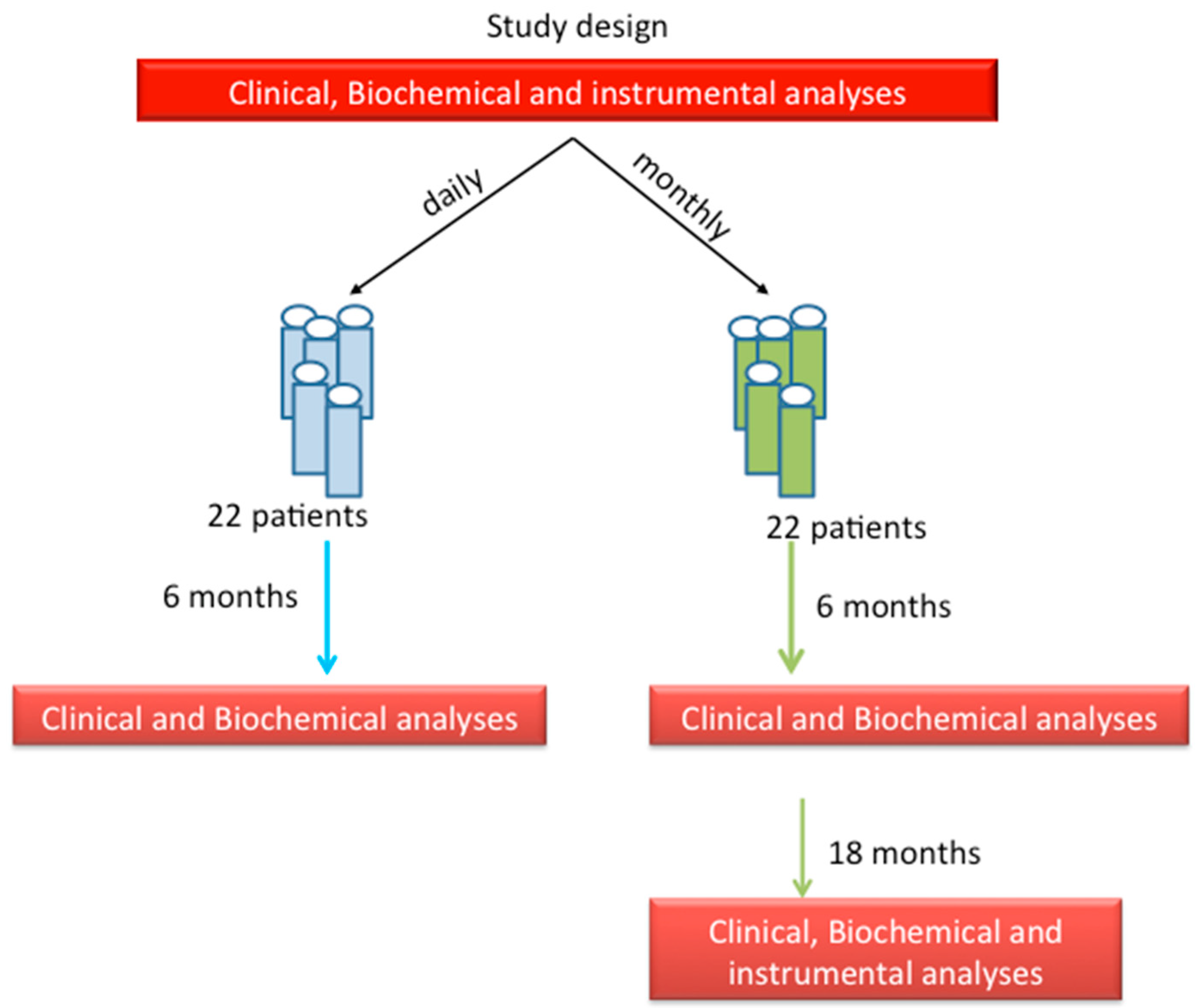
2. Patients and Methods
2.1. Biochemical Evaluations
2.2. Clinical Evaluations
2.3. Bone Densitometry
2.4. Statistical Analysis
3. Results
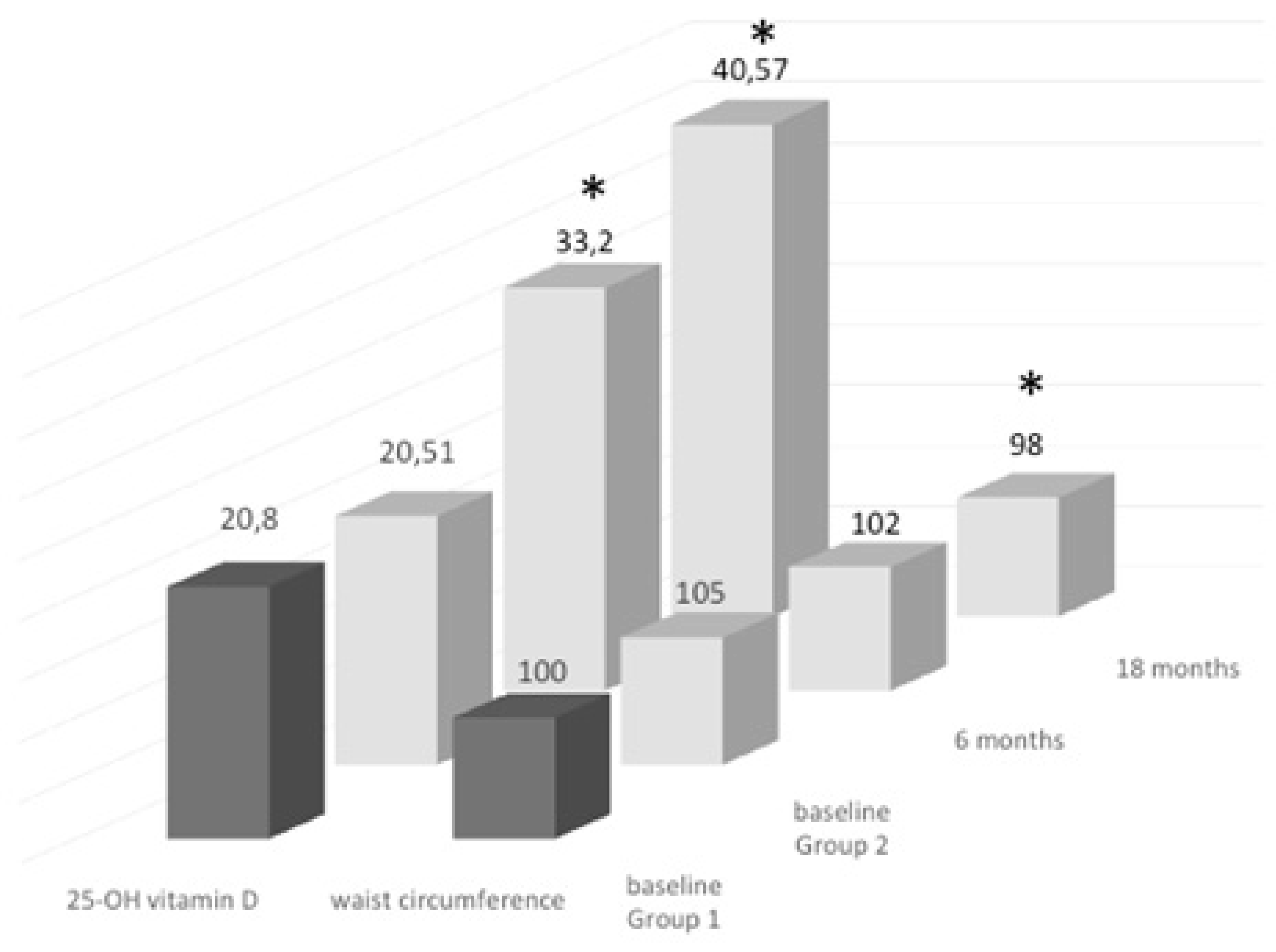
3.1. Groups of Treatment: Daily vs. Monthly
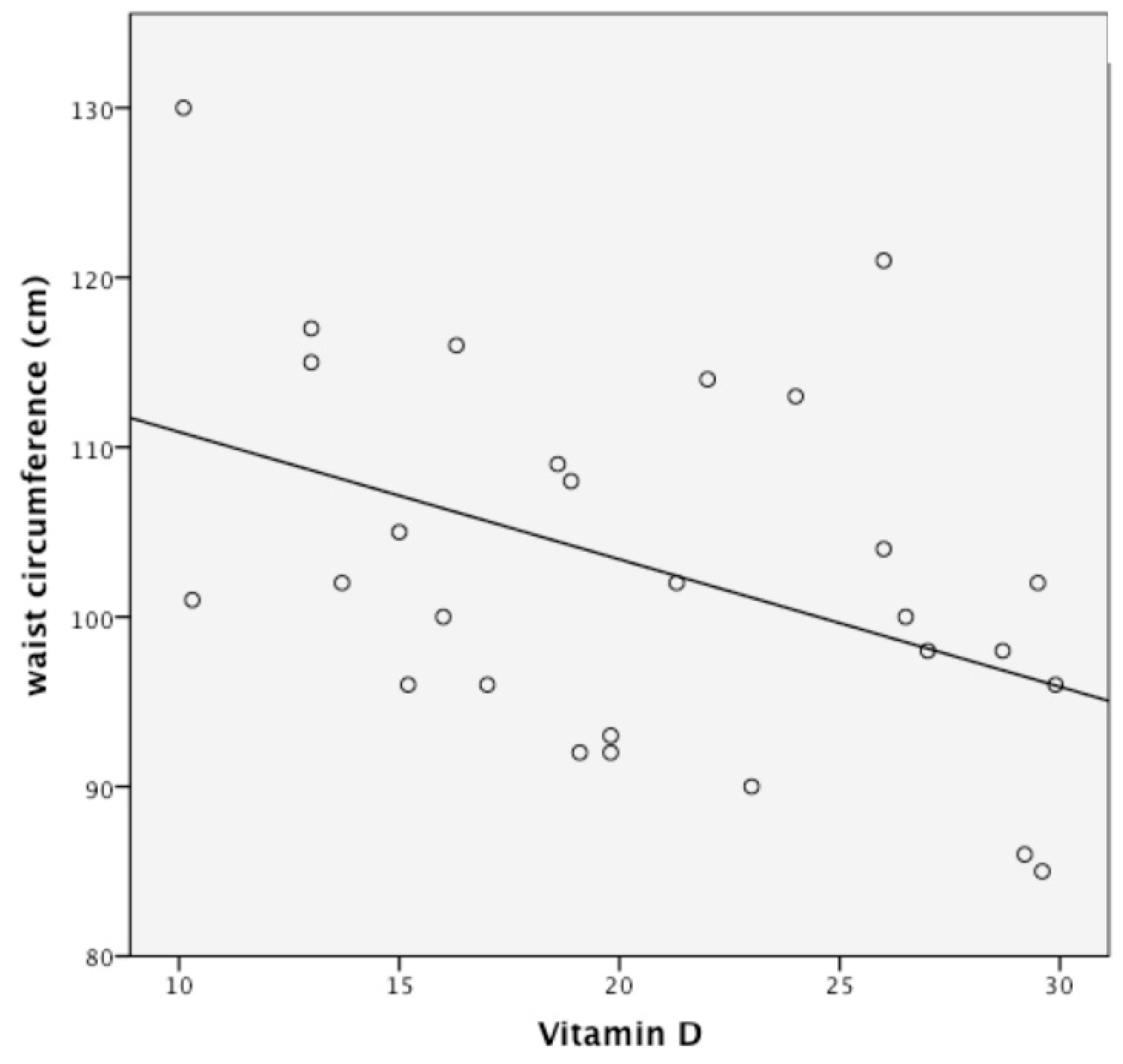
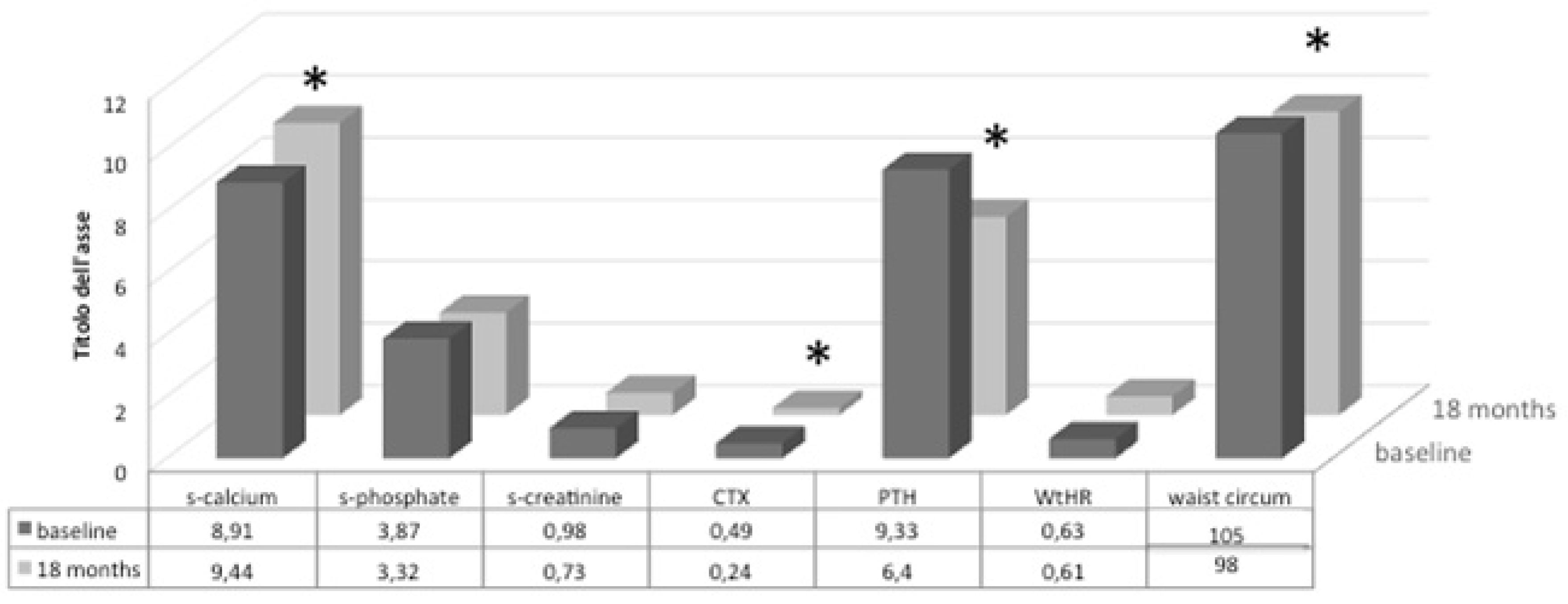
3.2. Follow-Up Study of Monthly Treatment
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cashman, K.D. Vitamin D requirements for the future-lessons learned and charting a path forward. Nutrients 2018, 10, 533. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Valenti, M.T.; del Forno, F.; Caneva, E.; Pietrobelli, A. Vitamin D: Daily vs. monthly use in children and elderly—What is going on? Nutrients 2017, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, P.; Adler, R.; Jones, G.; Liberman, U.A.; Mazziotti, G.; Minisola, S.; Munns, C.; Napoli, N.; Pittas, A.; Giustina, A.; et al. Management of endocrine disease: Therapeutics of vitamin D. Eur. J. Endocrinol. 2018, 179, R239–R259. [Google Scholar] [CrossRef] [PubMed]
- Posa, F.; Di Benedetto, A.; Cavalcanti-Adam, E.A.; Colaianni, G.; Porro, C.; Trotta, T.; Brunetti, G.; Lo Muzio, L.; Grano, M.; Mori, G. Vitamin D promotes MSC osteogenic differentiation stimulating cell adhesion and alphavbeta3 expression. Stem Cells Int. 2018, 2018, 6958713. [Google Scholar] [CrossRef] [PubMed]
- Posa, F.; Di Benedetto, A.; Colaianni, G.; Cavalcanti-Adam, E.A.; Brunetti, G.; Porro, C.; Trotta, T.; Grano, M.; Mori, G. Vitamin D effects on osteoblastic differentiation of mesenchymal stem cells from dental tissues. Stem Cells Int. 2016, 2016, 9150819. [Google Scholar] [CrossRef]
- Liu, P.; Oyajobi, B.O.; Russell, R.G.; Scutt, A. Regulation of osteogenic differentiation of human bone marrow stromal cells: Interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) in vitro. Calcified Tissue Int. 1999, 65, 173–180. [Google Scholar] [CrossRef]
- Rosen, C.J.; Ackert-Bicknell, C.; Rodriguez, J.P.; Pino, A.M. Marrow fat and the bone microenvironment: Developmental, functional, and pathological implications. Crit. Rev. Eukaryot Gene Expr. 2009, 19, 109–124. [Google Scholar] [CrossRef]
- Ge, C.; Cawthorn, W.P.; Li, Y.; Zhao, G.; Macdougald, O.A.; Franceschi, R.T. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARgamma transcription factors. J. Cell. Physiol. 2016, 231, 587–596. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Manfredi, M.; Caviglia, G.; Conte, E.; Robotti, E.; Marengo, E.; Cheri, S.; Zamboni, F.; Gabbiani, D.; Deiana, M.; et al. Can half-marathon affect overall health? The yin-yang of sport. J. Proteomics 2018, 170, 80–87. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for vitamin A. EFSA J. 2016, 14, 4547. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. MeTable 2013, 98, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Szlagatys-Sidorkiewicz, A.; Brzeziński, M.; Jankowska, A.; Metelska, P.; Słomińska-Frączek, M.; Socha, P. Long-term effects of vitamin D supplementation in vitamin D deficient obese children participating in an integrated weight-loss programme (a double-blind placebo-controlled study)—Rationale for the study design. BMC Pediatr. 2017, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Pietrobelli, A.; Faith, M.S.; Wang, J.; Brambilla, P.; Chiumello, G.; Heymsfield, S.B. Association of lean tissue and fat mass with bone mineral content in children and adolescents. Obes. Res. 2002, 10, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Feizabad, E.; Hossein-Nezhad, A.; Maghbooli, Z.; Ramezani, M.; Hashemian, R.; Moattari, S. Impact of air pollution on vitamin D deficiency and bone health in adolescents. Arch Osteoporos. 2017, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Iwasaki, N.; Ide, R.; Takizawa, M.; Tanaka, M.; Tetsuo, T.; Sato, A.; Uchigata, Y. Role of vitamin D in energy and bone metabolism in postmenopausal women with type 2 diabetes mellitus: A 6-month follow-up evaluation. J. Diabetes Investig. 2018, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.F.; Bauer, J.D.; Martin, I.; Rochester, S.; Duarte Romero, B.; Prins, J.B.; Wright, O.R.L. Association of sun exposure, skin colour and body mass index with vitamin d status in individuals who are morbidly obese. Nutrients 2017, 9, 1094. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef]
- Javaid, M.K. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: A longitudinal study. Lancet 2006, 367, 36–44. [Google Scholar] [CrossRef]
- Hart, P.H.; Lucas, R.M.; Walsh, J.P. Vitamin D in fetal development: Findings from a birth cohort study. Pediatrics 2015, 135, e167–e173. [Google Scholar] [CrossRef]
- Hewison, M.; Adams, J.S. Vitamin insufficiency and skeletal development in utero. J Bone Miner. Res. 2010, 25, 11–13. [Google Scholar] [CrossRef]
- Eggemoen, A.R.; Jenum, A.K.; Mdala, I.; Knutsen, K.V.; Lagerlov, P.; Sletner, L. Vitamin D levels during pregnancy and association with birth weight and body composition of the newborn: A longitudinal multiethnic population-based study. BMJ 2017, 117, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Hensen, K.E.; Johnson, M.G. An update on vitamin D for clinicians. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. 2017, 13, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J. Vitamin D and adipogenesis: New molecular insights. Nutr. Rev. 2008, 66, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Kawada, T.; Kazuki, R.; Ono, T.; Kato, S.; Sugimoto, E. Vitamin D receptor gene expression is upregulated by 1,25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 1993, 193, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Marcotorchino, J.; Tourniaire, F.; Landrier, J.F. Vitamin D, adipose tissue, and obesity. Horm. Mol. Biol. Clin. Investig. 2013, 15, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Power, C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: The role of obesity. Diabetes Care 2006, 29, 2244–2246. [Google Scholar] [CrossRef]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Fernández-Real, J.M. Adipocyte differentiation. In Adipose Tissue Biology; Symonds, M., Ed.; Springer: New York, NY, USA, 2012; pp. 17–28. [Google Scholar]
- Felicidade, I.; Sartori, D.; Coort, S.L.M.; Semprebon, S.C.; Niwa, A.M.; D’Epiro, G.F.R.; Biazi, B.I.; Marques, L.A.; Evelo, C.T.; Mantovani, M.S.; et al. Role of 1α,25-Dihydroxyvitamin D3 in adipogenesis of SGBS cells: New insights into human preadipocyte proliferation. Cell. Physiol. Biochem. 2018, 48, 397–408. [Google Scholar] [CrossRef]
- Kong, J.; Li, Y.C. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am. J. Physiol. Endocrinol. MeTable 2006, 290, E916–E924. [Google Scholar] [CrossRef]
- Duque, G.; Daly, R.M.; Sanders, K.; Kiel, D.P. Review article: Vitamin D, bones, and muscle: Myth versus reality. Australas. J. Aging 2017, 36, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Abrams, S.A.; Aloia, S.J.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; et al. IOM Committee members respond to Endocrine Society vitamin D guideline. J. Clin. Endocrinol. MeTable 2012, 97, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The effect of vitamin D supplementation on serum 25OHD in thin and obese women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, M.; Kraak, V.; Hulver, M.; Epling, J. Clinical management of low Vitamin D: A scoping review of physicians’ practices. Nutrients 2018, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Wu, C.C.; Liao, M.T.; Shyu, J.F.; Hung, C.F.; Yen, T.H.; Lu, C.L.; Lu, K.C. Role of nutritional vitamin D in osteoporosis treatment. Clin. Chim. Acta 2018, 484, 179–191. [Google Scholar] [CrossRef]
- Apostolakis, M.; Armeni, E.; Bakas, P.; Lambrinoudaki, I. Vitamin D and cardiovascular disease. Maturitas 2018, 115, 1–22. [Google Scholar] [CrossRef]
| Parameter | Group 1 (Daily) | Group 2 (Montly) | P |
|---|---|---|---|
| Number of subjects | 22 | 22 | NS |
| Sex (M/F) | 10/12 | 9/13 | NS |
| Age (yrs) | 77 ± 9 | 78 ± 8 | NS |
| Weight (kg) | 76 ± 18.6 | 77 ± 11.2 | NS |
| BMI (kg/m2) | 27.7 ± 6.5 | 27.3 ± 4 | NS |
| Waist circumference (cm) | 99.5 ± 13 | 105 ± 9 | NS |
| WtHR | 0.60 ± 0.78 | 0.63 ± 0.07 | NS |
| 25(OH) vitamin D (ng/mL) | 20.81 ± 6.98 | 20.51 ± 5.61 | NS |
| s-Calcium (mg/dL) | 8.99 ± 0.45 | 8.91 ± 0.29 | NS |
| s-Phoshate (mg/dL) | 2.52 ± 0.67 | 3.87 ± 0.56 | NS |
| s-Creatinine (mg/dL) | 0.96 ± 0.23 | 0.98 ± 0.25 | NS |
| CTX (ng/mL) | 0.50 ± 0.37 | 0.49 ± 0.31 | NS |
| PTH (pmol/L) | 8.39 ± 3.31 | 9.33 ± 6.76 | NS |
| ALP (U/L) | 85.25 ± 31.18 | 100.5 ± 74.24 | NS |
| Total Hip BMD (g/cm2) | 0.821 ± 0.105 | 0.873 ± 0.101 | NS |
| Total Hip T-score (SD) | −1.0 ± 0.9 | −0.3 ± 0.7 | NS |
| Femoral Neck BMD (g/cm2) | 0.656 ± 0.137 | 0.798 ± 0.201 | NS |
| Femoral Neck T-score (SD) | −1.7 ± 0.3 | −0.7 ± 0.7 | NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalle Carbonare, L.; Valenti, M.T.; Del Forno, F.; Piacentini, G.; Pietrobelli, A. Vitamin D Daily versus Monthly Administration: Bone Turnover and Adipose Tissue Influences. Nutrients 2018, 10, 1934. https://doi.org/10.3390/nu10121934
Dalle Carbonare L, Valenti MT, Del Forno F, Piacentini G, Pietrobelli A. Vitamin D Daily versus Monthly Administration: Bone Turnover and Adipose Tissue Influences. Nutrients. 2018; 10(12):1934. https://doi.org/10.3390/nu10121934
Chicago/Turabian StyleDalle Carbonare, Luca, Maria Teresa Valenti, Francesco Del Forno, Giorgio Piacentini, and Angelo Pietrobelli. 2018. "Vitamin D Daily versus Monthly Administration: Bone Turnover and Adipose Tissue Influences" Nutrients 10, no. 12: 1934. https://doi.org/10.3390/nu10121934
APA StyleDalle Carbonare, L., Valenti, M. T., Del Forno, F., Piacentini, G., & Pietrobelli, A. (2018). Vitamin D Daily versus Monthly Administration: Bone Turnover and Adipose Tissue Influences. Nutrients, 10(12), 1934. https://doi.org/10.3390/nu10121934







